Key words
|
| |
| Agglomeration, flowability, compactibility, bulk drug powder |
| |
INTRODUCTION
|
| |
| Tablets are the most common solid dosage form for many reasons including ease of manufacturing and better stability than liquids and parenteral dosage forms. Direct compression is the simplest and most economical method for the manufacturing of tablets because it requires less processing steps than other techniques such as wet granulation and roller compaction. However, most powder drugs cannot be compressed directly into tablets [1]. At present, the pharmaceutical industry tends to relate a powders physical functionality to the size of single particle properties, bulk properties, particle-particle interactions and powder morphology (particle size, specific surface area, porosity and particle shape). Several techniques are available for building the desired physical functionality to the powder to render it for direct compression powder. |
| |
| Agglomeration is the most widely used pretreatment method for the production of powders with altered solid state properties. Agglomeration is a size enlargement process in which primary particles stick together to form agglomerates [2]. In past, various techniques has been developed to form agglomerates that include melt extrusion [3], melt agglomeration [4], Crystallo-co-agglomeration [5], agglomeration and coagglomeration [6], spherical crystallization [7,8,9] etc. Thus, there are several options available to produce an agglomerated product and it can be difficult to narrow down the best method. |
| |
| Flowability is the ability of solids and powders to flow. Flow behavior is multi-dimensional in nature and it depends on many physical characteristic [10]. Particle size and particle size distribution, bulk density, angle of repose and compressibility play significant role in flowability of bulk solids. A study was carried out using Hausners ratio and angle of repose as indicator to determine powder flowability of two types of powders- spherical and angular by Geldart et al [11]. It was revealed that increase in the particle size of a powder results in decrease in cohesiveness. The same trends were observed for the angle of repose, which increased for both the materials as the mean particle size decreased. |
| |
| Compressibility and compactibility of a powder are influenced by the flow properties and in the microscale by the adhesion forces between the particles. Compressibility is the ability to reduce the volume under pressure and compactibility is the ability to build the solid agglomerate under pressure with sufficient strength and stability [12]. The two most commonly used measures of the relative importance of interparticulate interactions are the compressibility index often referred as Carrs index and Hausners ratio. |
| |
| In this study, solvent treated method was employed to form agglomerated powder. Mesalamine (MSA) original, was used as a model drug. Additionally, result of a study of the effect of shape and size of solvent treated agglomerated powder on powder flow and compressibility properties is presented. These properties were evaluated by comparing the MSA and wet granules of the drug (MSA-Wet). |
| |
MATERIAL AND METHODS
|
| |
|
Materials
|
| |
| Mesalamine was obtained from Swastic Chemicals, Nagpur, India. Ammonia, starch and other materials were purchased from S. D. Fine chemicals (Mumbai, India). |
| |
|
Solvent treated Agglomeration Method
|
| |
| Mesalamine powder was added to 5% aqueous ammonia solution as the solvent treatment. The slurry was stirred mechanically (at 100, 200 and 300 rpm) at room temperature for the specified time (1/2 h and 1 h). The ammonia-treated undissolved powder was separated using a sintered glass funnel, and left to dry at room temperature away from light. The dried agglomerate powder was stored in plastic bag. |
| |
|
Wet granulation method (MSA-Wet)
|
| |
| Mesalamine powder was granulated using 5 % soluble starch as binder solution. The granules were dried in a laboratory tray dryer, passed through 500 Gm screen using a sieve shaker and stored in plastic bag. |
| |
|
Powder characterization
|
| |
|
Particle size distribution
|
| |
| Particle size distribution of the powders were determined by sieve (mesh) analysis using laboratory sifter equipped with series of 6 screens and a pan. An approximate 10 gm sample was tested for total sifting time of 5 min. The method was carried out in triplicate (n=3). |
| |
|
Determination of particle shape parameters
|
| |
| The particle shape in the present work was measured by using Motic (optical) microscope with an attached digital camera (Olympus). The microscope was used to create 10 images, at a magnification of 10X or 40X. From the microscope images, approximately 100 particles were analyzed using the Image-Pro Plus software to determine the particle descriptors of major and minor axis length and perimeter. Aspect ratio and irregularity were calculated from these particle descriptors using equations |
| |
| Aspect Ratio = b/l (1) |
| |
| Irregularity P/l (2) |
| |
| In these equations b represents the length of the minor axis, l is the length of the major axis, and P is the perimeter. |
| |
|
Static Angle of Repose
|
| |
| The static angle of repose flowability test was performed following the procedure described in literature [13]. A conical funnel was mounted with its stem 6 cm from the horizontal surface. Between 50 and 100 grams of powder were poured through the funnel, enough that the top of the resulting pile reached the funnel outlet. The angle measured on the right and left hand sides of the pile were averaged to give a single static angle of repose. The angle of repose can be obtained from equation |
| |
| Tan θ = h/0.5d (3) |
| |
| Where h- height of the cone and d- diameter of the cone. The method was carried out in triplicate ( n=3). |
| |
|
Hausners Ratio and Carrs Index
|
| |
| The Hausners Ratio and Carrs Index are both calculated from compressibility data [14]. The test powder is gently loaded through a funnel into a 100 ml cylinder and weighed to calculate its bulk density. Next, the cylinder is tapped in a single platform tapped density meter till no change in the volume of powder is observed. The Hausner ratio is calculated from equation (4) and the Carr Index from equation (5), where BD is the powder bulk density and TD is the powder tapped density [15]. The method was carried out in triplicate (n=3). |
| |
| HR = TD/ BD (4) |
| |
| CI= TD-BD/TD x 100 (5) |
| |
|
Porosity
|
| |
| The porosities of the MSA, MSA-AG and MSA-Wet powder were calculated from their bulk and true densities. The porosity of the powder was calculated from true density of the powder using the following equation: |
| |
| Total Porosity (E) = 1- Bulk density/ True density (6) |
| |
|
Packability
|
| |
| Sample packability was assessed by analysis of the tapping process with the Kawakita [16] (Eq. 7) and Kuno [17] (Eq. 8) methods, and using the parameters a, b, and k in the equations. |
| |
| n/C = 1/ (ab) +n/a. (7) |
| |
| C = (Vo-Vn)/Vo, a =(Vo-V∞) /Vo. |
| |
| ρf- ρn= (ρf- ρo) . exp. (-kn) |
| |
| Where: a is the degree of volume reduction when the tap number is infinity, b and k are constants for the apparent packing rate, V0 and Vn are the volume in the initial loosely packed and the nth tapped state, and ρ0, ρ n, and ρ f are the apparent density in the initial state, the nth tapped state, and the most densely packed state. |
| |
|
Compact Preparation of Powder
|
| |
| Compact compression was performed on R&D tablet press tablet (model M/C-12 STN, Cemach Machineries Ltd, India). Six different compaction forces (from 1 ton to 6 ton) were used for MSA, MSAAG and MSA-Wet powders. The compact tablets were of 480 mg of powder and a flat-faced punch with a diameter of half inch (D-tooling) was used. The punch and die were lubricated with magnesium stearate before punching. Each compact was weighed accurately, and its dimensions (diameter and thickness) were measured with vernier caliper apparatus. |
| |
|
Compact characterization of MSA, MSA-Wet, MSA-AG
|
| |
|
Heckel Analysis
|
| |
| The following Heckel’s equation [18] was used to analyze the compression process of agglomerated crystals and wet granules, and assessed their compactibility. |
| |
| In [1/(1-D)]=KP+A (9) |
| |
| Where, D is the relative density of the tablets under compression Pressure, K is the slope of the straight portion of the Heckel Plot. The following equation gives the intercept obtained by extrapolating the straight portion of the plots. |
| |
| A=1n [1/(1-D0)]+B (10) |
| |
| Where, D0 is the relative density of the powder bed when P=0. The following equation gives the relative densities corresponding to A and B. |
| |
| DA=1-e-A (11) |
| |
| DB=DA-D0 (12) |
| |
|
Tablet Elastic Recovery Test
|
| |
| Each powder was placed, 450 mg, in a die with 12 mm diameter and compressed under 6 tons (Hc) pressure. The thickness and diameter of each tablet at initial and after 24 h of ejection (He) was measured. Following equation was used to calculate the elastic recovery ratio (ER). |
| |
| ER=[(He – Hc)/Hc]x100 (13) |
| |
| About 24 h after the tablet was ejected, its weight, diameter, and thickness were measured, and its apparent density ρa calculated. |
| |
|
Compact Hardness, Tensile strength, Friability and Disintegration time
|
| |
| Tablet hardness was determined using a Monsanto hardness tester. The tensile strength (T) of the compact was calculated using the following equation: |
| |
| T = 2F/ π Dt (14) |
| |
| in which D and t are the diameter and thickness of the compact, respectively, and F is the force fracturing the compact. |
| |
| The friability values of the tablets were determined using a Roche-type friabilator. It was rotated at 25 rpm for 4 min. Percent friability was calculated using the following equation: |
| |
| Friability = ([W0 – W]/ W0) x 100 (15) |
| |
| in which W0 is the weight of the tablets at time zero before revolution, and W is the weight of the tablets after 100 revolutions. |
| |
| The disintegration times of the tablet formulations were determined using a tablet disintegration test apparatus (Veego, Mumbai). |
| |
|
Content Uniformity
|
| |
| Ten tablets of MSA were weighed and powdered. Crushed powder of tablets equivalent to 0.15 gm was weighed and dissolved in pH 7.4 Phosphate buffer. Solution was filtered and diluted and drug content was analyzed spectrophotometrically at about 334.5 nm. Each sample was assayed to triplicate (n=3). |
| |
RESULTS AND DISCUSSION
|
| |
|
Preparations of agglomerates
|
| |
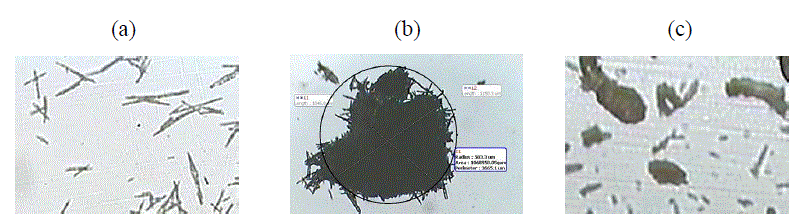
| It was found that crystal agglomeration was possible with solvent treated method. The MSA consisted of single, very small crystals and with an unfavorable habit for direct compression [Figure 1(a)]. The structure and particle size distribution of the agglomerated powder were determined by the parameters that depended on the stirring rate and the time of stirring. It was observed that less the initial time of stirring and larger stirring rate resulted in no or smaller size agglomerates. More time of stirring and slower stirring rate was favorable in the building-up of crystal agglomerates with a closed structure [Figure 1(b)]. On the basis of above results the agglomerates preparation was optimized considering stirring rate of 100 rpm and stirring time of 1 h and this agglomerate powder now onwards would be named as MSA-AG for further characterization. The agglomerates of wet granulation showed irregular structure [Figure 1 (c)] with relatively larger sizes than MSA. |
| |
|
Powder characterization of MSA, MSA-Wet, MSA-AG
|
| |
| The physical properties of the MSA, MSA-AG and MSA-Wet are summarized in Table 1. The mean diameters of the agglomerated particles were approximately 40–60 times higher than those of the untreated MSA crystals. |
| |
| The flow properties of particulate solids are known to depend on the size, shape and size distribution of particles [2]. Aspect ratio varies between 0 and 1, with a low value indicative of an elongated particle; a perfect circle has an aspect ratio of 1. Irregularity measures the surface area compared to the size of the particle; in this case, a perfect circle has an irregularity of π. Table 1 showed that the aspect ratio of MSA-AG powder was close to 1, indicating spherical shape while that of MSA-Wet had lower aspect ratio. Similarly MSA- AG was less irregular while MSA-Wet exhibited higher irregularities. The aspect ratio for MSA could not be calculated as they were small and needle shaped. |
| |
| According to Cain [14], a static angle of repose greater than 40° indicates a cohesive powder, whereas an angle greater than 50° indicates a very cohesive powder. The angle of repose of MSA crystals clearly shows that they form very cohesive powder. |
| |
| The bulk density and tapped density of MSA is lower than those of the MSA-AG and MSA-Wet indicating more porous nature of powder. The MSA had a Hausner ratio of 1.7 and a Carr index of 43.7 %. Both of these measurements are indicative of a cohesive powder [14]. MSA-AG and MSA-Wet had Hausner ratio of 1.28 and 1.20 whereas a Carr index of 22.19 % and 16.4 % respectively. These measurements indicate increased or good flow property. |
| |
| The equations of Kawakita and Kuno were used to analyze the tapping process. The value ‘a’ in Kawakita equation was lower for MSA-Wet compared to that of the MSA crystals, while ‘b’ in Kawatika equation and ‘k’ in Kuno equation were both higher for MSA-AG and MSA-Wet than MSA. This indicates that both MSA-AG and MSA-Wet had excellent flowability and packability. |
| |
|
Compact characterization of MSA, MSA-Wet, MSA-AG
|
| |
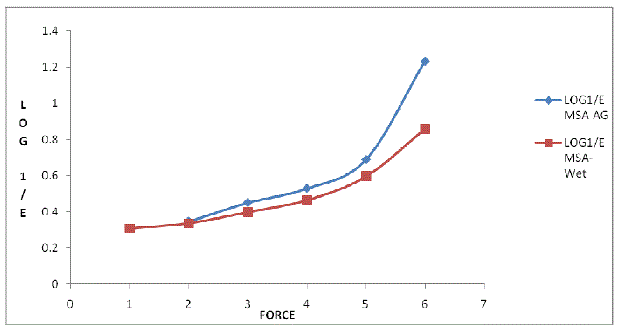
| In order to achieve uniformity in tablet weight, the feed powder crystals must flow and pack smoothly into the die cavity of the tablet machine. The MSA granules were not compressible. The Heckel plots for the MSA-AG and MSA-Wet are shown in Figure 2 and Table 1, shows the Heckel constants derived from the plots. The plots for the MSA-AG and MSAWet were similar, showing linearity over the compression range of 2-4 tons, indicating that the mechanism of consolidation of the material were similar, predominantly plastic deformation. The slope of the linear portion, K, can be correlated to the crushing strength of compacts; larger values of K usually indicate harder compacts [18]. The K values for the MSA-AG and MSA-Wet are comparable and would be expected to form harder compacts. On the basis of these findings, it could be concluded that good flowability and packability for agglomerates (MSA-AG) may be attributed to the spherical shape and the bigger particle size. |
| |
| A hardness study of tablets showed that the tablets prepared from MSA-AG and MSA-Wet had similar mechanical strength (see Table 2). As expected, the tensile strength of the MSA-AG compact was more than the MSA- Wet compacts. This may be a result of the stronger bonds formed between newly formed crystals of agglomerates. A friability study showed lower friability of the tablets prepared from the MSAAG, possibly owing to the better compaction of the spherical crystals. Disintegration tests showed that the tablets of MSA-AG and MSA-Wet were comparable and disintegrated within the prescribed official limits. |
| |
CONCLUSIONS
|
| |
| It was concluded that the selected solvent treated method for the formation of agglomerates of model drug improved the powder functionality properties of the of MSA drug powder such as flowability, packability and compactibility. Compacts by direct compression method could be formed successfully of agglomerated powder (MSA-AG) that was comparable with the wet granules (MSA-Wet). The original powder of model drug (MSA) failed to be compressed by direct compression method. Thus MSA-AG provided the requirements needed to convert wet granulated formulations to direct compression formulations, thus avoiding labor and energy intensive of wet granulation processes and is an alternative to develop cost effective formulations. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |








