Keywords
Incidental meningioma; Natural history; Tumor growth; Treatment modalities
Introduction
Meningioma is the second most common primary brain tumor [1], accounting for about 13 to 26% of all intracranial tumors [2]. It arises from arachnoid cells [3], predominates in women and its incidence increases with age [4]. It is estimated that up to 3% of patients older than 60 years may have an asymptomatic meningioma [5]. The number of incidentally discovered meningiomas (IDMs) is increasing due to the availability of modern imaging techniques and the greater longevity of current population. An incidental (asymptomatic) meningioma is one that is discovered when an image study is practiced to a patient with neurological symptoms that can not be linked to it [6-8], and nowadays, this is the form of presentation of almost 50% of diagnosed meningiomas [7,8]. Symptoms commonly encountered in those patients in whom the tumor is discovered are headache and dizziness [1,9].
Once the diagnosis is made, the neurosurgeon must face the challenge of finding the best treatment option for each patient. This, has been matter of debate, but the better knowledge of the natural history of these lesions has been clarifying this issue. The authors report 4 cases of IDMs and perform a review of the current literature concerning the natural history of these tumors, its growth patterns and management options.
Clinical Cases
Case 1
A 60-year-old male with a history of smoking and colon cancer diagnosed 2 years before, treated with surgery and chemotherapy with good control, was admitted to the Hospital after suffering a traffic accident (car dump). The head computed tomography (CT) scan did not show traumatic brain lesions but revealed a right frontal parasagital mass. He was lucid without any focal deficit. The chest, abdomen and pelvis CT discarded local recurrence of his tumor as well as compromise of others parenchymas. The magnetic resonance imaging (MRI) showed that the intracranial mass had dural contact and intense and homogeneous enhancement with gadolinium (Figure 1).
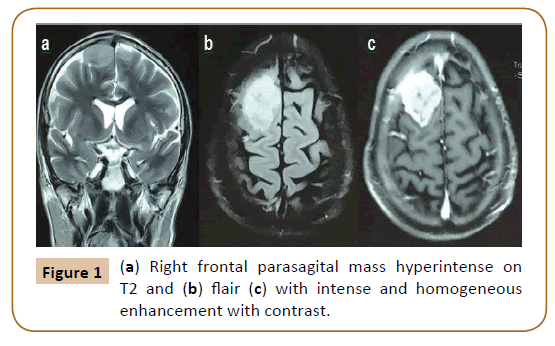
Figure 1: (a) Right frontal parasagital mass hyperintense on T2 and (b) flair (c) with intense and homogeneous enhancement with contrast.
Given the possibility of a dural metastasis considering the neoplastic history of the patient we decided to operate this incidental mass. We achieved complete resection of the tumor with its dural implantation in the convexity duramater and we performed a duraplasty with pericranium. The histopathological examination revelead a World Health Organization (WHO) grade II meningioma. The clinical evolution was satisfactory and control MRI showed the complete removal of the lesion (Figure 2). The patient is under close follow-up given the diagnosis of an atypical meningioma.
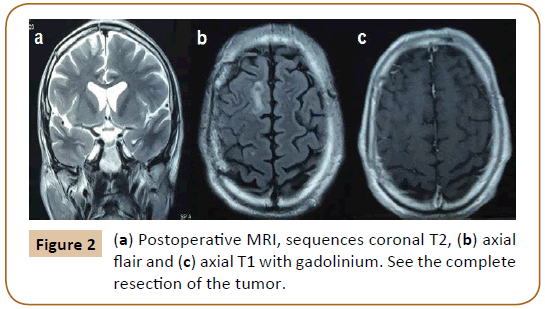
Figure 2: (a) Postoperative MRI, sequences coronal T2, (b) axial flair and (c) axial T1 with gadolinium. See the complete resection of the tumor.
Case 2
A 71-year-old man with a history of hypertension with irregular controls and moderate to severe renal chronic disease was admitted to the emergency medical center for respiratory symptoms and a viral impregnation syndrome. At physical examination he had mental confusion and because of this the emergency physician requested a cranial CT and a right pterional meningioma was found. He was diagnosed with a respiratory infection and once it was treated, his mental status improved. The evaluation of the lesion was completed with resonance (Figure 3) and after that we discussed the management options.
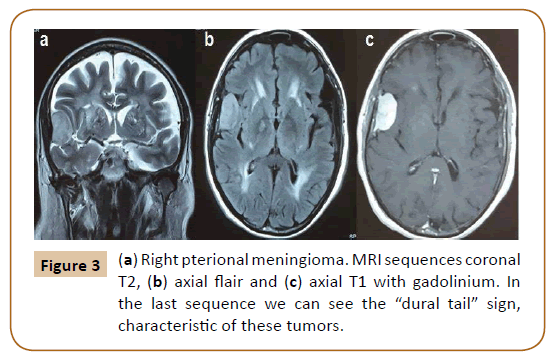
Figure 3: (a) Right pterional meningioma. MRI sequences coronal T2, (b) axial flair and (c) axial T1 with gadolinium. In the last sequence we can see the “dural tail” sign, characteristic of these tumors.
This incidental meningioma was hyperintense on T2-weighted MRI image, a feature associated with a higher growth rate. Anyway, this incidental tumor sits in a 71-year-old patient with a bad medical condition; therefore initial observation was considered the best management option.
Case 3
A 49-year-old female, carrier of sickle cell anemia, was derived by neurologist. She presented a history of dizziness of two months of evolution. The clinical examination did not reveal cranial nerves or focal spinal deficits. MRI showed a right sphenoid wing meningioma with extension to the anterior clinoid process and cavernous sinus, surrounded by edema (Figure 4).
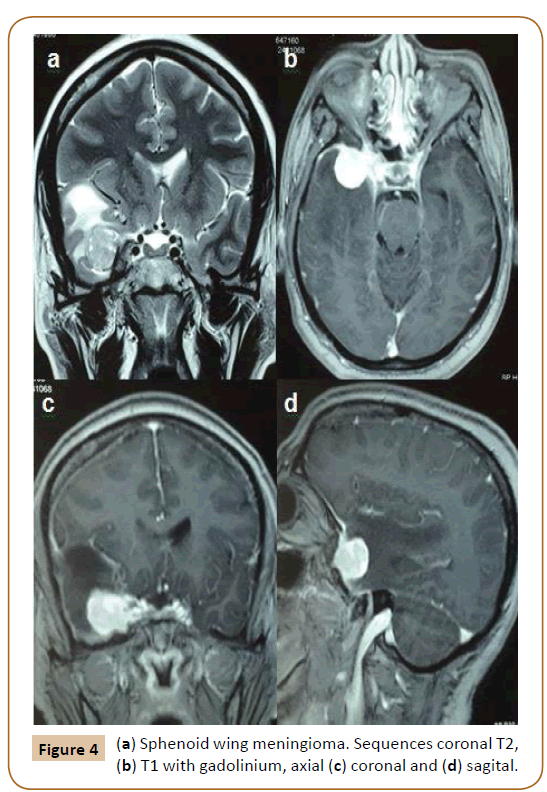
Figure 4: (a) Sphenoid wing meningioma. Sequences coronal T2, (b) T1 with gadolinium, axial (c) coronal and (d) sagital.
In this case, we chose surgical treatment taking into account the age of the patient, high signal of the tumor on T2-weighted MRI image and presence of brain edema. Even though, this location may be associated with increased surgical morbidity, early intervention can allow us to achieve a resection with a lower grade on the Simpson scale.
We performed a pterional craniotomy with extra-intradural approach to remove the lesion. The inmediate postoperative course was uneventful and the pathological anatomy confirmed a WHO grade I meningioma. An MRI was requested to evaluate tumor resection (Figure 5). After discharge, the patient presented right V1 pain, which has remained stable but requiring specific analgesia. The patient is being followed by neurologist and neurosurgeon in the outpatient clinic.
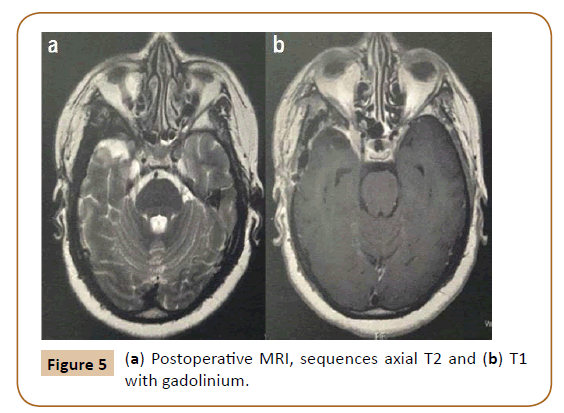
Figure 5: (a) Postoperative MRI, sequences axial T2 and (b) T1 with gadolinium.
Case 4
An 80-year-old male with a past history of smoking, hypertension and chronic obstructive pulmonary disease, came to our outpatient clinic. He carried a calcified left frontal meningioma, diagnosed 12 years before, asymptomatic.
In recents months adds dizziness and a new image is requested (Figure 6). The same did not show changes compared with previous images.
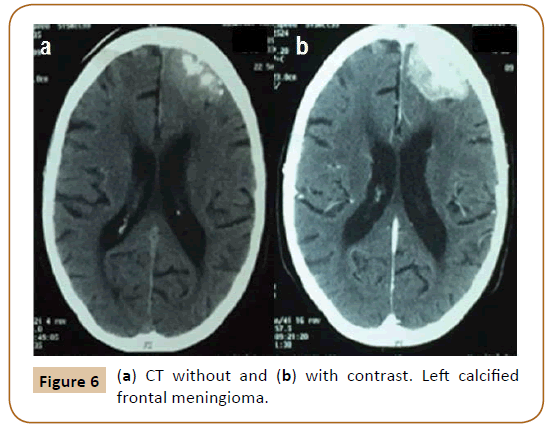
Figure 6: (a) CT without and (b) with contrast. Left calcified frontal meningioma.
Considering age, comorbidities, symptoms not related to the mass, which is a calcified meningioma that has remained stable for the last years, we decided for an expectant management.
The most important variables considered to define treatment in each case are summarized in (Table 1).
| Case |
Variable |
Treatment |
| 1 |
Differential diagnosis |
Surgery |
| 2 |
Age, comorbidities |
Observation |
| 3 |
Age, T2 signal, edema |
Surgery |
| 4 |
Age, comorbidities, calcifications |
Observation |
Table 1: Main variables considered for decision making and treatment adopted, according to the case.
Discussion
When dealing with an incidental meningioma we have to consider factors related to the patient and factors related to the tumor and its biology.
Age is one of the most important factors to consider in these patients. In cases 2 and 4 we adopted an expectant management because patients were old and had singnificant comorbidities. Asymptomatic meningiomas in patients older than 70 years have lower growth rates and the surgical morbidity in these patients is higher compared with younger ones, therefore, an strategy of “wait and see” is recommended by the majority of authors [9,10-12]. If the patient becomes symptomatic during follow-up interventional treatment must be considered.
On the other hand, in younger patients tumor growth is greater and tumor doubling time is shorter [4,13]. In one study the age less than or equal to 60 was associated with a significant risk of tumor growth [14]. This was one of the most important reasons to choose microsurgery in case 3, in addition to the presence of edema.
In relation to the factors linked to the tumor we have to consider the following aspects: natural history, tumor growth rate, location, initial size, presence of calcifications, signal intensity on T2-weighted MRI images and associated edema.
As we said, modern imaging techniques have allowed us to better understand the natural history of meningiomas.
Most IDMs remain stable or grow very slowly, according to the available literature [1,6,10,12]. In one study conducted by Yoneoka and collegues, 9 of 37 patients (24.3%) with incidental meningiomas showed significant growth [15]. In a series of 603 asymptomatic meningiomas, 63% did not experience growth and only 6% of the patients developed symptoms (3,9 years of followup) [10]. In another study, 65 patients with IDMs were followed for at least 10 years. The authors reported growth in 35.4% of the cases and the 15-year growth rate was 75%, concluding that long-term growth appears to be higher than expected, suggesting a more agressive treatment in young patients [1]. In this study, 84% of the patients that showed progression were treated.
Tumor growth has been defined as an increase of 2 or 5 mm in diameter [7], an anual growth rate greater than 1 cc [15] or an increase of 15% of the volume [16]. Volumetric analysis has shown to be more sensitive than the measurement of the linear diameter when estimating tumor growth, particularly in skull base meningiomas which can adopt irregular shapes. In one of the studies with more number of conservatively treated meningiomas, Oya et al. observed tumor growth in 74% of the cases using volumetric analysis compared with 44% when diameter measurement was used, showing the higher sensitivity of the former method [14].
The growth pattern of meningiomas can be variable. Some tumors grow exponentially, others follow a linear pattern and others can exhibit a more complex pattern; with an initial exponential growth, then a linear growth and finally reaching a plateau17. This latter pattern could explain the higher growth seen in younger patients [6,15,17] and in smaller tumors [6,12,17,18], but this last point is supported only by some authors.
Location of the tumor is not a minor aspect. Falx meningiomas may have an agressive behaviour, requiring early intervention and tuberculum sellae tumors can compromise visual pathway quickly, so surgery is recommended once they are diagnosed [8]. The potential invasion of a vascular structure, like a dural sinus can limit complete resection [13]. Skull base meningiomas may have a relatively indolent clinical course, reaching important volumes without significant symptoms [19]. In a study of 110 incidental meningiomas, Hashimoto et al. observed that skull base ones had significantly slower growth compared to other locations (p=0.002) and tumor doubling time was significantly higher (p=0.008) [20]. On the other hand, in a series of iniatially untreated petroclival meningiomas, Van Havenbergh and collegues observed growth in 76% of the cases during a mean follow-up period of 82 months [5,21]. The morbidity associated with surgery in skull base meningiomas can be greater compared with other locations, with risk of cranial nerve deficit and higher incidence of cerebrospinal fluid (CSF) leak. However, we must keep in mind that surgery in late stages when the tumor has grown significantly, can limit the resection with the lesser Simpson grade posible [4]. This was another reason considered to choose surgery in the patient with the sphenoid wing meningioma. Some authors suggest that skull base meningiomas will grow sooner or later resulting in significant morbidity and so they recommend treatment with microsurgery, emphasizing that dissection over neurovascular structures is easier in early stages [5].
Continuing with the analysis of the features linked to the tumor itself, we have to mention the role of the initial tumor size. Its predictive value to estimate tumor growth is controversial [13]. In the series of Oya et al. which included 244 patients with 273 meningiomas, one of the variables associated with higher annual growth rate was an initial tumor diameter greater than 2.5 cm (p<0.0001) [14]. However, most authors agree that this variable has no relationship with tumor enlargement [1,3,6], but it seems to be associated with symptoms onset. In one study, initial tumor size lesser than 2.5 cm was correlated with a silent behaviour in the first 5 years of follow-up [18].
One of the most important predictive factors is the presence of calcifications. The percentage of calcifications in asymptomatic meningiomas usually is higher compared with symptomatic ones. Calcified tumors have been correlated with a lower MIB-1 index labeling [22] and therefore they have less tendency to enlarge [3,4,16]. Case 4 is an example of this issue, showing us a stable asymptomatic tumor mass for several years.
On the other hand, high tumor signal on T2-weighted MRI image is one of the most significant risk factors for high tumor growth rate [11].
The patient of case 3 carried a history of dizziness, with an MRI showing us a right sphenoid wing meningioma with perilesional edema. Considering that symptoms were nonspecific, we managed this lesion as an incidental meningioma and the presence of edema was one of the variables that led us to propose surgery. This factor has been correlated with a higher growth rate [14].
A recent meta-analysis of 9 studies with a total of 777 patients with asymptomatic meningiomas concluded that only the presence of calcifications and a low intensity signal on T2- weighted MRI images were associated with slow growth, suggesting a conservative management in such cases. On the other hand, they do not find correlation between tumor growth and other factors such as sex, skull base location and peritumoral edema [3].
In the first case of this paper we adopted an early intervention, due mainly to the posibility of a dural metastasis taking into account the patient’s past history of cancer. Dural metastasis may have the same enhancement pattern as meningiomas, including dural tail [4], so anatomopathological diagnosis was considered a priority in that case, given its therapeutic implications. Another tumor that can mimic meningioma is hemangiopericytoma. Initially considered a variant of the former, has a more agressive behaviour, with high recurrence rate and ability to metastasize [23].
With respect to follow-up of IDMs several algorithms have been proposed. Authors like Abolfotoh and Al-Mefty propose an initial MRI 3 months after diagnosis to discard fast growth or lesions that can mimic meningioma and if the lesion remains stable a new MRI is requested at 9 months. After that, an annual or year by resonance can be performed if the patient remains asymptomatic during the first 5 years. According to that authors, if tumor shows enlargement but the patient is still asymptomatic an MRI is performed every 6 months and if progressive and significant growth is documented over time, treatment is necessary [8].
The management options available in patients with incidental meningiomas are basically three: observation with close clinical and imagenological follow-up, surgery and radiosurgery.
As we said previously, most incidental meningiomas remain stable over time, so initial observation is a reasonable option and is supported by the literature [6,8-14,16].
In those lesions that become symptomatic and/or demonstrate sustained growth during follow-up period, we must adopt an interventional treatment and microsurgery offers the patient the best possibilities of cure. It is documented that once the meningioma is growing the risk of permanent neurological morbidity after treatment is much higher if the patient is symptomatic than if it is not [11]. This is an important argument considered by some authors in favor of interventional treatment once the lesion has started to grow.
Surgery has evolved significantly in the past decades. In a recently published work, the recurrence rates for WHO grade I meningiomas after resections grade I, II, III and IV in Simpson scale were 5%, 22%, 31% and 35%, respectively, in a period of time of more than 15 years [24].
Surgery has fewer complications in asymptomatic patients compared with symptomatic ones [25], and as in cases 1 and 3 sometimes can be performed once the incidental meningioma is diagnosed [6,11], especially in patients lesser than 60-years-old [25].
In skull base meningiomas, although is a matter of debate, there are authors who advocate for early surgery, because these tumors can grow into the skull base foramina or adopt an en plaque distribution, significantly increasing surgical risks [5].
The authors of the present work think that in young asymptomatic patients, with many years of life ahead, microsurgery should be the first treatment option before the tumor generates its own morbidity, knowing that current microsurgical techniques in well trained neurosurgeons decrease the risk of procedural complications, achieving good ocutcomes.
Gamma knife radiosurgery (GKRS) is a therapy that has begun to be used as initial treatment in small incidental meningiomas [11,26] since it can control tumor growth [11,27]. Cerebral edema [25,27] and risk of malignant transformation are some of the short and long term complications of this therapy, respectively [11,26,28,29]. In opinion of the authors, GKRS as first treatment option should be reserved for very selected cases, like older patients with significant comorbidities non suitable for surgery, because although tumor control can be achieved, this modality of treatment is not without short and long-term risks and does not allow complete removal of the meningioma.
Conclusion
The knowledge of the natural history of incidental meningiomas and its predictive growth factors are very important when dealing with these kind of lesions.
Most IDMs remain stable over time, so they can be managed conservatively with close follow-up. Interventional treatment is recommended when the lesion becomes symptomatic or shows sustained growth.
In order to achieve the goal of complete resection, microsurgery is the treatment of choice. GKRS is an alternative treatment option, especially in elderly patients with poor medical condition.
21745
References
- Jadid KD, Feychting M, Höijer J, Hylin S, Kihlström L, et al. (2015) Long term follow-up of incidentally discovered meningiomas. Acta Neurochir 157: 225-230.
- Chang V, Narang J, Schultz L, Issawi A, Jain R, et al. (2012) Computer-aided volumetric analysis as a sensitive tool for the management of incidental meningiomas. Acta Neurochir 154: 589-597.
- Zeng L, Liang P, Jiao J, Chen J, Lei T (2015) Will an Asymptomatic Meningioma Grow or Not Grow? A Meta-analysis. J Neurol Surg A 76: 341-347.
- Chamoun R, Krisht KM, Couldwell WT (2011) Incidental meningiomas. Neurosurg Focus 31: E19.
- Borba AB, Cavalcanti DD (2013) The Role of Microsurgery in the Management of Small Skull-Base Meningiomas. Controversies in Neurosurgery II. New York: Thieme, pp: 60-63.
- Nakamura M, Roser F, Michel J, Jacobs C, Samii M (2003) The natural history of incidental meningiomas. Neurosurg 53: 62-70.
- Yano S, Kuratsu JI (2011) Natural course of untreated meningiomas. In: DeMonte F, McDermott MW, Al-Mefty O (eds.), Al-Mefty’s Meningiomas. 2nd edn. New York: Thieme, pp: 63-67.
- Abolfotoh M, Al-Mefty O (2013) Observation of Incidental Meningiomas. In: Al-Mefty O (eds.), Controversies in Neurosurgery II. New York: Thieme, pp: 60-63.
- Firsching RP, Fischer A, Peters R, Thun F, Klug N (1990) Growth rate of incidental meningiomas. J Neurosurg 73: 545-547.
- Yano S, Kuratsu J (2006) Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg 105: 538-543.
- Jo KW, Kim CH, Kong DS, Seol HJ, Nam DH, et al. (1999) Treatment modalities and outcomes for asymptomatic meningiomas. Acta Neurochir 153: 62-67.
- Olivero WC, Lister JR, Elwood PW (1995) The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg 83: 222-224.
- Rubin G, Herscovici Z, Laviv Y, Jackson S, Rappaport ZH (2011) Outcome of untreated meningiomas. Isr Med Assoc J 13: 157-160.
- Oya S, Kim SH, Sade B, Lee JH (2011) The natural history of intracranial meningiomas. J Neurosurg 114: 1250-1256.
- Yoneoka Y, Fujii Y, Tanaka R (2000) Growth of incidental meningiomas. Acta Neurochir (Wien) 142: 507-511.
- Hashiba T, Hashimoto N, Izumoto S, Suzuki T, Kagawa N, et al. (2009) Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. J Neurosurg 110: 675-684.
- Hashimoto N, Yoshimine T (2012) Incidentally Discovered Meningiomas: Growth Rates and Patterns. In: Hayat MA (ed.), Tumors of the Central Nervous System, Volume 7: Meningiomas and Schwannomas. Springer, pp: 87-94.
- Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, et al. (2010) Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg 113: 1036-1042.
- Bindal R, Goodman JM, Kawasaki A, Purvin V, Kuzma B (2003) The natural history of untreated skull base meningiomas. Surg Neurol 59: 87-92.
- Hashimoto N, Rabo CS, Okita Y, Kinoshita M, Kagawa N, et al. (2011) Slower growth of skull base meningiomas compared with non–skull base meningiomas based on volumetric and biological studies. J Neurosurg 116: 574-580.
- Van Havenbergh T, Carvalho G, Tatagiba M, Plets C, Samii M (2003) Natural history of petroclival meningiomas. Neurosurg 52: 55-62.
- Lee EJ, Kim JH, Park ES, Kim YH, Lee JK, et al. (2017) A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg 127: 971-980.
- Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, et al. (2011) Predictors of mortality following treatment of intracranial hemangiopericytoma. Clinical article. J Neurosurg 113: 333-339.
- Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, et al. (2017) Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg 126: 201-211.
- Zeng L, Wang L, Ye F, Chen J, Lei T, et al. (2015) Clinical characteristics of patients with asymptomatic intracranial meningiomas and results of their surgical management. Neurosurg Rev 38: 481-488.
- Hasegawa T (2013) Stereotactic Radiosurgery for Small Meningiomas. In: Al-Mefty O (ed.). Controversies in Neurosurgery II. New York: Thieme, pp: 56-60.
- Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, et al. (2008) Radiosurgery as definitive management of intracranial meningiomas. Neurosurg 62: 53-58.
- Couldwell WT, Cole CD, Al-Mefty O (2007) Patterns of skull base meningioma progression after failed radiosurgery. J Neurosurg 106: 30-35.
- Kunert P, Matyja E, Janowski M, Marchel A (2009) Rapid growth of small, asymptomatic meningioma following radiosurgery. Br J Neurosurg 23: 206-208.











