Keywords
Marchiafava-bignami; Alcohol; Dementia; Corpus callosum
Introduction
Marchiafava-Bignami disease (MBD) is a rare disease, with overall high mortality and disability rates, where many pathogenic and prognostic factors are somewhat obscure. Both the clinical and the imaging features can provide useful information about the evolution. However the clinical significance of the presence of extra-callosal lesions is still uncertain [1]. Our patient has stabilized into a chronic dementia, despite the presence of favorable and unfavorable prognostic hints.
Case Report
A 44 year-old Italian man with a 25-year history of alcohol abuse (he declared cheap Italian red wine, 2 liters/day) was admitted to the emergency room (ER) of our hospital. In the past two weeks he had been increasingly showing asthenia, mental slowing and gait impairment. Relatives reported a weight loss of 4 kg in 10 days, secondary to complete fasting.
On clinical examination, he seemed cachectic (body weight = 48 kg) and dehydrated. He was confused and disoriented in space and time. He appeared overall slow from the psychomotor point of view, and unable to execute complex orders. On neurological examination, he showed an ataxic gait, and a diffuse muscle wasting and weakness (more proximal than distal). His face was hypo-mimic, and his speech was dysarthric and slow. Tendon reflexes were diffusely weak, while plantar responses were normal. Cranial nerves examination was normal. There were no sensation defects. Meningeal signs were absent.
A CT scan of the head showed a low-density lesion in the splenium of the corpus callosum. Moreover, there were lowdensity lesions, without perilesional edema, in the lateral frontal lobe on both sides (Figures 1A and 1B).
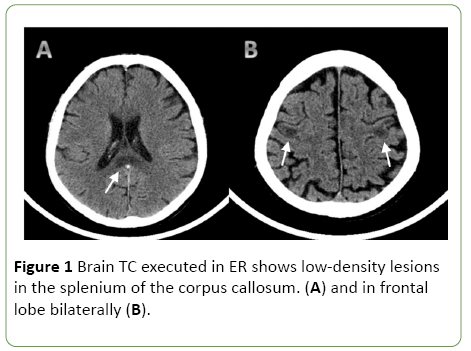
Figure 1: Brain TC executed in ER shows low-density lesions in the splenium of the corpus callosum. (A) and in frontal lobe bilaterally (B).
Laboratory investigations revealed a normal serum glucose (96 mg/dL), low sodium (132 mEq/L) and potassium (3.3 mEq/L) levels. The alcohol blood content was zero. Haepatic, renal, and thyroid functions were normal. B1, B12 vitamins and folate were in the normal range. Autoimmunity screening (ANA, ENA, AMA, pANCA, cANCA) proved no abnormalities. The cerebrospinal fluid was normal. An EEG showed diffuse slow waves of 6-7 Hz, with a slight prevalence in frontotemporal regions, without epileptiform discharges.
On day 2 from admission, a cranial MRI with diffusionweighted sequences showed restricted water diffusion in the splenium and the body of corpus callosum with a decreased apparent diffusion coefficient (ADC) value (Figure 2). T2 and Fluid Attenuated Inversion Recovery (FLAIR) weighted sequences confirmed hyperintense lesions of the splenium and the body of corpus callosum, with relative sparing of the dorsal and ventral layers, which represented the typical “sandwich sign” (Figure 3) [2]. In FLAIR images, the frontal cortical-sottocortical lesions seen on the CT scan were evident as hyperintense areas (Figure 4).
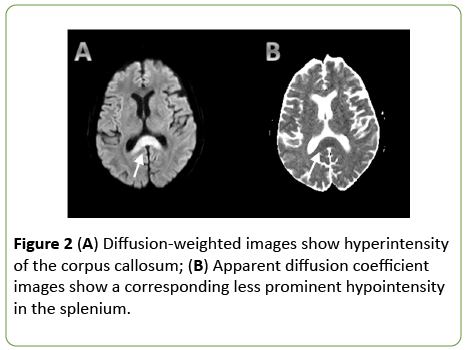
Figure 2: (A) Diffusion-weighted images show hyperintensity of the corpus callosum; (B) Apparent diffusion coefficient images show a corresponding less prominent hypointensity in the splenium.
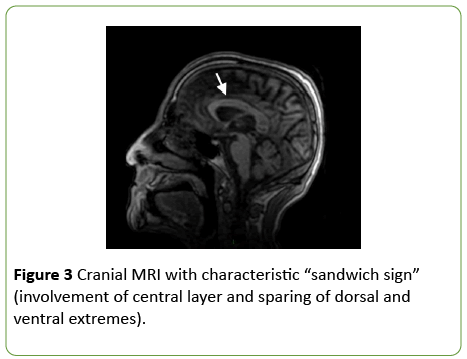
Figure 3: Cranial MRI with characteristic “sandwich sign” (involvement of central layer and sparing of dorsal and ventral extremes).
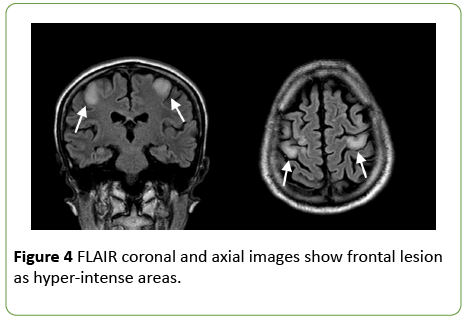
Figure 4: FLAIR coronal and axial images show frontal lesion as hyper-intense areas.
After 15 days of hospitalization the patients underwent a basic neuropsychological assessment with a Mini Mental State Examination (MMSE), which disclosed a severe cognitive impairment (score = 14/30), and a Frontal Assessment Battery (FAB), that showed a marked deficit of frontal lobe functions (score = 5/18). A more detailed neuropsychological evaluation was impossible because of the patient inability to understand the tasks and to maintain an adequate attention.
On the basis of the above-mentioned features, a diagnosis of Marchiafava-Bignami disease was made.
Because of the history of alcohol abuse, a diagnosis of Wernicke Encephalopathy was initially considered but excluded on the basis of clinical and MRI findings. The same applied to the hypothesis of an alcohol withdrawal syndrome. Similarly, other diseases involving the corpus callosum, such as multiple sclerosis, encephalitis, lymphoma, infarction or solid tumors were not substantiated by the history, clinical features and clinical findings.
On admission, the patient was promptly treated with parenteral thiamine (300 mg/day), hydration and parenteral nutrition with vitamin supplement, so the electrolyte balance was quickly restored. We did not use steroid therapy. Three days after hospitalization the patient was started on a physical therapy program. The program started with Early Active Mobilization Protocol; gradually the rehabilitation program included also the Frenkel and Rhythmic Stabilization Exercises.
Seven to ten days after admission, we noticed a progressive improvement of the ideomotor performance. The patient was able to walk slowly without support. He was more ready to interact with the medical and nursing staff. He continued being disoriented in space and time, but there were no clear signs of hemispheric disconnection. Two weeks later admission, the patient underwent to brain TC as a control, without significant changes. After 25 days in the neurological ward, his conditions were such to allow him to be discharged, in the context of a rehabilitative program. At the release, the neurocognitive performance were slight better, although a complete evaluation was not possible (MMSE: 15/30, FAB 7/18).
Six month later his clinical conditions were stable: He lived in a nursing home, depending on the activities of daily living; his relatives rejected additional follow up investigations.
Discussion and Conclusion
MBD is characterized by a degenerative lesion of the corpus callosum, probably related to deficiency of nutritional factors [3]. It is considered a form of toxic demyelination seen in alcoholic patients, especially males between 40 and 60 years of age. MBD was observed in persons of all ethnic groups and drinking any type of alcoholic beverage, and even in rare nonalcoholic patients [4,5].
MBD can present in two main clinical forms according to Heinrich’s classification:
Type A, with impairment of consciousness, seizures, hemiparesis, poor prognosis and T2W hyperintense swelling of the entire corpus callosum, sometimes with extracallosal lesions;
Type B, with slight impairment of consciousness, progressive dementia, pyramidal and cerebellar dysfunctions, better outcome and partial callosal lesions on MRI [6,7].
Neuroimaging can help in diagnosis and staging of MBD. In the acute phase, related to cellular damage and cytotoxic edema, MRI lesions are located across the corpus callosum (body and genu, then splenium), hypointense in T1 and hyperintense in T2 sequences, with restricted diffusion in DWI without a mass effect and rarely they may have contrast enhancement. Occasionally, similar lesions can involve other areas, such as anterior and posterior commissures, brachium pontis [8], optic chiasm, putamen [9], and frontal cortex [1,10]. In the chronic phase, because of the progression of demyelinating process, it possible to observe necrotic and cystic areas, haemosiderin stores, atrophy of corpus callosum [11,12].
Each imaging profile has been associated with a given clinical and prognostic evolution. A partial lesion of corpus callosum would imply a chronic disease, with variable cognitive recovery, and a survival in the order of years. A complete injury to the corpus callosum, together with extracallosal lesions, would rather imply an aggressive course, with poor outcome [1].
Cortical lesions, uncommon in MBD, are usually associated with complete involvement of corpus callosum. Few reports of partial damage to the corpus callosum and associated cortical lesions with good outcome are described [13-15].
In our patient an early MRI showed a partial involvement of the corpus callosum, described as favorable sign, and cortical lesions, related to severe disease and poor outcome. We hypothesize that a prompt therapy can be efficient to stop the demyelinating process and, subsequently, the disease progression.
MRI imaging, alone, albeit very useful in the diagnostic process, cannot be considerate an infallible predictor of outcome; it need to be evaluate with the type of onset and the clinical course.
18442
References
- Johkura K, Naito M, Naka T (2005) Cortical involvement in Marchiafava-Bignami disease. AJNR Am J Neuroradiol 26: 670-673.
- Gopal-krishnaMurthy KP (2014) Magnetic resonance imaging in marchiafava-bignami syndrome: A cornerstone in diagnosis and prognosis. Case Rep Radiol 2014: 609708.
- Bignami A, Marchiafava E (1903) Sopra un’ alterazione del corpocallosoosservata in soggettialcoolisti. Riv. Patol. nerv. ment 8: 544-549.
- Caulo M, Briganti C, Notturno F (2009) Non-alcoholic partially reversible marchiafava-bignami disease: Review and relation with reversible splenial lesions. A case report and literature review. Neuroradiol J 22: 35-40.
- Jorge JM, Gold M, Sternman D (2015) Marchiafava-Bignami disease in a trauma patient. J Emerg Trauma Shock 8: 52-54.
- Delay J, Brion S, Escourolle R (1961) Corticocallosal lesions of alcoholism. Corpus callosum degeneration of Marchiafava-Bignami and laminary cortical sclerosis of Morel. World Neurol 2: 662-671.
- Heinrich A, Runge U, Khaw AV (2004) Clinico-radiologic subtypes of Marchiafava-Bignami disease. J Neurol 251: 1050-1059.
- Victor M, Adams RD, Collins GH (1989) The Wernicke-Korsakoff Syndrome and related neurologic disorders due to alcoholism and malnutrition. J NeurolNeurosurg Psychiatry 52: 1217-1218.
- Ihn YK, Hwang SS, Park YH (2007) Acute Marchiafava-Bignami disease: Diffusion-weighted MRI in cortical and callosal involvement. Yonsei Med J 48: 321-324.
- Delay J, Brion S, Escourolle R (1959) Rapports entre la degenerescence du corps calleux de Marchiafava-Bignami et la sclerose luminaire corticale de Morel. Encephale 49: 281.
- Hillbom M, Saloheimo P, Fujioka S (2014) Diagnosis and management of Marchiafava-Bignami disease: A review of CT/MRI confirmed cases. J NeurolNeurosurg Psychiatry.
- Kohler C, Ances B, Coleman R (2000) Marchiafava-Bignami disease: Literature review and case report. Cognitive and behavioral neurology 13: 67-76.
- Kakkar C, Prakashini K, Polnaya A (2014) Acute Marchiafava-Bignami disease: Clinical and serial MRI correlation. BMJ Case Rep pII: Bcr2013203442.
- Nicoli F, Vion-Dury J, Chave B (1994) Marchiafava-Bignami disease: Interhemispheric disconnection, Balint syndrome, spontaneously favourable outcome. Rev Neurol150: 157-161.
- Ruiz MJ, Martínez Pérez-Balsa A, Ruibal M (1999) Marchiafava-Bignami disease with widespread extracallosal lesions and favourable course. Neuroradiology 41: 40-43.









