Keywords
Medium chain triglycerides; Serum lipid profile; Fatty acids; Eicosapentaenoic acid; Docosapentaenoic acid
Introduction
Medium chain triglyceride (MCT) oil is synthetic oil made from medium chain C8 and C10 fatty acids. It has been traditionally used as a neutral “placebo” in lipid clinical trials. Thus, we had selected MCT oil capsules as a “placebo”, intended for comparison with an omega-3 polyunsaturated fatty acid-containing supplement, to measure fatty acid profiles and inflammation in healthy people. This was a preliminary trial to establish the supplement’s safety in healthy people before it was used in a trial whose participants had Crohn’s disease.
MCTs were introduced into clinical nutrition in the 1950s for the dietary treatment of malabsorption syndromes, because of their rapid absorption and solubility [1]. The definition of MCTs considers these as being composed of C8 and C10 fatty acidcontaining triglycerides [2]. Used for nutritional and other commercial purposes, they are derived from tropical oils such as coconut and palm kernel oils [3]. In the process of producing MCTs, these oils are hydrolyzed to fatty acids and glycerol. The glycerol is drawn off from the resultant mixture, and the medium-chain fatty acids (MCFA) are fractionally distilled, separating them from the major component, lauric acid. The MCFA fraction used commercially is mainly comprised of the 8 carbon caprylic or octanoic acid and the 10 carbon capric or decanoic acid. There are also smaller amounts of the six carbon caproic or hexanoic acid and the 12 carbon lauric acid in the commercial products [2,4].
When MCT’s are metabolized they are split by lipase in the body to produce fatty acids and a diglyceride [5]. The molecular weight of MCTs is lower than the molecular weight of long-chain triglycerides (LCTs), thereby facilitating the action of pancreatic lipase. Consequently, MCTs are hydrolyzed both faster and more completely than LCTs, and as fast as glucose [1,5,6]. The half-life of the MCT’s has been estimated to be 11 minutes [7]. Subsequent to absorption, MCFA are secreted directly into the portal circulation without undergoing reesterification in the enterocyte. Due to their water solubility, MCFA do not require albumin for transportation from capillaries to peripheral tissues. Similar to long-chain fatty acids (LCFA), MCFA also undergo oxidation in mitochondria but do not require carnitine for mitochondrial transport. All of these factors result in rapid metabolism of MCFA from ingested MCT and form the basis for their use in clinical nutrition and parenteral nutrition as well as their proposed emerging role in weight management [6,8-10].
These factors appeared to provide an appropriate rationale for utilizing an MCT capsule as a placebo, in a study designed to test the specific effect of a long chain omega-3 polyunsaturated fatty acid on parameters of cardiovascular health. MCT oil has an appropriate safety profile and long use in clinical trials [2,11-14]. The data for the omega-3 supplement will be presented elsewhere and we focus here on the results of the MCT.
Materials and Methods
Participants were recruited from the Nutrigenomics New Zealand dataset, and/or from advertisements in local Auckland papers as necessary. They comprised equal numbers of males and females (15 of each), giving a total of 30 participants, aged between 20-65 years.
The median ages for the groups were 49 years and 50 years respectively. They had no history of cancer in last 5 years, or of gastrointestinal disorders (Ulcerative colitis, Crohn’s Disease or Irritable Bowel Syndrome). Any prescribed medication was stable, with no changes in the previous 3 months. They had not taken antibiotics in the month prior to the study commencement and were not on blood thinning medication (e.g. Aspirin). They were also not currently taking any vitamin D, fish oil/flax seed oil supplements, or eating more than 4 servings of oily fish (salmon, mackerel) each week.
In the first group, the 15 participants took the omega-3 supplement according to the manufacturer’s recommendations, for four weeks. This was followed with a four week period of no supplementation and then another four week period of taking a similarly encapsulated MCT capsule, intended as a placebo. The second group took the encapsulated MCT for the first four weeks, followed by a four week period of no supplementation. The omega-3 supplement was then taken for the remaining four weeks, according to the manufacturer’s recommendations. All participants were blinded to the treatment regime they were on. For either type of capsule, participants were asked to take two capsules each day, with food, at lunch or dinner. These were recommended to be taken without blood thinning medication (Figure 1).
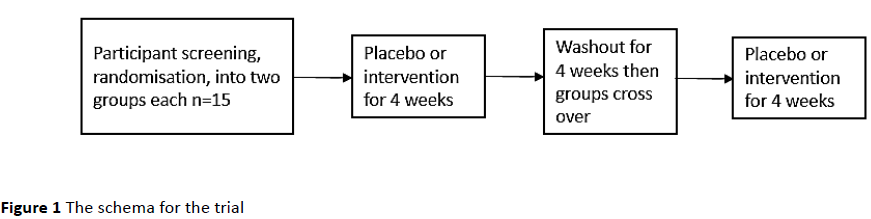
Figure 1: The schema for the trial
The MCT oil was purchased from Croda, Singapore, as CRODAMOL GTCC-LQ-(SG), product code: GE83907/0190/8S02. It was described as a Caprylic/Capric triglyceride, with guaranteed limits of heavy metal contamination as less than 10 ppm. The major components were FAD-C6:0 Caproic 0.0 2.0%; FAD-C8:0 Caprylic 50.0-80.0%; FAD-C10:0 Capric 20.0-50.0%; FAD-C12:0 Lauric 0.03.0%.
There were 4 times when blood was drawn. These were labelled in chronological order as T1, T2, T3 and T4. Once the intervention was identified, it was labelled T start at the beginning of the intervention and T end at the end of the intervention. These collection points were conducted:
1. before any intervention
2. after 4 weeks of the first intervention and before washout
3. after washout and before the second intervention
4. at the end of the second intervention
C-reactive protein (CRP) and fatty acid levels were measured in all the collected samples. Serum aliquots of 500 μl were stored in Eppendorf tubes at -80°C for later evaluation using the analysis of fatty acid methyl esters (FAME). FAME is based on the esterification of lipids followed by gas chromatography analysis. This method allows the identification of metabolic changes associated with each intervention, which can be used to assess the extent to which a fatty acid is taken up and utilized. CRP levels in blood plasma were measured using the CRP assay kit from Roche. The CRP analysis was performed by LabPlus (https://www.labplus.co.nz/, Auckland, NZ).
The Ethics approval number for this trial was: NTY/ 11/11/109.
Results
Although the vast majority of tested variables showed no difference before and after the MCT consumption, initial analyses suggested changes either statistically significant or approaching significance in levels of four fatty acids (Table 1).
| Chain |
Common Name |
Median (25% and 75% percentiles) |
P value |
| Placebo T start |
Placebo T end |
| C14:1 |
Myristoleic acid |
0 (0 and 0.10) |
0.0856
(0 and 0.11) |
0.058 |
| C20:5 |
Eicosapentaenoic acid |
1.021
(0.836 and 1.326) |
0.885
(0.756 and 1.289) |
0.007 |
| C22:5 |
Docosapentaenoic acid |
0.568
(0.549 and 0.65) |
0.564
(0.492 and 0.605) |
0.063 |
| C22:6 |
Docosahexaenoic acid |
2.282
(1.937 and 2.68) |
2.025
(1.791 and 2.278) |
0.053 |
Table 1: Comparison of individual placebo T start and T end levels of the fatty acids after MCT consumption using the Wilcoxon Signed Rank Test.
Subsequently as the data were not normally distributed (P<0.050) a Kruskal-Wallis one way analysis of variance (ANOVA) on ranks was conducted between the placebo T start and placebo T end difference between the four fatty acids (Table 2).
| Chain |
Common Name |
N |
Median |
25% |
75% |
| C14:1 |
Myristoleic acid |
27 |
0 |
-0.0001 |
0.0936 |
| C20:5 |
Eicosapentaenoic acid |
27 |
-0.0771 |
-0.281 |
0.0389 |
| C22:5 |
Docosapentaenoic acid |
27 |
-0.0191 |
-0.0866 |
0.0134 |
| C22:6 |
Docosahexaenoic acid |
27 |
-0.144 |
-0.38 |
0.131 |
Table 2: Kruskal-Wallis one way analysis of variance on ranks on differences between time points placebo T start and placebo T end after consumption of MCT.
The ANOVA indicates that there is a significant difference between the two initial and post supplementation data for eicosapentaenoic acid and docosapentaenoic acid (significantly different P<0.05). That is, EPA and DPA have shown significant decreases as a result of MCT consumption.
Discussion
MCTs have a reduced chain length which means that they are more rapidly absorbed and metabolized by the body than LCTs, and also produce about 10% less calories. These energyenhancing aspects of MCTs are thought to be a result of not needing carnitine, as LCTs do, to cross the double membrane of the mitochondria. This feature of MCTs results in an excess of acetyl-coA, and consequent increased ketone production from the metabolic pathways that acetyl-coA contributes to. This means that MCTs are ketogenic and, for this reason, have been used as the basis of a ketogenic diet [15,16]. However, the current data raise some questions about their widespread use.
Dietary sources of EPA and DHA for humans are oily fish, fish oil, seaweed and commercially produced microalgae and breast milk. The EPA and DHA in fish are from the plankton they eat [17-19]. DHA is an important structural component of the human brain, retina, testicles, sperm and skin [20,21]. A deficit in DHA has been associated with cognitive deterioration [22]. Severely depressed people also have shown reduced levels of DHA in their brain tissue [23]. DPA food sources are fish, meat and poultry [24]. In the human body, EPA, DHA and DPA are derived from alpha-linolenic acid. However, the human body absorbs these fatty acids more easily from foods that contain them. The capacity of the human body to metabolize EPA and DHA also appears to be significantly limited when people experience conditions like diabetes [25]. There are few studies about the metabolism of DPA in humans, although there is a suggestion that DPA may act as a reservoir for EPA and DHA [26]. The rest of this discussion will center on EPA and DHA.
Diets that are rich in EPA and DHA are associated with decreased severity and incidence of chronic diseases such as cancer and cardiovascular disease [27]. Both these fatty acids lower inflammation through their effects on expression of adhesion molecules, leukocyte chemotaxis and leukocyteendothelial adhesive interactions. EPA also seems to reduce the activity of pro-inflammatory genes e.g. nuclear factor kappa-light-chain-enhancer of activated B cells, and activate anti-inflammatory transcription factors e.g. peroxisome proliferator-activated nuclear receptor gamma. Some of their beneficial effects are also thought to be through their metabolites e.g. leukotrienes, prostaglandins, thromboxanes and resolvins [27-30]. EPA has also been thought to be helpful in mental health conditions like depression and schizophrenia [31-33]. EPA has been used for people on cancer chemotherapy as it improves their responses and has been shown to suppress cachexia that is often associated with the disease [34].
Harris and Schacky proposed the Omega-3 Index as a new risk factor for death from coronary heart disease [35]. They proposed that the red blood cell EPA+DHA (which they termed the Omega-3 Index) be considered a new risk factor for death from cardiovascular disease (CHD). They conducted clinical and laboratory experiments and considered several published primary and secondary prevention studies to validate this assertion. Their evidence showed that the Omega-3 Index was inversely associated with risk for CHD mortality. An Omega-3 index of ≥8% was associated with protection from heart disease, especially for sudden death, while an index of ≤4% showed the converse effect.
Medium chain triglycerides are the only edible liquid oils contained in exclusively saturated fatty acids. It was recognized early on that MCT is a unique lipid and is different from all the fats and oils commonly used [1]. Conventional fats and oils such as long chain triglycerides are absorbed via the lymphatic system, require chylomicrons formation and are carnitine dependent. MCT does not require chylomicrons formation and is transported to the liver directly by the portal system. Early workers described the physiological considerations and the potential therapeutic applications for MCT. Several research workers showed the MCT is absorbed and metabolized as rapidly as glucose, whereas long chain triglycerides, the conventional fats and oils are utilized more slowly [1,11]. It was also reported that MCT oil lowered serum cholesterol and tissue cholesterol levels in animals and so MCT or was considered a relatively good placebo or neutral or to use when studying the effects of other oils and fats on lipid biomarkers. The results of our current experiments demonstrated that in fact MCT oil is not neutral and is not suitable for a placebo.
Despite many positive claims about the neutral effects of pure MCTs on lipid levels, Cater and co-workers reported the converse effect [36]. They found that although MCTs have long been described as having neutral effects on serum cholesterol concentrations, experimental evidence supporting this claim is limited. In a randomized, crossover, metabolic-ward study, they compared the lipid effects of a natural food diet supplemented with MCTs, palm oil, or high oleic acid sunflower oil in nine middle-aged men with mild hypercholesterolemia. Rather than having a neutral effect, MCT oil produced total cholesterol concentrations that were not significantly different from those produced by palm oil, but significantly higher than those produced by high oleic acid sunflower oil. Low-density-lipoprotein (LDL) cholesterol concentrations paralleled those of total cholesterol. MCT oil tended to result in higher triglyceride concentrations than either palm oil or high oleic acid sunflower oil, although this difference was not statistically significant. Tholstrup et al. [37] found that, compared with the intake of high-oleic sunflower oil, MCT intake resulted in a higher plasma total cholesterol, higher LDL and VLDL cholesterol, a higher ratio of LDL to HDL cholesterol, higher plasma total triacylglycerol, and higher plasma glucose.
Conclusion
MCT oil was not neutral in its effect on blood lipid biomarkers. The results were surprising and unexpected and have ramifications for the claims made about the positive benefits of MCT oils, and the extrapolations currently being used by some marketers to promote coconut oil and its’ derivatives.
Funding Sources
About Health Supplements Ltd, New Zealand Ministry of Business, Innovation and Employment
9935
References
- Bach AC, Babayan VK (1982) Medium-Chain Triglycerides: An Update. Am J ClinNutr 36: 950-962.
- Marten B, Pfeuffer M, Schrezenmeir J (2006) Medium-Chain Triglycerides. Int Dairy J 16: 1374-1382.
- Silverman JR, John G (2015) Biobased Fat Mimicking Molecular Structuring Agents for Medium-Chain Triglycerides (MCTs) and Other Edible Oils. J Agric Food Chem 63: 10536-10542.
- Eyres L, Eyres MF, Chisholm A, Brown RC (2016) Coconut Oil Consumption and Cardiovascular Risk Factors in Humans. Nutrients.
- Ooi EM, Watts GF, Ng TW, Barrett PHR (2015) Effect of Dietary Fatty Acids on Human Lipoprotein Metabolism: A Comprehensive Update. Nutrients 7: 4416-4425.
- Wang W, Yan X, Ni Y, Guo K, Ke C, et al. (2013) Effects of Lipid Emulsions in Parenteral Nutrition of Esophageal Cancer Surgical Patients Receiving Enteral Nutrition: A Comparative Analysis. Nutrients 6: 111-123.
- Mingrone G, de Gaetano A, Greco AV, Capristo E, Castagneto M, et al. (1995) Medium-Chain Triglycerides for Parenteral Nutrition: Kinetic Profile in Humans. Nutrition 11: 418-422.
- Kasai M, Nosaka N, Maki H, Negishi S, Aoyama T, et al. (2003) Effect of Dietary Medium-and Long-Chain Triacylglycerols (MLCT) on Accumulation of Body Fat in Healthy Humans. Asia Pac. J ClinNutr 12: 151-160.
- Edmunds CE, Brody RA, Parrott JS, Stankorb SM, Heyland DK (2014) The Effects of Different IV Fat Emulsions on Clinical Outcomes in Critically Ill Patients. Crit Care Med 42: 1168-1177.
- Mumme K, Stonehouse W (2015) Effects of Medium-Chain Triglycerides on Weight Loss and Body Composition: A Meta-Analysis of Randomized Controlled Trials. J AcadNutr Diet115: 249-263.
- Tsai Y, Park S, Kovacic J, Snook JT (1999) Mechanisms Mediating Lipoprotein Responses to Diets with Medium-Chain Triglyceride and Lauric Acid. Lipids 34: 895-905.
- Ozturk B, Argin S, Ozilgen M, McClements DJ (2015) Nanoemulsion Delivery Systems for Oil-Soluble Vitamins: Influence of Carrier Oil Type on Lipid Digestion and Vitamin D 3 Bioaccessibility. Food Chem 18: 499-506.
- Boisrame-Helms J, Said A, Burban M, Delabranche X, Stiel L, et al. (2014) Medium-Chain Triglyceride Supplementation Exacerbates Peritonitis-Induced Septic Shock in Rats: Role on Cell Membrane Remodeling. Shock 42: 548-553.
- Traul K, Driedger A, Ingle D, Nakhasi D (2000) Review of the Toxicologic Properties of Medium-Chain Triglycerides. Food and chemical toxicology 38: 79-98.
- Ward D, English J (2013) Medium Chain Triglycerides (MCTs). Beneficial Effects on Energy, Arthersclerosis and Aging. Nutr Rev.
- Huttenlocher PR, Wilbourn AJ, Signore JM (1971) Medium-Chain Triglycerides as a Therapy for Intractable Childhood Epilepsy. Neurology 2: 1097-1103.
- Iguchi K, Okumura N, Usui S, Sajiki H, Hirota K, et al. (2001) Myristoleic Acid, a Cytotoxic Component in the Extract from SerenoaRepens, Induces Apoptosis and Necrosis in Human Prostatic LNCaP Cells. Prostate 47: 59-65.
- Sczaniecka AK, Brasky TM, Lampe JW, Patterson RE, White E (2012) Dietary Intake of Specific Fatty Acids and Breast Cancer Risk among Postmenopausal Women in the VITAL Cohort. Nutr Cancer60: 1131-1142.
- Halliday J (2007) Water 4 to Introduce Algae DHA/EPA as Food Ingredient.
- Guesnet P, Alessandri J (2011) Docosahexaenoic Acid (DHA) and the Developing Central Nervous System (CNS)–Implications for Dietary Recommendations. Biochimie 93: 7-12.
- Singh M (2005) Essential Fatty Acids, DHA and Human Brain. Indian J Pediatr 72: 239-242.
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, et al. (2005) A Role for Docosahexaenoic Acid-Derived Neuroprotectin D1 in Neural Cell Survival and Alzheimer Disease. J Clin Invest 115: 2774-2783.
- McNamara RK, Hahn C, Jandacek R, Rider T, Tso P, et al. (2007) Selective Deficits in the Omega-3 Fatty Acid Docosahexaenoic Acid in the Postmortem Orbitofrontal Cortex of Patients with Major Depressive Disorder. Biol Psychiatry 62: 17-24.
- Rahmawaty S, Charlton K, Lyons-Wall P, Meyer BJ (2013) Dietary Intake and Food Sources of EPA, DPA and DHA in Australian Children. Lipids 48: 869-877.
- Malasanos TH, Stacpoole PW (1991) Biological Effects of Ω-3 Fatty Acids in Diabetes Mellitus. Diabetes Care 14: 1160-1179.
- Miller E, Kaur G, Larsen A, Loh SP, Linderborg K, et al. (2013) A Short-Term N-3 DPA Supplementation Study in Humans. Eur J Nutr 52: 895-904.
- Vanden HJ (2011) Nutrigenomics and Nutrigenetics of Ω3 Polyunsaturated Fatty Acids. ProgMolBiolTranslSci 108: 75-112.
- Mas E, Croft KD, Zahra P, Barden A, Mori TA (2012) Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood Following N-3 Fatty Acid Supplementation. ClinChem 58: 1476-1484.
- Maskrey BH, Megson IL, Rossi AG, Whitfield PD (2013) Emerging Importance of OmegaÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂ3 Fatty Acids in the Innate Immune Response: Molecular Mechanisms and Lipidomic Strategies for their Analysis. MolNutr Food Res 57: 1390-1400.
- Nowak JZ (2011) Biosynthesis and Characteristics of Anti-Inflammatory Proresolving Derivatives of Omega-3 and Omega-6 Polyunsaturated Fatty Acids. Mil Pharm Med 3: 30-41.
- Peet M, Brind J, Ramchand C, Shah S, Vankar G (2001) Two Double-Blind Placebo-Controlled Pilot Studies of Eicosapentaenoic Acid in the Treatment of Schizophrenia. Schizophr Res 49: 243-251.
- Song C, Zhao S (2007) Omega-3 Fatty Acid Eicosapentaenoic Acid. A New Treatment for Psychiatric and Neurodegenerative Diseases: A Review of Clinical Investigations. Expert OpinInvestig Drugs 16: 1627-1638.
- Martins JG (2009) EPA but Not DHA Appears to be Responsible for the Efficacy of Omega-3 Long Chain Polyunsaturated Fatty Acid Supplementation in Depression: Evidence from a Meta-Analysis of Randomized Controlled Trials. J Am CollNutr 28: 525-542.
- Hardman WE (2004) (N-3) Fatty Acids and Cancer Therapy. J Nutr 134: 3427S-3430S.
- Harris WS (2008) The Omega-3 Index as a Risk Factor for Coronary Heart Disease. Am J ClinNutr 87: 1997S-2002S.
- Cater NB, Heller HJ, Denke MA (1997) Comparison of the Effects of Medium-Chain Triacylglycerols, Palm Oil, and High Oleic Acid Sunflower Oil on Plasma Triacylglycerol Fatty Acids and Lipid and Lipoprotein Concentrations in Humans. Am J ClinNutr 65: 41-45.
- Tholstrup T, Ehnholm C, Jauhiainen M, Petersen M, Hoy CE, et al. (2004) Effects of Medium-Chain Fatty Acids and Oleic Acid on Blood Lipids, Lipoproteins, Glucose, Insulin, and Lipid Transfer Protein Activities. Am J ClinNutr 79: 564-569.







