Ivan Khoo Yi1, Hao Li1, Ming Yann Lim1, Ernest Fu Weizhong1, Nandini CL Rao2, Yaw Khian Chong and Manish Mahadeorao Bundele2*
1Department of Otorhinolaryngology, Tan Tock Seng Hospital, Singapore
2Department of Pathology, Tan Tock Seng Hospital, Singapore
*Corresponding Author:
Manish Mahadeorao Bundele
Department of Pathology
Tan Tock Seng Hospital, Singapore
Tel: +6581263459
E-mail: manishbundele@yahoo.com
Received Date: November 22, 2018; Accepted Date: January 21, 2019; Published Date: January 23, 2019
Citation: Li H, Yi IK, Lim MY, Weizhong EF, Rao NCL, et al. (2019) Merkel Cell Carcinoma Occurring Within A Squamous Papilloma of the External Ear Canal - A Case of Spontaneous Regression. Ann Clin Lab Res 7:281. DOI: 10.21767/2386-5180.100281
Keywords
Merkel Cell Carcinoma (MCC); External auditory canal; Malignant neuroendocrine tumours; Blue cell tumours
Introduction
Originally described in 1972 as “Trabecular Carcinoma of the skin” [1], there are no specific histological features to differentiate Merkel cell carcinomas from other small round blue cell tumours [2]. They have a predilection for occurrence in the head and neck region, and in men above the age of 65, in contrast to most other small round blue cell tumours which tend to occur in children or young adults [3-9]. Treatment is with surgery and radiotherapy for local disease. There is a high incidence of local recurrence, early metastases and poor prognosis [8-14].
Case Report
An 82-year-old male with a background history of rheumatic heart disease, coronary artery disease and a recent cerebrovascular accident presented with a one month history of otorrhea and a large, violaceous 7 cm mass extending from the left external auditory canal to the parotid and post-auricular area. A flexible nasoendoscopy of the anterior and posterior nasal space, larynx and hypopharynx was normal. The oropharyngeal examination was also unremarkable.
A computed tomography was arranged but the patient was unable to complete the scan and subsequently defaulted the follow-up. He presented again 2 months later with clinical resolution of the intraparotid component. The external auditory canal component was still present, measuring 3 cm in diameter and was friable with contact bleeding. A biopsy was declined at that time.
The patient was seen 3 months later, 5 months after the initial presentation, with only a small remnant external auditory canal tumour measuring 1 cm by 0.5 cm. Patient had been on a regime of daily alginate dressing and Gentrisone application, with the only significant clinical change being an improvement of his functional status of his other comorbidities. The remnant tumour was excised under local anaesthesia and the base cauterized with silver nitrate. A chest X-ray performed on the patient was normal, and he declined further computed tomography of the head and neck.
The excised tumour was sent for aerobic culture, fungal culture, acid-fast bacilli smear and culture as well as histology. All cultures and smears were negative. Histological examination showed polypoidal tissue lined with keratinizing squamous epithelium and papillomatosis, variable hypergranulosis, hyperkeratosis and focal parakeratosis, consistent with a squamous papilloma. Within this papilloma, there were 3 foci of subepithelial infiltrates, composed of nests and sheets of monomorphic small round blue cell tumour, largest measuring 0.1 cm in maximum dimension. The cells showed enlarged vesicular nuclei with fine granular chromatin, discernable nucleoli and scanty cytoplasm with a high mitotic count of 10/high powered field. No squamous dysplasia or carcinoma in situ component was identified. No lymphovascular emboli were identified. On immunostaining, synaptophysin and chromogranin were diffuse and strongly positive with AE1/3 and cytokeratin CK20 showing dot like positive staining. CD117 showed weak positive staining. LCA, terminal deoxynucleotidyl transferase (TdT), hematopoietic progenitor cell antigen (CD34) were negative, thus excluding a haematolymphoid neoplasm. Cytokeratin 7 (CK7) and thyroid transcription factor -1(TTF1) were also negative. The patient declined all further investigations and was reviewed 3 months post excision of the ear mass with no clinical evidence of local or regional recurrence.
Discussion
First described in 1972, Merkel Cell Carcinomas have been well characterised by over 2000 case reports. The incidence appears to be increasing with a current estimate of 0.6 per 100,000 based on data available from 1986 to 2006. This increased incidence has been attributed in part to increasing clinical and histopathological awareness as well as availability of histochemical biomarkers [15-21]. However, due to the overall rarity of this tumour, it remains to be seen if the incidence rate is truly increasing.
Merkel cell carcinomas are believed to originate from Merkel cells which are neuroendocrine cells in the epidermis, hypothesized to be involved in mechanotransduction of touch sensation as well as neuromodulatory and developmental functions through the secretion of numerous neuroactive peptides [22-28]. Risk factors for MCC include ultraviolet light exposure, immunosuppression and advanced age [29]. Patients with inflammatory connective tissue diseases and diabetes are at the highest risk for MCC [30] (Figures 1 and 2).
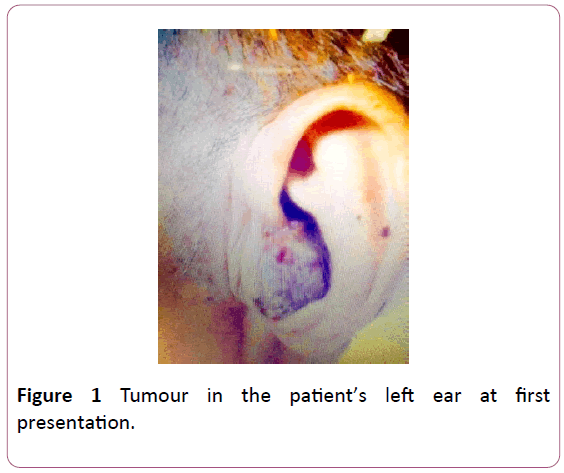
Figure 1: Tumour in the patient’s left ear at first presentation.
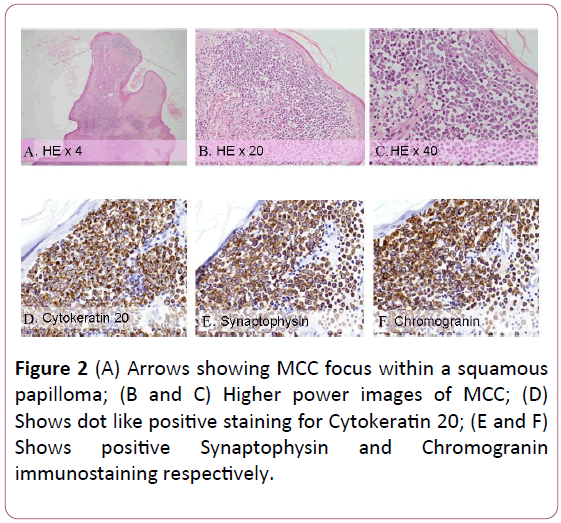
Figure 2: (A) Arrows showing MCC focus within a squamous papilloma; (B and C) Higher power images of MCC; (D) Shows dot like positive staining for Cytokeratin 20; (E and F) Shows positive Synaptophysin and Chromogranin immunostaining respectively.
MCC has also been found to be associated with the Merkel cell polyomavirus (MCPyV) [31-34]. Approximately 80% of MCC are positive for clonally integrated MCPyV [31-33,35]. Higher levels of MCPyV-specific antibodies, suggestive of the rigor of the adaptive immune response, may correlate with better prognosis in MCPyV-positive MCC However, one study reported that levels of MCPyV in blood predict a worse overall response [36,37]. Further investigation is needed to determine the influence of viral status on survival and its potential as a prognostic biomarker.
The aetiology of virus-negative MCC, as investigated in multiple recent studies, shows similarities with other skin cancers. In particular, virus-negative MCC development is linked to exposure to UV radiation, and primary MCC lesions typically develop on sun-exposed skin [16,18].
Histologically, the abundance of malignant round cells with scant cytoplasm that stain blue on hemotoxylin and eosin preparation is nonspecific and results in Merkel cell carcinomas being classified broadly as a type of small round blue cell tumour. This group of tumours include numerous other malignancies such as Ewing’s sarcoma, medulloblastoma, neuroblastoma, small cell lung carcinoma, retinoblastoma and non-Hodgkin’s lymphoma [38]. Due to the significant histological similarities between these various differential diagnoses, immunohistochemical stains are integral to providing the right diagnosis. Immunostaining panel of CK7, CK20, TTF1, synaptophysin and chromogranin have been suggested as the basic markers to differentiate Merkel cell carcinoma from metastatic small cell carcinomas, neuroendocrine tumours, basal cell carcinomas and adenocarcinomas [39]. Merkel cell carcinomas classically show CK20 positivity in a paranuclear dot pattern, positivity for neuroendocrine markers such as synaptophysin, chromogranin and negative staining for CK7 and TTF1. Leukocyte common antigen (CD45) and terminal deoxynucleotidyl transferase (TdT) negativity are used to differentiate MCC from hematological malignancies and lymphoma. Vascular markers like CD34 are used to prognosticate tumours in the absence of lymphovascular invasion [40]. CD117 positive staining has also been reported in Merkel cell carcinoma, which is present in our case [41].
Clinically MCC presents as asymptomatic, expanding masses often in the presence of relative immunosuppression in a predominantly older population and often with a history of sun/ultraviolet exposure [41,42]. These features are well captured by Heath et al. in the pneumonic “AEIOU” [43]. Nearly half of reported MCCs occur in the head and neck, with an overall 5-year survival rate of 62%. Regional and distant spread is frequently present in up to 35% of patients at first presentation, with 5-year relative survival in Stage III (distant) at 25% compared to 75% for Stage I (localized) disease [3].
Perhaps due to its rarity, there is currently no consensus on the staging of Merkel cell carcinomas, with 6 existing staging systems [44-49]. Although varied, the various staging systems agree that MCC is an aggressive tumour with a high incidence of regional and distal metastases and with a poor prognosis if not confined to the primary site [50].
The mainstay of local treatment is surgical resection with clear margins, with a debate between Mohs surgery versus wide local excision. In Mohs surgery for MCC, local recurrence rates have varied from 4-14% in several small studies [51-53]. Which are comparable with the 9-10% local recurrence rates in larger retrospective studies conducted at the Memorial Sloan- Kettering Cancer Center [14]. Despite a lack of controlled trials on best surgical management, the National Comprehensive Cancer Network guidelines of the United States recommend wide local excision with a 1-2 cm gross margin as the treatment of primary MCC disease [54].
MCC tumours are radiosensitive and radiotherapy has a proven role in the treatment of inoperable local disease, recurrent disease, and as adjuvant therapy [55-60].
For regional disease, NCCN guidelines recommend routine sentinel lymph node biopsy for node negative patients and a comprehensive neck dissection to node positive patients. Adjuvant radiation to the dissected neck is usually considered when there is more than one nodal metastasis or any number of nodes showing extranodal extension [54]. The role of chemotherapy in the treatment of MCC is yet to be clearly defined but recently, immunotherapy agents such as Avelumab have been approved in the United States for the treatment of distantly metastatic MCC. They hold the promise of more durable response with less toxicity and provide an alternative to cytotoxic chemotherapy for the patients with distant disease [54]. Although a high level of disease recurrence is known, there have been reports of spontaneous MCC regression, including that of metastatic lesions [61,62]. These cases provide evidence that the adaptive immune response may successfully regress MCC.
In our case, the lesion showed histological and immunohistochemical characteristics of MCC. However, the presentation was atypical, with a self-involuting tumour despite a lack of aggressive intervention in the form of wide local excision, radiotherapy or chemotherapy. The only significant change in the patient during the clinical course of his MCC was an improvement in the severity of his pre-existing comorbidities. Notwithstanding the lack of more comprehensive radiological imaging, the lesion has not recurred at the excision site or in the adjacent nodal basins after a simple excision and the patient is currently disease free after 3 years of follow-up.
We propose three explanations for this unusually good clinical course. First, the MCC might be an incidental finding within the squamous papilloma and excised before it became clinically significant, such that the size of the largest tumour focus was only 0.1 cm, much smaller than the close to 2 cm size of a typical MCC at initial presentation [63,64]. Thus, an excision with narrow margin sufficed as adequate oncological treatment. Second, spontaneous resolution of the MCC might have occurred and be related to the possible viral origin of this tumour. It is plausible that the improvement of the patient’s health and immune status led to suppression of the viral-driven oncogenesis, resulting in a regression in the MCC. Third, the spontaneous regression occurred in the squamous papilloma and the underlying mechanism lead to the concomitant regression of the MCC. These are hypotheses that need further prospective clinical or laboratory validation.
Conclusion
In conclusion, Merkel cell carcinoma is a rare primary skin cancer arising from an epidermal Merkel cell or dermal neuroendocrine cell or a pleuripotent stem cell with a possible viral link to the Merkel cell polyomavirus [63,64]. To our knowledge, Merkel cell carcinoma has not been reported in a squamous papilloma and its spontaneous regression is uncommon [63,64]. Although the consensus on the primary treatment of MCC is surgical excision with wide margins with or without adjuvant radiotherapy, the atypical presentation of a spontaneously regressing tumour, foci of MCC occurring within a squamous papilloma in our case report may represent the role of the immune system in modulating viral-associated tumours or the fortuitous excision of microscopic foci of MCC with clear margins.
Conflict of Interest
The authors declare no conflict of interests.
23976
References
- Toker C (1972) Trabecular carcinoma of the skin. Arch Dermatol 105(1): 107-110.
- Lewis KG, Weinstock MA, Weaver AL, Otley CC (2006) Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol 142(6): 693-700.
- Agelli M, Clegg LX, Becker JC, Rollison DE (2010) The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer 34: 14-37.
- Anderson LL, Phipps TJ, McCollough ML (1992) Neuroendocrine carcinoma of the skin (Merkel cell carcinoma) in a black. J Dermatol Surg Oncol 18: 375-380.
- Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, et al. (2010) Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 37(1): 20-27.
- Hussain SK, Sundquist J, Hemminki K (2010) Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age-dependent increases. J Invest Dermatol 130(5): 1323-1328.
- Smith PD, Patterson JW (2001) Merkel cell carcinoma (neuroendocrine carcinoma of the skin). Am J Clin Pathol 11(5): 68-78.
- Enzinger FM, Weiss SW (1995) Primitive neuroectodermal tumors and related lesions. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors, 3rd ed. St. Louis: Mosby–Year Book pp: 929-964.
- Nikitakis NG, Salama AR, O'Malley BW Jr, Ord RA, Papadimitriou JC (2003) Malignant peripheral primitive neuroectodermal tumor-peripheral neuroepithelioma of the head and neck: A clinicopathologic study of five cases and review of the literature. Head Neck 25(6): 488-498.
- Lewis KG, Weinstock MA, Weaver AL, Otley CC (2006) Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol 142(6): 693-700.
- O’Connor WJ, Roenigk RK, Brodland DG (1997) Merkel cell carcinoma. Comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg 23(10): 929-933.
- Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK (1991) Merkel cell carcinoma: Prognosis and management. Arch Surg 126(12): 1514-1519.
- Akhtar S, Oza KK, Wright J (2000) Merkel cell carcinoma: A report of 10 cases and review of the literature. J Am Acad Dermatol 43(5): 755-767.
- Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, et al. (2005) Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. Journal of Clinical Oncology 23(10): 2300-2309.
- Hodgson NC (2005) Merkel cell carcinoma: Changing incidence trends. J Surg Oncol 89(1): 1-4.
- Agelli M, Clegg LX (2003) Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol 49(5): 832-841.
- Miller RW, Rabkin CS (1999) Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol Biomarkers Prev 8(2):153-158.
- Lemos B, Nghiem P (2007) Merkel cell carcinoma: More deaths but still no pathway to blame. J Invest Dermatol 127(9): 2100-2103.
- Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, et al. (2010) Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 37(1): 20-27.
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, et al. (2009) Merkel cells are essential for light-touch responses. Science 324(5934): 1580-1582.
- Hartschuh W, Weihe E, Yanaihara N, Reinecke M (1983) Immunohistochemical localization of vasoactive intestinal polypeptide (VIP) in Merkel cells of vari- ous mammals: evidence for a neuromodulator function of the Merkel cell. J Invest Dermatol 81(4): 361-364.
- Chew SB, Leung PY (1991) Immunocytochemical evidence of a met-enkephalin-like substance in the dense-core granules of mouse Merkel cells. Cell Tissue Res 265(3): 611-614.
- Fantini F, Johansson O (1995) Neurochemical markers in human cutaneous Merkel cells. An immunohis-tochemical investigation. Exp Dermatol 4(6): 365-371.
- English KB, Wang ZZ, Stayner N, Stensaas LJ, Martin H, et al. (1992) Serotonin-like immunoreactivity in Merkel cells and their afferent neurons in touch domes from the hairy skin of rats. The Anatomical Record 232(1): 112-120.
- García-Caballero T, Gallego R, Rosón E, Fraga M, Beiras A (1989) Calcitonin gene-related peptide (CGRP) immunoreactivity in the neuroendocrine Merkel cells and nerve fibres of pig and human skin. Histochemistry 92(2): 127-132.
- Hartschuh W, Weihe E, Yanaihara N (1989) Immunohistochemical analysis of chromogranin A and multiple peptides in the mammalian Merkel cell: further evidence for its paraneuronal function?. Arch Histol Cytol 52(Suppl): 423-431.
- Alvarez FJ, Cervantes C, Villalba R, Blasco I, Martínez-Murillo R, et al. (1988) Immunocytochemical analysis of calcitonin generelated peptide and vasoactive intestinal polypeptide in Merkel cells and cutaneous free nerve endings of cats. Cell and tissue research 254(2): 429-437.
- Schadendorf D, Lebbé C, Hausen AZ, Avril MF, Hariharan S, et al. (2017) Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer 71: 53-69.
- Sahi H, Sihto H, Artama M, Koljonen V, Bohling T, et al. (2017) History of chronic inflammatory disorders increases the risk of Merkel cell carcinoma, but does not correlate with Merkel cell polyomavirus infection. Br J Cancer 116(2): 260-264.
- Feng H, Shuda M, Chang Y, Moore PS (2008) Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319(5866): 1096-100.
- Becker JC, Houben R, Ugurel S, Trefzer U, Pföhler C, et al. (2009) MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermato 129(1): 248-250.
- Kassem A, Schöpflin A, Diaz C, Weyers W, Stickeler E, et al. (2008) Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res 68(13): 5009-5013.
- Garneski KM, Warcola AH, Feng Q, Kiviat N, Leonard JH, et al. (2009) Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol 129(1): 246-248.
- Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, et al. (2012) Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest 122(12): 4645-4653.
- Touze A, Le Bidre E, Laude H, Fleury MJ, Cazal R, et al. (2011) High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol 29(12): 1612-1619.
- Laude HC, Jonchère B, Maubec E, Carlotti A, Marinho E, et al. (2010) Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog 26(6): 1001076.
- Chen QR, Vansant G, Oades K, Pickering M, Wei JS, et al. (2007) Diagnosis of the small round blue cell tumors using multiplex polymerase chain reaction. J Mol Diagn 9(1): 80-88.
- North J, McCalmont TH (2013) Histopathologic Diagnosis. In: Alam, Murad (ed.), Merkel Cell Carcinoma, Springer, New York, USA. pp: 65-86.
- Ng L, Beer TW, Murray K (2008) Vascular density has prog-nostic value in Merkel cell carcinoma. Am J Dermatopathol 30(5): 442-445.
- Lanoy E, Costagliola D, Engels EA (2010) Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer 126(7): 1724-1731.
- Engels EA (2009) Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS 23(3): 385-393.
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, et al. (2008) Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol 58(3): 375-381.
- Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK (1991) Merkel cell carcinoma: Prognosis and management. Arch Surg. 126(12): 1514-1519.
- Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, et al. (2005) Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J Clin Oncol 23(10): 2300-2309.
- Greene FL, Page DL, Fleming ID (2002) AJCC cancer staging manual. (6th edn). New York: Springer, USA.
- Allen PJ, Zhang ZF, Coit DG (1999) Surgical management of Merkel cell carcinoma. Ann Surg 229(1): 97-105.
- Clark JR, Veness MJ, Gilbert R, O’Brien CJ, Gullane PJ (2007) Merkel cell carcinoma of the head and neck: Is adjuvant radiotherapy necessary?. Head and Neck 29(3): 249-257.
- Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, et al. (2010) Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 63(5): 751-761.
- Ibrahim SF, Siegrid SY (2013) Staging. In: Alam, Murad (ed.), Merkel Cell Carcinoma, Springer, New York, USA. pp: 53-64.
- Roy R, Kuzel TM (2013) Radiation therapy (Primary and recurrent disease). In: Alam, Murad (ed.), Merkel Cell Carcinoma, Springer, New York, USA. pp: 119-133.
- Boyer JD, Zitelli JA, Brodland DG, D’Angelo G (2002) Local control of primary Merkel cell carcinoma: review of 45 cases treated with Mohs micrographic surgery with and without adjuvant radiation. J Am Acad Dermatol 47(6): 885-892.
- Snow SN, Larson PO, Hardy S, Bentz M, Madjar D, et al. (2001) Merkel cell carcinoma of the skin and mucosa: A report of 12 cutaneous cases with 2 cases arising from the nasal mucosa. Dermatol Surg 27(2): 165-170.
- Cotlar AM, Gates JO, Gibbs Jr FA (1986) Merkel cell carcinoma: combined surgery and radiation therapy. Am Surg 52(3): 159-164.
- Raaf JH, Urmacher C, Knapper WK, Shiu MH, Cheng EW, et al. (1986) Trabecular (Merkel cell) carcinoma of the skin: Treatment of primary, recurrent and meta-static disease. Cancer 57(1): 178-82.
- Koh CS, Veness MJ (2009) Role of definitive radiotherapy in treating patients with inoperable Merkel cell carcinoma: the Westmead Hospital experience and a review of the literature. Australas J Dermatol 50(4): 249-256.
- Eng TY, Naguib M, Fuller CD, Jones III WE, Herman TS (2004) Treatment of recurrent Merkel cell carcinoma: an analysis of 46 cases. Am J Clin Oncol 27(6): 576-583.
- Tai P, Yu E, Assouline A, Lian JD, Joseph K, et al. (2010) Multimodality management for 145 cases of Merkel cell carcinoma. Med Oncol 27(4): 1260-1266.
- Mojica P, Smith D, Ellenhorn JD (2007) Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol 25(9): 1043-1047.
- O'Rourke MG, Bell JR (1986) Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol 12(9): 994-997.
- Pang C, Sharma D, Sankar T (2015) Spontaneous regression of Merkel cell carcinoma: A case report and review of the literature. Int J Surg Case Rep 7: 104-108.
- Lester DR, Thompson Bruce M, Wenig Diagnostic pathology (2016) Diagnostic Pathology: Head and Neck, 2nd ed pp: 82-84.
- https://www.elsevier.com/books/mckees-pathology-of-theskin/calonje/978-1-4160-5649-2.








