Samer Abdel Al1*, Wafa Asha2, Mohamad K Abou Chaar1, Hani Al-Najjar1 and Maysa Al-Hussaini3
1Department of Surgery, King Hussein Cancer Center, Amman, Jordan
2Department of Radiation Oncology, King Hussein Cancer Centre, Amman, Jordan
3Department of Pathology and Laboratory Medicine, King Hussein Cancer Center, Amman, Jordan
- Corresponding Author:
- Samer Abdel Al
Consultant Orthopedics Oncology
Department of Surgery, King Hussein Cancer Center
Amman, Jordan.
E-mail:hanialnajjar88@gmail.com samer24al@ yahoo.com
Received: January 02, 2020, Accepted: January 17, 2020, Published: January 24, 2020
Citation:Samer Abdel Al, Asha W, Chaar MKA, Al-Najjar H, Al-Hussaini M (2020) Massive Fungating Malignant Peripheral Nerve Sheath Tumor Arising from the Forearm: A Case Report and Literature Review. Arch Cancer Res. Vol.8 No.1:2 doi: 10.36648/2254-6081.8.1.2
Keywords
Malignant peripheral nerve sheath tumors; Heterologous tumor; Forearm
Abbreviation
MPNST: Malignant Peripheral Nerve Sheath Tumor; NF1: Neurofibromatosis type-1; FCR: Flexor Carpi Radials;
KHCC: King Hussein Cancer Center; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; EDCM: Extensor Digitorum Comminus Muscle; FCR: Flexor Carpi Radials; EDC: Extensor Digitorum Communis; DNR: Do Not Resuscitate
Introduction
Malignant peripheral nerve sheath tumor (MPNST) accounts for 5-10% of all soft tissue sarcomas. It is defined as an aggressive and uncommon malignant tumor arising from a peripheral nerve or showing nerve sheath differentiation [1]. Most of patients diagnosed with MPNST fall between the ages of 20 to 50 years with rare paediatric cases [2]. The typical presentation is an enlarging mass that might be associated with pain, paraesthesia, weakness or radicular pain [3]. It was noted that patients with NF1 has greater lifetime risk of developing MPNST and worse prognosis in comparison to the general population. However; and regardless of association with NF1, MPNST has a low 5-year disease specific survival (16-38% versus 42-57% respectively) [1,4-9].
Case Presentation
A 51-year-old right-handed male patient noticed a nodule on the proximal radial side of his right forearm that persisted for 18 months without seeking any medical advice. Initially, the painless nodule was 1.0 x 1.0 cm that started to grow, it was excised at an outside facility and showed spindle cell tumor with features suggestive of MPNST. The patient presented to King Hussein Cancer Center (KHCC) four months after the initial excision with an oval-shaped large exophytic, and fungating, haemorrhagic, firm, and painful mass measuring 10 cm x 15 cm at the same site of the excised nodule (Figure 1). The neurovascular function was intact and no evidence of local lymph nodes enlargement. Magnetic resonance imaging (MRI) demonstrated evidence of subcutaneous soft tissue mass depicted at the proximal ulnar aspect of the right forearm mainly subcutaneous in location with evidence of surrounding muscular facial involvement and irregularity of the overlying skin layers without any invasion of the surrounding bony structures (Figure 2a-c). The mass measured 8 cm x 3.3 cm in axial dimension and 9.8 cm in craniocaudal dimension. Chest computed tomography (CT) scan showed multiple bilateral variably sized pulmonary nodules consistent with pulmonary metastasis, the largest of which measured 1.6 x 1.4 cm in trans-axial dimension adjacent to right oblique fissure (Figure 3). Bone scan showed no evidence of bone metastasis. A multidisciplinary committee discussed with the patient and his family the treatment plan including starting with chemotherapy followed by surgery. He opted to proceed with the surgical excision first since he could not tolerate the foul smell from the mass and was anxious to develop severe infection if it was left untreated. With the aid of bloodless field using tourniquet without exsanguination, we outlined our wide local excision about 3 cm away from the tumor. An elliptical incision was made while carefully inspecting the soft tissue mass to avoid any iatrogenic dissection through the tumor. Multiple frozen sections were sent during the operation from the deep proximal and deep distal ends and came back negative for malignancy. The mass was taken en-block with a safety margin from the extensor digitorum comminus muscle (EDCM) (Figure 4). An approximation of the muscles was done to cover the exposed tendon, as well as undermining and approximation of skin in burse-string manner to decrease the surface area of the defect using absorbable sutures (Figure 5) followed by vacuum pressure dressing. The pathology report was issued with the final diagnosis of malignant nerve sheath tumor grade 3 with heterologous bony elements and negative resection margins (Figure 6a-c).
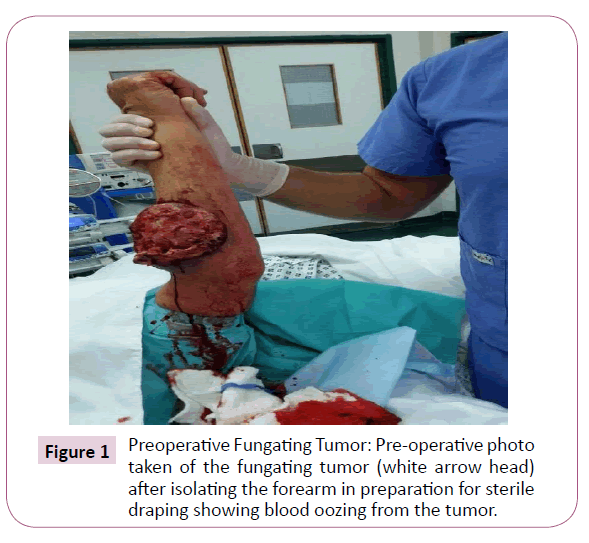
Figure 1: Preoperative Fungating Tumor: Pre-operative photo taken of the fungating tumor (white arrow head) after isolating the forearm in preparation for sterile draping showing blood oozing from the tumor.
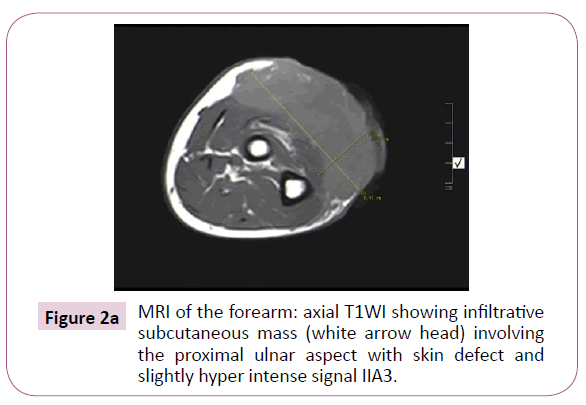
Figure 2a: MRI of the forearm: axial T1WI showing infiltrative subcutaneous mass (white arrow head) involving the proximal ulnar aspect with skin defect and slightly hyper intense signal IIA3.
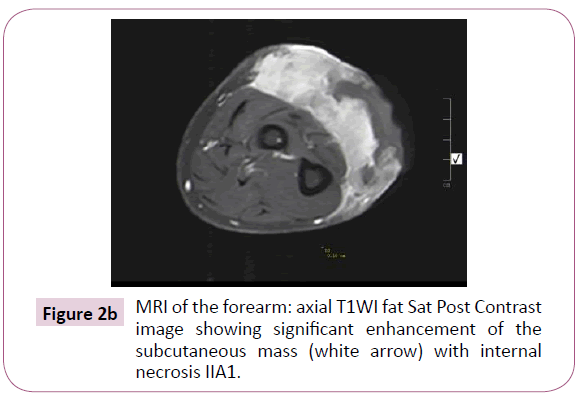
Figure 2b: MRI of the forearm: axial T1WI fat Sat Post Contrast image showing significant enhancement of the subcutaneous mass (white arrow) with internal necrosis IIA1.
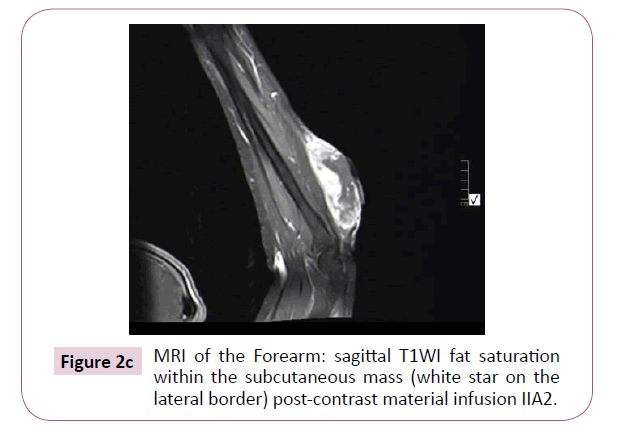
Figure 2c: MRI of the Forearm: sagittal T1WI fat saturation within the subcutaneous mass (white star on the lateral border) post-contrast material infusion IIA2.
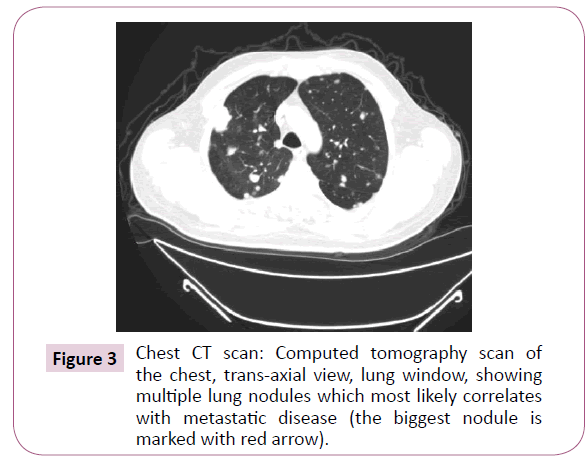
Figure 3: Chest CT scan: Computed tomography scan of the chest, trans-axial view, lung window, showing multiple lung nodules which most likely correlates with metastatic disease (the biggest nodule is marked with red arrow).
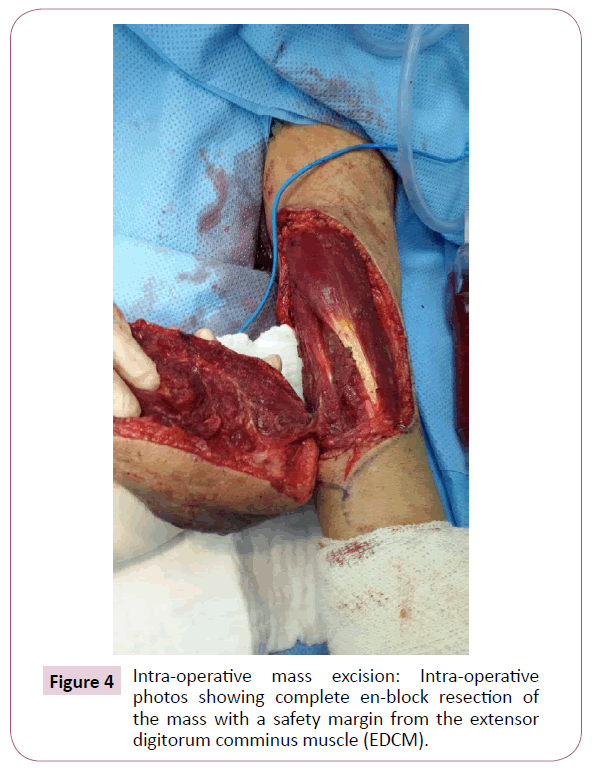
Figure 4: Intra-operative mass excision: Intra-operative photos showing complete en-block resection of the mass with a safety margin from the extensor digitorum comminus muscle (EDCM).
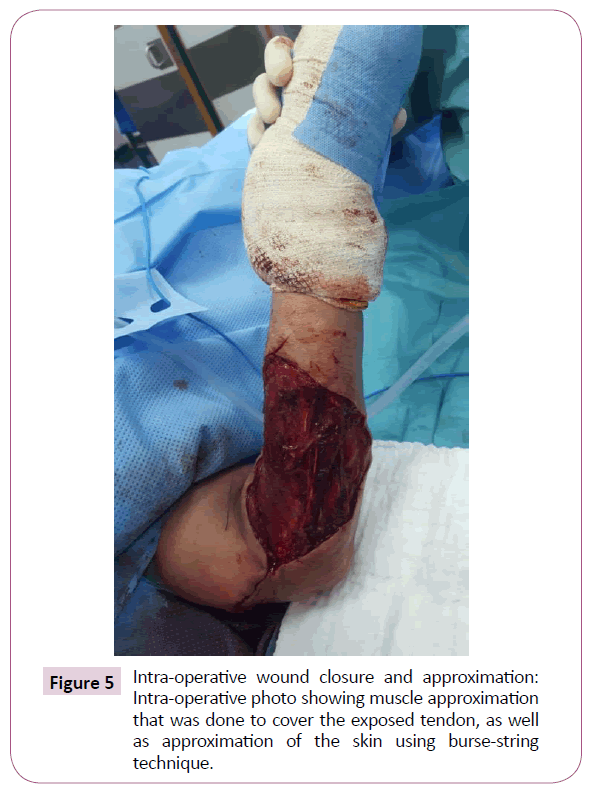
Figure 5: Intra-operative wound closure and approximation: Intra-operative photo showing muscle approximation that was done to cover the exposed tendon, as well as approximation of the skin using burse-string technique.
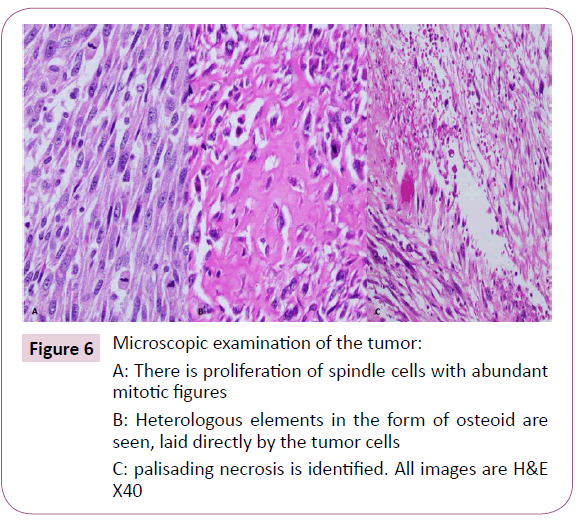
Figure 6: Microscopic examination of the tumor: A: There is proliferation of spindle cells with abundant mitotic figures
B: Heterologous elements in the form of osteoid are seen, laid directly by the tumor cells
C: palisading necrosis is identified. All images are H&E X40
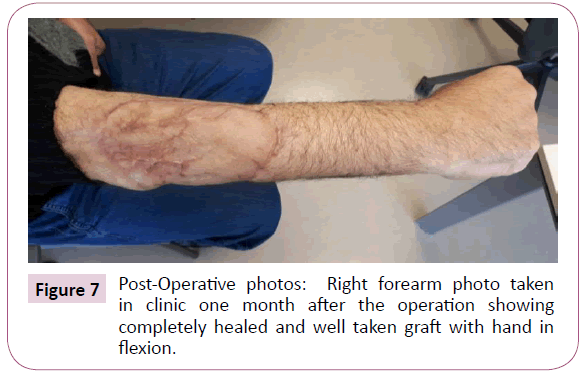
Figure 7: Post-Operative photos: Right forearm photo taken in clinic one month after the operation showing completely healed and well taken graft with hand in flexion.
The patient was then staged for another procedure for both flexor carpi radials (FCR) tendon transfer to extensor digitorum communis (EDC) tendon as well as soft tissue defect coverage with split thickness skin graft harvested from the ipsilateral thigh. The patient was splinted for one month and he was seen every 2 weeks to check for the skin graft until it was completely healed (Figure 7). Physiotherapy and occupational therapy resumed after the splint was removed on bi-weekly basis and our patient regained his full range of elbow motion as well as most of his finger extension. The patient doesn’t have any history of radiation exposure (treatment related, or work related) and patient NF-1 negative. The patient was then started on multiple palliative chemotherapy lines (2 cycles of ifosfamide and etoposide then doxorubicin) that failed due to disease progression. The patient then developed respiratory type 2 failure and elected a “do not resuscitate” (DNR) status, then he was referred for palliative care. A month later, the patient passed away at home.
Discussion and Conclusion
Malignant peripheral nerve sheath tumor (previously known as neurogenic sarcoma, neurofibrosarcoma, malignant schwannoma) are relatively rare, accounting for 5–10 % of all soft tissue sarcomas with an incidence rate of 0.001% in the general population and 4.6% in patients with NF1 [5] with an age range at presentation between 20 to 50 years. It is most commonly located on the extremities (45%) followed by the trunk (34%) and the head and neck area (19%) as reported by Stucky et al. [2]. However, Kim et al. who analysed a large cohort of patients over 30 years concluded that peripheral nerve tumors have higher prevalence in upper limb in comparison to lower limb [6] Gosk et al. however; reviewed 94 cases of MPNST, 6 of which had tumour in the forearm [7].
Due to the rapid pace of tumor progression, most patients seek medical advice when the tumor increases in size or when it starts to cause neuropathic symptoms ranging from paraesthesia to radicular pain [3]. Stucky et al. reported 67 patients who had metastasis, either at the time of presentation or on follow-up, out of 175 total patients with lung being the most common site for distant metastasis (55%), followed by intra-abdominal viscera, and osseous metastases [2]. Sordillo et al. also reported the lung as the most common site of metastasis followed by the liver, the peritoneum, and subcutaneous tissue [1].
MPNST can resemble benign tumors, both histologically and radiologically. Demir et al. suggested that the diagnosis is easily made in patients without NF1 who present with a palpable mass and pain, however; the diagnosis is frequently delayed in patients with NF1 due to potential confusion of these lesions as the classical neurofibroma and/or plexiform neurofibroma [8]. To ensure an early diagnosis of MPNST, MRI evaluation and biopsy should be performed immediately when malignancy is suspected [9,10]. However, both plexiform neurofibroma and MPNSTs have similar MRI findings, which demonstrate areas of low-tointermediate signal intensity on T1W sequence and high signal intensity on T2W sequence with heterogeneous enhancement. Wasa et al reported that patients with MPNST generally have giant masses, and presence of peripheral enhancement pattern, perilesional edema-like zone, and intratumoral cystic lesions [11]. Histologically, MPNST shows fasciculated growth pattern with hyperchromatic spindle cells. Nuclei are elongated, and perivascular hypercellularity is often seen. Marked mitotic activity and geographic tumor necrosis are also commonly present [2].
Prognosis in MPNST is poor, especially for tumors that cannot be totally resected. Compared with other soft tissue sarcomas, MPNST has the highest risk of sarcoma-specific death [6,7]. Anghileri et al. reported that large tumor size, truncal location, and positive surgical margins were significant factors predicting local recurrence while recurrence at presentation, tumor size, and tumor grade as significant factors for distant metastases [12]. This is similar to findings reported by Stucky et al. [2]. It should be noted that both studies identified local recurrence, size >5 cm, and truncal location of the primary as being associated with adverse survival. Additionally, Stucky et al. commented that high tumor grade also affects survival, keeping in mind that NF1-associated MPNST patients have a significantly worse disease free survival as compared to those with sporadic disease [1,2,4-9].
The mainstay of treatment is an aggressive surgical approach with negative margins (R0 resection) followed by adjuvant radiotherapy for tumors with high grade, large size, and positive margins and addition of chemotherapy for those who can tolerate [2]. Robert A. Chase indicated that limb salvage surgery can be attempted if blood supply is adequate for vascular anastomoses or grafts, the residual elements of the extremity can be salvaged to serve their original intended purpose, and keeping in mind assessing the importance and likelihood of success of limb salvage in relationship to the patient well-being and other possible disabilities (age, dominance of hand, loss of contralateral extremity, blindness, intended future occupation, and patient's personal needs) [13]. This was one of the major reasons for which we opted to perform a novel limb salvage surgery for a huge fungating MPNST for a metastatic patient.
We believe that if our patient presented earlier to our center for management, we would have been able to completely excise the tumor, preserve his hand function, and possibly control his lung metastases. Unfortunately, in the Middle East, patients are frequently get referred to tertiary cancer centers when it is too late with disease has already progression, so that is beyond control [14-17].
Acknowledgement
Not applicable
Statement of Ethics
This is a case report. We have obtained a written informed consent from the patient to publish this report
Disclosure Statement
Authors have nothing to disclose
Funding Sources
Not applicable
Author Contributions
MK performed the literature search and wrote parts of the manuscript. MH obtained and reviewed the pathology samples and provided the images. HN obtained the written consent and IRB approval. SA oversaw the manuscript inception, guided the literature search, counseled the patient. WA wrote the manuscript, and provided major revisions. All authors read and approved the final manuscript. Abdel Al Oversaw the manuscript inception, guided literature research, Counselled patient. Asha wrote the manuscript and provided major revisions. Chaar Performed the literature search and wrote parts of the manuscript. Al-Najjar Obtained the retained consent and IRB approval and reviewed clinical images. Al- Hussaini obtained and reviewed the pathology samples.
26519
References
- Sordillo PP, Helson L, Haijdu SI (1981) Malignant schwannoma-clinical characteristics, survival, and response to therapy. J Cancer 47: 2503-509.
- Hueman MT, Ahuja N, Martin RF (2008) Soft Tissue Sarcomas. J SurgClin North Am 88: 503-04.
- Kleihues P, Cavenee WK (2000) World Health Organization classification of tumours: pathology and genetics: tumours of the nervous system, USA: Oxford University Press.
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Istrup DM (1986) malignant peripheral nerve sheath tumors. J Cancer 57: 2006-21.
- Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG (2005) A series of 397 peripheral neural sheath tumors: 30-Year experience at Louisiana State University Health Sciences Center. J Neurosurg 102: 246-55.
- Gosk J, Gutkowska O, Mazurek P, Koszewicz M, ZióÅÃÂÃÂÃÂâÂÂÃÂâââÂÂìÃÂ
áÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
ákowski P (2015) Peripheral nerve tumours: 30-year experience in the surgical treatment. J Neurosurg Rev 38: 511-20.
- Demir HA, Varan A, Yalçn B, Akyüz C, Kutluk T, et al. (2012) Malignant Peripheral Nerve Sheath Tumors in Childhood. J Pediatr Hematol Oncol 34: 204-7.
- Gnanalingham K, Bhattacharjee S, O'Neill K (2004) intraosseous malignant peripheral nerve sheath tumor (MPNST) of the thoracic spine: a rare cause of spinal cord compression. 29: 402-5.
- Moharir M, London K, Howman-Giles R, North K (2010) Utility of positron emission tomography for tumour surveillance in children with neurofibromatosis. Eur J Nucl Med Mol Imaging 37: 1309-17.
- Wasa J, Nishida Y, Tsukushi S, Shido Y, Sugiura H, et al. (2010) MRI Features in the Differentiation of Malignant Peripheral Nerve Sheath Tumors and Neurofibromas. AJR Am J Roentgenol 194: 1568-74.
- Anghileri M, Miceli R, Fiore M (2006) Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. J Cancer 107: 1065-74.
- Chase RA (1970) The Severely Injured Upper Limb:To Amputate or Reconstruct: That is the Question. J Arch Surg100: 382-92.
- Hruban RH, Shiu MH, Senie RT, Woodruff JM (1990) Malignant peripheral nerve sheath tumors of the buttock and lower extremity. J Cancer 66: 1253-65.
- Carli M, Ferrari A, Mattke A (2005) Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 23: 8422-30.
- Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL (1998) Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J RadiatOncol Biol Phys 42: 351-60.
- Evans DG, Baser ME, McGaughran J, Sharif S, Howard E (2002) Malignant peripheral nerve sheath tumours in neurofibromatosis. J Med Genet 39: 311-4.














