Keywords
Burn; Infection; Microbial contamination; Patients; Prevalence; Taif; Wound
Introduction
Burns provide a suitable site for bacterial multiplication and are more persistent richer sources of infection than surgical wounds, mainly because of the larger area involved and longer duration of patient stay in the hospital [1]. Infection is a major cause of morbidity and mortality in hospitalized burn patients [2]. It has been estimated that about 75% of the mortality associated with burn injuries is related to sepsis especially in developing countries [3]. In addition, overcrowding in burns units is an important cause of cross infection which necessitates a regular monitoring of bacterial species and their antibiotic susceptibilities because significant shifts in these data may be correlated with changes in clinical management with respect to drug choice for therapy [4]. Infection causes 50% to 60% of deaths in burn patients in spite of intensive therapy with antibiotics both topically as well as intravenous [5]. The pattern of infection differs from hospital to hospital; the varied bacterial flora of infected wound may change considerably during the healing period [6]. Despite the advances in patient care and the use of a large number of antimicrobial agents, infections which complicate the clinical course of patients who had sustained severe thermal injures continue to be a major unsolved problem. Historically, Staphylococci and beta hemolytic Streptococci were the commonest organisms causing burn wound infection in early part of the century [7]. Burns are one of the most common and devastating forms of trauma. Patients with serious thermal injury require immediate specialized care in order to minimize morbidity and mortality. Data from the National Center for Injury Prevention and Control in the United States show that approximately 2 million fires are reported each year which result in 1.2 million people with burn injuries [8-11]. Moderate to severe burn injuries requiring hospitalization account for approximately 100,000 of these cases, and about 5,000 patients die each year from burn related complications [11]. The survival rates for burn patients have improved substantially in the past few decades due to advances in modern medical care in specialized burn centers. Improved outcomes for severely burned patients have been attributed to medical advances in fluid resuscitation, nutritional support, pulmonary care; burn wound care, and infection control practices. As a result, burn-related deaths, depending on the extent of injury, have been halved within the past 40 years [12]. In patients with severe burns over more than 40% of the Total Body Surface Area (TBSA), 75% of all deaths are currently related to sepsis from burn wound infection or other infection complications and/or inhalation injury [13]. Immediately following injury, Gram-positive bacteria colonize the burn wound [14]. Following Gram-negative bacteria also rapidly colonize the burn wound surface in the first few days after injury [15,16]. Wound colonization by yeasts and fungi usually occurs later due to the use of broad spectrum antibiotic therapy [17]. Microorganisms routinely isolated from burn wounds include aerobic organisms like Staphylococcus aureus, Streptococcus pyogenes, E. coli, Klebsiella Spp., Proteus, Pseudomonas aeruginosa, anaerobic organisms like Bacteroides fragilis, Peptostreptococcus, Propionibacterium Spp., Fusobacterium Spp. and fungi like Aspergillus niger, Candida Spp and Zygomycetes. It has been found by many investigators that the distribution of various species of bacteria from burn wound surfaces is similar to that from blood specimens [18]. Children have a much higher risk of being burned than adults [4-7]. In the United States in 2001 to 2002, an estimated 92,500 children aged 14 years and under required emergency care for burn-related injuries, and approximately 500 of these children died. Approximately twothirds of these children sustained thermal injuries, while children less than 4 years of age are particularly prone to scald injury. Male children have a higher risk of burn injury and burn related death than females, and obese boys represented a disproportionate number of the patients admitted to a pediatric burn center from 1991 to 1997 [19]. The efficacy of various topical antimicrobials in common use in modern burn centers is dynamic due to the ability of microorganisms to develop resistance rapidly. The sustained potency of individual agents depends on the extent of use and the resident nosocomial flora within any specialized burn center [20]. In order to accurately detect and track emerging trends in topical antimicrobial resistance in modern burn units, it is essential that standard reproducible methods be published for clinical implementation [21]. The aim of the present study was to study the microbial profile of burn wound infections in burn patients, and to evaluate the antibiotic sensitivity of causative agents, Taif, Saudi Arabia.
Materials and Methods
Study population
This study included 220 patients who were admitted in the burn unit in Al-Hada Military Hospital Taif, Saudi, during the 6 months period from March 2015 to August 2015, suffering various forms of acute burn injuries, age from 5-59 Year, all included in study. Selection criteria for the patients included in the study, (i) Any patient admitted in the burn unit in Al-Hada Military Hospital without delay more than six hours with acute burn injury covering less than 50% of the Total Body Surface Area (TBSA). (ii) No history of diabetes mellitus, immunosuppressive disease or receiving any immunosuppressive therapy in the preceding 6 months. (iii) Patients who followed the open dressing method or underwent surgical coverage procedure as split thickness skin graft in the first 24 days post burn were excluded from the study. (iv) Pregnant women, newborns or nursing mothers with infants younger than two months of age were excluded as they cannot use silver sulfadiazine which is standardized for dressing in all patients because of the risk of sulfonamide kernicterus. Initial evaluation of all selected patients included primary stabilization of the patient’s general condition, emphasizing on support of the airway, respiration, and circulatory stability followed by a burn-specific secondary survey to determine the site, size and depth of the burn in addition to all investigations that would aid the clinical assessment. Clinical evaluation of the burn depth was done. All available information was recorded, including the patient’s personal data and the mechanism of injury. Color photographs of the burn injuries were taken. This was followed by the usual regimen of local burn wound care in the form of clothing removal, wound cleaning by washing with sterile saline solution (0.9% NaCl), chemoprophylaxis and dressing.
Chemoprophylaxis
Tetanus immunization was updated in patients with wounds deeper than a superficial partialthickness burn. Human tetanus immunoglobulin was given (250 to 500 IU) to provide immediate passive immunization regardless of the patient’s active immunization status. Routine penicillin IV administration at the dose of 1.2 million IU every 6 hours for the first 72 hours, as it is believed to contribute in further reducing the incidence of burn wound infection involving group a beta haemolytic Streptococci [22].
Sample collection
All samples were collected from different wound sites admitted in the burn unit in Al-Hada. Military Hospital Taif, 1- On day of patient’s admission, surface swabs were taken from all patients included in the study: A quantitative microbiology culture report was done to provide the CFU per cm2 of wound surface area. Therefore, during sample collection, tip of the swab was to be rolled on its side for one full rotation over 4 cm2 of the part of the wound granulation tissue with the most obvious signs of infection and/or inflammation. The swabs were transported within 1 hour to the Microbiology department. Swabs for anaerobic culture were transported in thioglycolate broth in well-sealed bottles. Afterwards, the samples were plated on culture media as soon as possible, according to Steer et al. [23]. 2- On suspicion of having septicemia or fungaemia, two blood samples were taken from each patient. The samples were collected under complete aseptic conditions.
Sample processing
Direct examination of specimens
The first swab was used to prepare two direct smears. One was examined after adding 10% KOH solution for fungal identification. The other was stained by Gram stain for bacterial examination and detection of PMNL which is an important feature in case of bacterial infection rather than in bacterial colonization [24].
Culture
• The second swab was placed in 1 cm of Tween 80 and well shaken using the vortex for a minute. Then, 0.1 ml and 0.01 ml amounts of the bacterial suspensions were inoculated evenly over different agar plates aerobically; MacConkey and 5% Blood agar and Sabouraud Dextrose Agar supplemented by chloramphenicol (Saudi Prepared Media Laboratory, Saudi Arabia, and Riyadh (SPML).
• While the third swab in thioglycolate was incubated for 24 hrs, then placed on Blood and MacConkey agar and incubated anaerobically for 2-4 days (Saudi Prepared Media Laboratory, Saudi Arabia, and Riyadh (SPML).
• The blood samples were inoculated in 2 different blood culture bottles for aerobic (Oxoid signal blood culture bottle) and anaerobic (thioglycolate blood culture bottle) isolates
Species identification
• Bacterial growths and fungal yields were identified according to standard conventional procedures.
• The species identification was based on Gram-stain, catalase test, oxidase test, indole test, lactophenol cotton blue for microscopy and staining molds, strep latex test kit (BBL Streptocard),staphyloslide test kit (BBL Staphyloslide), germ tube test, sugar assimilation test, sugar fermentation test, and KOH test for fungi identification.
• Identified isolates were stored on nutrient agar slant at room temperature for subsequent susceptibility testing. According to guidelines of the CDC 2010.
• Commercial identification kits were used to identify the isolates up to species level Different type of API kits Analytab product,Plainview), and Vitek system, different card for identification of gram-positive bacteria, gramnegative bacteria, yeast. Afterwards, the sensitivity to the antibiotics was accomplished by disk diffusion test performed for all the isolates by the method are commended by Clinical and Laboratory Standard Institute (CLST). A suspension of each isolate was made so that the turbidity was equal to 0.5 McFarland turbidity standards and then plated onto Muller-Hinton agar (Saudi Prepared Media Laboratory, Saudi Arabia, and Riyadh (SPML). Antibiotic disks (Oxoid) were applied to each plate. After incubation at 37ºC for 24 h, inhibition zone size was measured. The patients received the proper antibiotic thereafter [25]. Twenty two types of antibiotics were used in both Gram-negative rod and Gram-positive cocci. Amoxicillin/Clavulinicacid (20/10 μg), Gentamicin (10 μg), Oxacillin (1 μg), Sulfamethoxazole/Trimethoprim (1.25/23.75 μg), Ciprofloxacin (5 μg), Clindamycin(2 μg), Vancomycin (30 μg), Cefoxitin (30 μg), Cefoxithin (30 μg), Ceftazidime (30 μg), Cefotaxime (30 μg), Amikacin (30 μg), Ceftriaxone (30 μg), Ceftazidime (30 μg), Ampicillin/ Sulbactam (10/10 μg), Cefotaxim (30 μg), Ticarcillin/ clavulanicacid (75/10 μg), Imipenem (50 μg), Cefepime (10 μg), Ampicillin/Sulbactam (10/10 μg), Aztreonam (30 μg), Piperacillin/tazobactam (100/10 μg), (Oxoid, United).
Quality control
To maintain the quality of data every sample was processed in triplicates and every result was cross checked by the principal investigator and the coinvestigator. Enterococcus faecalis (ATCC 29212), Staphylococcus aureus(ATCC 24923), Streptococcus pyogenes (ATCC 19615), E. coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) were used as quality control throughout the study for culture, Gram stain. All the strains were obtained from the (ATCC, The essential of live science research, USA).
Data analysis
Statistical analyses were performed using the Statistical Package for the Social Science (SPSS), Version 16 for Windows. Continuous variables were summarized using descriptive statistics in terms of means, ± standard deviations, T.test; 95% confidence intervals (95% CI), P value<0.05 were considered significant.
Results
This study included 220 patients, 159 males (72.2%) and 61 females (27.7%), their ages ranged from 5 years to 59 years. Total Body Surface Area (TBSA) was less than 50% with a mean of 24%.
Direct microscopic examination
It was done routinely in all surface swabs using Gram stain and 10% KOH. The relation between Gram stain and culture results in the current study were found to be reasonable, 72% of the Gram stain results provided an index of the type of organisms expected whether Gram positive cocci, Gram negative bacilli or even fungal isolates. It also provided a good indication of microbial colonization, distinguishing it from infection side by side with clinical symptoms and signs. However, the degree of correlation between surface swab KOH examination and culture results of yeast, and fungal yields were found to be fair, only 31.5% of the positive fungal cultures were found to be positive by direct examination.
Surface swab culture
A quantitative microbiology culture was used to provide the CFU per cm2 of wound surface area. Colony counts are done to obtain the predominant potential pathogens isolated, while, the isolates of very low counts were ignored. The surface swab taken from the patients showed 9 bacterial isolates from 220 different patients (4%). On the other hand, fungal culture showed fungal colonization by Candida glabrat in ten cases (4.5%), and Aspergillus sp. six cases (2.7%) (Table 1). The socio-demographic characteristics (age in years) of 220 burn patients admitting Al- Hada Military Hospital Taif, was presented in Table 1. The mean age of the participants was years 5 ranging 5-59 years. The majority of the participants were the age of 27-37 year (54.5%), followed the 16-26 year 60 (27.2%), while the remaining were 5-15 year 26 (12.2%), 38-48 year 10 (4.5%), and 49-59 year 3 (1.3%). There is statistical significant difference between age and frequency of burn infection at age 38-48, P=0.01 (Table 2). The predominant site of infection was femur130 (59%), followed by thigh, and knee with carriage rate 21 (9.5%), 20 (9%) respectively. Other sites present in low percentage groin 10 (4.5%), abdomen 9 (4%), peritoneal fluid 8 (3.6%), pleural fluid, and pelvis 6 (2.75), sacral sore 4 (1.8%), and J-vac drain, and stomach 3 (1.3%) was presented in Table 2. There is statistical significant difference between sites of infection, and burn injuries infection at femur, groin and frequency of P=0.01, P=0.02 respectively (Table 3).
| Socio-demographic Characteristics |
Frequency N= 220 |
Percent (%) P |
value |
| 1- Age in years |
|
|
| May-15 |
27 |
12.2 |
|
| 16-26 |
60 |
27.2 |
|
| 27-37 |
120 |
54.5 |
|
| 38-48 |
10 |
4.5 |
0.01 |
| 49-59 |
3 |
1.3 |
|
| total |
220 |
100 |
|
| Age/years; Mean 2.5; ±Std 1.5 |
Table 1: Socio-demographic characteristics (age in years) of patients in burn unit, N=220.
| Sites |
Frequency N=220 |
Percent (%) |
P value |
| Peritoneal fluid |
8 |
3.6 |
|
| Pleural fluid |
6 |
2.7 |
|
| J-vac drain |
3 |
1.3 |
|
| Knee/swab |
20 |
9 |
|
| Stomach/swab |
3 |
1.3 |
|
| Thigh/swab |
21 |
9.5 |
|
| Pelvis/swab |
6 |
2.7 |
|
| Abdomen/swab |
9 |
4 |
|
| Femur/swab |
130 |
59 |
0.01 |
| Groin/swab |
10 |
4.5 |
0.02 |
| Sacral sore/swab |
4 |
1.8 |
|
| total |
220 |
100 |
|
| Mean 6; sd+1.4 |
Table 2: sits of infection and percentage.
| Causes of burns |
Frequency N= 220 |
Percent (%) |
P value |
| R.T.A |
150 |
68.1 |
|
| Hot water |
40 |
18.1 |
|
| Steam |
10 |
4.5 |
0.03 |
| Hot food |
20 |
9 |
|
| |
|
|
|
| total |
220 |
100 |
|
| Mean 2.5;Sd+1.2 |
Table 3: Causes of burns and percentage.
The predominant causes of burn infection in this study were Road Traffic Accident (RTA) with high rate of 150 (68.1%), followed by hot water with carriage 40 (18.1%). In contrast burn due to hot food presented in low rate of 20 (9%), while burn by steam gave very low rate of 10 (4.5%) was presented in Table 3. There is statistical significant difference between type of causes and burn infection in burn patients by steam P=0.03 (Table 4).
| Sociodemographic Characteristics |
Age/ year (n=220,%) |
| 3- Sex / male N=159(72.2%) |
|
|
|
|
|
| |
5-15Y |
16-26 Y |
27-37 Y |
38-48Y |
49-59 |
| |
10(4.5%) |
40(18.1%) |
100(45.4%) |
7(3.1%) P 0.03 |
2(0.9%) |
| 4- Sex /female N= 61(27.7%) |
17(7.7%) |
20(9%) |
20 (9%) |
3(1.3%) P 0.01 |
1(0.4%) |
| |
27 (12.2% |
60(27.2%) |
120(54.5%) |
10(4.5%) |
3(1.3%) |
| Total=220 |
|
|
|
|
| Sex /male; Mean 3/31; ±Sd 1.5/40 ; Sex /female ; Mean 3/12; ±Sd 1.5/9.4 |
Table 4: Socio-demographic characteristics (sex, gender) of burn patients,Taif. N=220.
The socio-demographic characteristics (sex, gender) of 220 patients admitted in burn unit at Al-Hada military Hospital, Taif. The total number of male patient was 159 (72.2%). The main group of the burn patient was male at age of 27-37 year 100 (45.5%), while female in less in age 16-26 year, and 27-37 year 20 (9%). The majority of the participants in male were the year 27-37 year 100 (45.4%), ,followed the 16-26 year 40 (18.1%), while year 5-15 gave rate of 10 (4.5%), and year 38-39 and 49- 59 year found in low rate 7(3.1%), 2(0.09%) respectively, was presented in Table 4. There is statistical significant difference between type of causes and burn infection in burn patients by steam P 0.03. The total number of female patient was 61. The majority of the participants in female were the year 16-26 year, and 27-37 year with the same rate of 20 (9%), followed the 5-15 year 17 (7.7%), while year 38-48 P=0.03, and 49-59 year, found in low rate 3 (1.3%), and 1 (0.4%) respectively, There is statistical significant difference between age and gender in burn patient at 38-48 year P=0.01 (Table 5). The socio-demographic characteristics of nationality (Saudi, non-Saudi) of 220 patients admitted in burn unit at Al-Hada military Hospital, Taif. Saudi citizen was predominant in burn patient 180 (81.1%), adult high rate 160 (72.2%), and Child 20 (9%). In contrast less rate in non- Saudi citizen 40 (18.1%), adult 30 (13.6%), and low rate in child 10 (4.5%), was presented in Table 5. There is statistical significant difference between age and gender in both Saudi and non-Saudi groups in chilled P=0.03 (Table 6).
| Nationality |
Frequency N= 220 |
Percent (%) |
P value |
| Saudi |
|
|
|
| child |
20 |
9 |
|
| adult |
160 |
72.2 |
|
| Total |
180 |
81.1 |
|
| Non-Saudi |
|
|
|
| child |
10 |
4.5 |
0.03 |
| adult |
30 |
13.6 |
|
| Total |
40 |
18.1 |
|
| Total all |
220 |
100 |
|
Table 5: Demographic pattern of burn patients, nationality (Saudi/ non Saudi) of patients admitted in burn unit,Taif. N=220.
| Degree of burn |
Frequency |
Percentage % |
P value |
| First- Degree |
50 |
22.7 |
|
| Second-Degree |
159 |
72.1 |
|
| Third- Degree |
6 |
2.7 |
0.03 |
| Fourth- Degree |
5 |
2.2 |
|
| Total |
220 |
100 |
|
| Mean 2.5/55 Sd+ 1.29/72.2 |
Table 6: Distribution of the burn patients according to degree of burn.
The main group of the burn degree of was Second-Degree 159 (72.1%), while First- Degree followed by rate of 50 (22.7%), In contrast, Third- Degree, and Fourth- Degree found in low rate 2.7%, and 2.2% respectively. There is statistical significant difference between degree of burn and culture results at Third- Degree P=0.03 (Table 7, Figure 1).
| Isolated Microorgainsms Bacterial isolates |
Frequency N= 220 |
% Percentage |
P value |
| Gram-positive cocci |
| Staphylococcus epidermidis |
50 |
22.2 |
|
| Staphylococcus aureus |
44 |
20 |
|
| Enterococcus facium |
10 |
4.5 |
|
| Gram-negative rod |
| Escherichia coli |
89 |
40 |
|
| Morganellamorganii |
25 |
11.3 |
|
| Pseudomonas aeruginosa |
87 |
39.5 |
|
| Klebsiellapneumoniae |
62 |
28.1 |
|
| Acinetobacterbaumannii |
43 |
19.5 |
0.001 |
| Proteus mirabilis |
35 |
16 |
|
| Yeast and Fungal isolates |
| Candida glabrata |
10 |
4.5 |
0.001 |
| Aspergillus sp. |
6 |
2.7 |
|
Table 7: lists the most common microorganisms colonizing and infecting burn wounds,Taif, N=220.
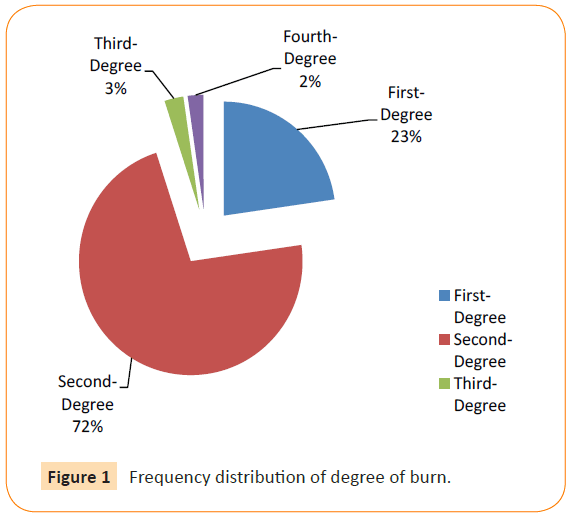
Figure 1: Frequency distribution of degree of burn.
A total of 220 burn patients admitting burn unit were all positive culture including Gram-positive cocci, Gram-negative rod, yeast and fungi was presented in Table 7.
Comparison of Average Incidence of Major Groups of Organisms
Gram-positive bacteria
Staphylococcus epidermidis was the predominant organisms with rate of 50 (22.2%), followed by Staphylococcus aureus 44 (20%), Enterococcus facium 10 (4.5%).
Gram-negative rod
E. coli was the predominant organisms with high rate of 89 (40%), followed by Pseudomonas aeruginosa 87 (39.5%), klebsiella pneumoniae 62 (28.1%), Acinetobacter baumannii 43 (19.5%), Proteus mirabilis 35 (16%), and Morganella morganii 25 (11.3%).
3-Yeast
Only Candida glabrata was the predominant organisms with rate of 10 (4.5%), and one species of mold Aspergillus sp. with very low rate 6(2.7%). There is statistical significant difference between isolated microorgainsms and frequency of infection in burn patient, Acinetobacter baumannii, and Candida glabrata P 0.001 (Figure 2). Among the bacterial isolates recovered from burn wound infections, E.coli, Pseudomonas aeruginosa, Klebsiella pneumoniae were most frequent of bacterial growths, each representing, 89 (40%), 87 (39.5%), 62 (28.1%) respectively followed by coagulase negative Staphylococci; Staphylococcus epidermidis, Staphylococcus aureus, Acinetobacter baumannii representing 50 (22.2%), 44 (20%), 43 (19.5%), respectively (Table 7).
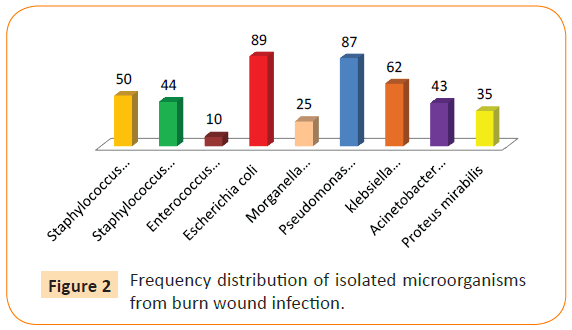
Figure 2: Frequency distribution of isolated microorganisms from burn wound infection.
The following organisms isolated in low percentage Proteus mirabilis 35(16%) Morganella morganii 25 (11.3%). Enterococcus facium 10(4.5%), only Candida glabrata was the predominant organisms with rate of 10 (4.5%), and one species of mold Aspergillus sp. with very low rate 6(2.7%). Out of 16 patients (7.2%) suffering from fungal wound invasion, 10 acquired Candida glabrata representing (4.5%). Six cases suffered from Aspergillus sp. representing 6(2.7%). Among the 220 patients enrolled in the study, it was observed that the frequency of bacterial infection was greater than that of fungal infection reaching 68.5%, while yeast, and fungal growths represented only 31.5%, that rise showed high significance when compared to hospital stay.
Antibiotic sensitivity
Disk diffusion method was performed to all bacterial isolates causing infection. Among these isolates, many were found to be resistant to more than one antibiotic (Table 8). The susceptibility pattern of 9 bacteria isolated from burn patient against 22 antimicrobial agents.
| Antibiotics |
Resistant Staphylococcus aureus N=44 -20% |
Resistant Coagulase negative Staphylococci N=50 (22.7%) |
Resistant Enterococcus facium N=10 (4.5%) |
| Amoxicillin/ Clavulanic acid (20/10 μg) |
9(20%) |
10(20%) |
2(20%) |
| Cephalothin (30 μg) |
8 (18.1%) |
5(10%) |
1(10) |
| Oxacillin (1 μg) |
S |
S |
S |
| Gentamicin (10 μg) |
8 (18.1%) |
5(10%) |
2(20%) |
| Amikacin (30 μg) |
5 (11.3%) |
3(6%) |
2(20%) |
| Ciprofloxacin (1 μg) |
9 (20%) |
5(10%) |
1(10) |
| Ceftriaxone (30 μg) |
3(6.8%) |
5(10%) |
1(10) |
| Co-trimoxazole (1.2/23.8 μg) |
5 (11.3%) |
5(10%) |
2(20%) |
| Ceftazidime (30 μg) |
3(6.8%) |
2(4%) |
1(10) |
| Ampicillin/Sulbactam (10/10) |
8(18.1%) |
2(4%) |
2(20%) |
| Cefotaxim (30μg) |
5(11.3%) |
1(2%) |
1(10) |
| Ticarcillin/clavulanic acid ( 75/10 μg) |
2(4.5%) |
S |
S |
| Piperacillin/tazobactam (100/10 μg) |
2(4.5%) |
2(4%) |
S |
| Imipenem (50 μg)) |
1(2.2%) |
S |
S |
| Cefepime (10 μg) |
2(4.5%) |
S |
S |
| Clindamycin (2 μg) |
1(2.2%) |
S |
S |
| Vancomycin (30 μg) |
S |
S |
S |
| Ampicillin/Sulbactam (10/10 μg) |
S |
S |
S |
| *S; Sensitive |
Table 8: Antibiotic susceptibility pattern (%) of resistant Gram positive isolates in burn patients.
Gram-positive cocci
Staphylococcus aureus found to be resistant in 44 out of 220 cases representing 20%. However, coagulase negative staphylococci were 50 out of 220 yields representing 22.7%, while Enterococcus facium were recovered from 10 out of 220 cases (4.5%). The result of this study revealed that, resistant of Staphylococcus aureus to 15 antibiotic, and fully susceptible to oxacillin, vancomycin, and apmecillin/sulbactam. In Table 7, while coagulase negative Staphylococci was fully susceptible to oxacillin, vancomycin, and apmecillin/sulbactam, Imipenem, Cefepime, Ticarcillin/clavulanic acid, and Clindamycin. In contrast, Enterococcus facium was fully susceptible to oxacillin, vancomycin, and apmecillin/sulbactam, piperacillin/tazobactam,imipenem, cefepime, ticarcillin/ clavulanic acid, and clindamycin. All strains were susceptible to some antibiotic used in study, and resistance was observed in some strains of Gram-positive cocci, show the different isolates’ resistance to various antibiotics in percent study (Table 9).
| Antibiotics |
Resistant E.coli N= 89 (40.4%) |
Resistant Pseudomonas aeruginosa N=87 (39.5%) |
Resistant Klebsiella pneumoniae N=62 (28.1%) |
Resistant Proteus mirabilis N=35 (12.7%) |
Resistant Morganella morganii N=25 (11.3%) |
Resistant Acinetobacter baumannii N= 43 (19.5%) |
| Aztreonam (30 μg) |
15(16.8%) |
17(19.5%) |
10(16%) |
2(5.6%) |
2(8%) |
20(47.5%) |
| Cephalothin (30 μg) |
18(20.2%) |
16(18.3%) |
15(24.1%) |
5(14.2%) |
3(12%) |
20(47.5%) |
| Cefoxithin (30 μg) |
10(11.2%) |
14(16%) |
10(16%) |
7(20%) |
3(12%) |
43(100%) |
| Gentamicin (10 μg) |
15(16.8%) |
16(18.3%) |
8(12.9%) |
5(14.2%) |
4(16%) |
10(23.2%) |
| Amikacin (30 μg) |
17(19.1%) |
15(17.2%) |
10(16%) |
3(18.5%) |
5(20%) |
43(100%) |
| Ciprofloxacin (5 μg) |
30(33.7%) |
16(18.3%) |
15(24.1%) |
7(20%) |
2(8%) |
20(47.5%) |
| Ceftriaxone (30 μg) |
19(21.3%) |
14(16%) |
10(16%) |
7(20%) |
3(12%) |
20(47.5%) |
| Co-trimoxazole (1.2/23.8 μg) |
20(22.4%) |
22(25.2%) |
20(32.2%) |
9(25.7%) |
5(20%) |
20(47.5%) |
| Ceftazidime (30 μg) |
15(16.8%) |
16(18.3%) |
8(12.9%) |
2(5.6%) |
1(4%) |
20(47.5%) |
| Ampicillin/Sulbactam (10/10 μg) |
1(1.1%) |
14(16%) |
8(12.9%) |
2(5.6%) |
1(4%) |
43(100%) |
| Cefotaxim (30 μg) |
15(16.8%) |
16(18.3%) |
10(16%) |
2(5.7%) |
2(8%) |
20(47.5%) |
| Ticarcillin/clavulanic acid (75/10 μg) |
2(2.2%) |
1(1.1%) |
1(1.6%) |
5(14.2%) |
2(8%) |
20(47.5%) |
| Piperacillin/tazobactam (100/10 μg) |
1(1.1%) |
1(1.1%) |
1(1.6%) |
2(5.6%) |
S |
20(47.5%) |
| Imipenem (10 μg) |
1(1.1%) |
1(1.1%) |
1(1.6%) |
S |
S |
10(23.2%) |
| Cefepime (10 μg) |
1(1.1%) |
1(1.1%) |
1(1.6%) |
S |
S |
10(23.2%) |
| *S; Sensitive |
Table 9: Antibiotic susceptibility pattern (%) of resistant Gram positive isolates in burn patients.
Gram-negative rod
E. coli found to be resistant in 89 out of 220 cases representing 40.4%. Pseudomonas aeruginosa 87 (39.5%). klebsiella pneumoniae 62 (28.1%), while Proteus mirabilis 33 (12.7%). In contrast, Morganella morganii found to be resistant in 22 out of 220 cases representing 11.3%. Acinetobacter baumannii 43 (19.5%).
Discussion
Burns are one of the most common and devastating forms of trauma. Patients with serious thermal injury require immediate specialized care in order to minimize morbidity and mortality. Although survival rates for burn patients have improved substantially in the past few decades due to advances in modern medical care in specialized burn centers, still, nosocomial infections represent a major challenge for a burn team in burn patients, which are known to cause over 50% of burn deaths. The burn wound is considered one of the major health problems in the world [26]. In the current study burn infection in males was 159 cases (72.2%), while in females it was 61 (27.7%). This result was in agreement with the finding reported by Ghaffar et al. who found that burn wound infection in males was 189 (62.4%) while burn wound infection in females 114 (37.6%) [27]. In a similar study, Macedo and Santos [28] found that burn wound infection in males 120 (59.1%) was more than burn wound infection in females 83 (40.9%). Also, Vostrugina et al. [29] found that burn infection in males was (76%) while burn infection in females was (24%). This may be due to that males are exposed more to burns and wear loose fitting clothes, but in our country may be due driving the car, rather than female [29]. Other study reported by Bagdonas et al. [30] found that burn wound infection in males was 1447 (64.4%) while burn wound infection in females were 799 (35.6%). In contrast to Rajupt et al. [3-8] showed that burn infection in females (60%) was more than male (40%) in India. In this study, it was found that the lowest distribution of burn wound infection found within the age group 5-15 years 27 (12.2%). This result was in agreement with the findings reported by Al- Akayleh [31] that showed that the age group<10 years had the highest distribution of burn wound infection in burn patients. In the other hand, Ghaffar et al. and Shakibaie et al. [27,32] found that the age group 10-19 years was more susceptible to burn wound infection than other age groups. In this study, it was found that the highest distribution of burn wound infection found within the age group 27-37 years 120 (54.5%), flowed by 16-26 year 60 (27.2%). This result was in agreement with the findings reported by K wong and Chung [33] found that the age group 19-40 years 23 (55%) were more susceptible to burn wound infection than other age groups. Children were the most susceptible group to burn wound infection in Sana’a city 70 (35%), followed by students 49 (24.5%) and house wife 45 (22.5%). Few cases were recorded in waiters working in restaurants 5 (2.5%), engineers 2 (1%), soldiers and peasants (0.5% for each). The relationship between patient's job and had burns wound infections showed no statistical significant P>0.05 [9]. Data in this investigation showed that 159 (72.1%) of burn infection patients had second degree burn, 50 (22.7%) had first degree burn, 6 (2.7%) had third Degree burn and 5 (2.2%) had fourth degree burn. Similarly, Al- Akayleh, showed that the highest distribution of burn wound infection found in burn patients who had second-degree burn (53.9%) [31]. R.T.A burns were the most common type in burn infection patients in the present study 150(68.1%), followed by hot water scald burns 40 (18.1%), hot food 20 (9%) and Steam burns 10 (4.5%). In the present study, found that R.T.A was more common types in burn infection patients, due to high speed driving. These findings are in little pit different with Ghaffar et al. [27] in India who found flame burns were the most common types in burn infection patients, Kerosene was the main accelerant accounted for burns [32]. This is probably because kerosene is cheap and easily accessible and more use of kerosene stove and kerosene lamp by the people of low socioeconomic status in rural area, where obsolete and unsafe uses of fire for cooking and light are still prevalent. Staphylococcus aureus (47.8%) was the most commonly isolated bacteria among burn patients with burn wound infection in Sana’a city, followed by P. aeruginosa (23%), E. coli, Serratia sp., P. mirabilis, S. epidermidis, Bacillus sp., Acinetobacter sp., S. faecalis, Klebsiella sp., Citrobacter freundii, Salmonella sp., and S. pyogenes [34]. This result was similar to that reported by Bagdonas et al. [30], Elsayed et al. [35] who found that the most prevalent bacteria among burn patients was S. aureus [30-37]. In the other hand, AL-Akayleh [31] and Sharma et al. [37] found that the most prevalence isolated bacteria from burn wound patients were P. aeruginosa, Klebsiella sp. S. aureus, P. mirabilis, while the least prevalence isolated bacteria was E. coli [31,38]. Our study showed that C. glabrata, and Aspergillus sp. were the only yeast and mold isolated. In the present study, Staphylococcus epidermidis was the predominant organisms isolated with rate of 50 (22.2%), followed by Staphylococcus aureus 44 (20%), Enterococcus facium 10 (4.5%). This finding was in agreement with Elsayed et al. S. aureus is a versatile human pathogen [36]. It was the predominant cause of burn wound infection in pre antibiotic era and still persists as an important pathogen, strongly considered as a major cause of nosocomial infection. Burn units have become major reservoir for S. aureus that has the special characteristics for spreading quickly in a hospital environment [39]. This pathogen has been reported as a major cause of nosocomail infection in Europe [40]. Edwards- Jones and Greenwood mentioned that burns become infected because of the environment at the site of the wound is ideal for the multiplication of infecting organisms [41]. The immune-suppressive status of the patient and the immediate lack of antibodies allow the microorganisms to multiply freely. There are plentiful supplies of moisture and nutrients in the physical environment; the temperature, gaseous requirements are ideal for growth. Bacteria will proliferate rapidly; the mean cell generation time in optimum conditions is approximately 20 min. Therefore, a single bacterium cell can increase in numbers within a 24 h period to over 10 billion cells.
Conclusion
In burn patients, an effective surveillance for infection control and accordingly to wipe and tissue culture sampling for control purposes at least twice a week5 are recommended. In burn patients, the fact that post-traumatic fever and white blood cell level have a more than higher course causes to overlook the infections that may develop at an early stage, therefore it is indicated that quantitative screening cultures performed with tissue biopsy give more rational results with respect to diagnosis at an early stage.
This led to a delayed identification of the infections, which occurred on the days when culture sampling was not performed, in burn patients whose clinical conditions went through an extremely dynamic process; this may also have caused an increase in the quantitative values of bacteria in the living tissue. The current study showed that contamination of the burn wound is almost the rule rather than an exception in burn wounds. In spite of the fact that all burned patients were routinely cleaned with an antiseptic solution and had 1% silver sulphadiazine cream applied to their wounds, 100% of the patients studied had microorganisms invasion of their burn wounds, at least once, by the end of the 4th week after admission. The susceptibility of burn wound to such opportunistic invasion or colonization by bacteria yeast and fungi might result from several factors including the presence of coagulated proteins, the absence of blood-borne immune factors, and the a vascularity of the burn wound.
8879
References
- Agnihotri N, Gupta V, Joshi RM (2004) Aerobic bacterial isolate from burn wound infections andtheir antibiograms - a five - year study. Burns 30: 241-243.
- Mc Manus AT, Mason AD, McManus WF, Pruitt BA (1994)A decade of reduced Gram-negative infections and mortality improved isolation of burned patients. Arch Surg 129: 1306-1309.
- Donati L, Scammazo F, Gervasoni M, Magliano A, Stankow B, et al. (1993) Infection andantibiotic therapy in 4000 burned patients in Milani Italy between 1976 and 1988. Burns 4: 345-348.
- Gupta M, Gupta OK, YaduVanshi RK, Upadhyaya J (1993) Burn epidemiology: the Pink Cityscene. Burns 19: 47-51.
- Arturson MG (1985)The pathophysiology of severe thermal injury. J Burn Care Rehabil 6:129-134.
- Kumar V, Bhatnagar SK, Singh AK, Kumar S, Mishra RK (2001) Burn Wound Infection: Astudy of 50 cases with special reference to antibiotic resistance. Indian Journal of BioResearch 46: 66-69.
- Riaz I, Babar AH (2015)Burn Wound Infections and Antibiotic Susceptibility Patterns at Pakistan Institute of Medical Sciences, Islamabad, Pakistan. World J PlastSurg 4: 9-15.
- Hunt JL(2000)The 2000 presidential address. Back to the future: the ABA and burnprevention. J Burn Care Rehabil 21:474-483.
- Atiyeh BS, Gunn SW, Hayek SN(2005) State of the art in burn treatment.World J Surg 29:131-148.
- Baker CC, Miller CL, Trunkey DD (1979) Predicting fatal sepsis in burn patients. J Trauma 19: 641-648.
- Bang RL, Sharma PN, Sanyal SC, Al Najjadah I (2002) Septicaemia after burn injury: a comparative study. Burns 28: 746-751.
- Barret JP, Herndon DN (2003) Effects of burn wound excision on bacterial colonization and invasion. PlastReconstrSurg 111: 744-750.
- Wysocki AB (2002) Evaluating and managing open skin wounds: colonization versus infection. AACN Clin Issues 13: 382-397.
- Manson WL, Coenen JM, Klasen HJ, Horwitz EH (1992) Intestinal bacterial translocation in experimentally burned mice with wounds colonized by Pseudomonas aeruginosa. J Trauma 33: 654-658.
- Ramzy PI, Wolf SE, Irtun O, Hart DW, Thompson JC, et al. (2000) Gut epithelial apoptosis after severe burn: effects of gut hypoperfusion. J Am CollSurg 190: 281-287.
- Ekenna O, Sherertz RJ, Bingham H (1993) Natural history of bloodstream infections in a burn patient population: the importance of candidemia. Am J Infect Control 21: 189-195.
- Barillo DJ, Burge TS, Harrington DT, Coffey EC, Shirani KZ, et al. (1998) Body habitus as a predictor of burn risk in children: do fat boys still get burned? Burns 24: 725-727.
- Brown TP, Cancio LC, McManus AT, Mason AD Jr (2004) Survival benefit conferred by topical antimicrobial preparations in burn patients: a historical perspective. J Trauma 56: 863-866.
- Altoparlak U, Erol S, Akcay MN, Celebi F, Kadanali A (2004) The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 30: 660-664.
- Nermin H Ibrahim,Tarek A Amer (2008) Frequency of bacterial and fungal infections of burn wounds at Cairo University Burn Center Egyptian Journal of Medical Microbiology 17: 4573.
- Steer JA, Papini RP, Wilson AP, McGrouther DA, Parkhouse N (1996) Quantitative microbiology in the management of burn patients.II. Relationship between bacterial counts obtained by burn wound biopsy culture and surface alginate swab culture, with clinical outcome following burn surgery and change of dressings. Burns 22: 177-181.
- Frank E Berkowitz, Robert C Jerris (2015) Practical Medical Microbiology for Clinicians. Wiley-Blackwell 465.
- Julia A Kiehlbauch, George E Hannett, Max Salfinger, Wendy Archinal, Catherine Monserrat, et al. (2000) Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J ClinMicrobiol 38: 3341-3348.
- Jorgensen JH, Ferraro MJ (2009) Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices 49: 1749-1755.
- Ghaffar UB, Husain M, Rizvi S (2002) Thermal burn: An epidermiological prospective study. Indian J Academy of Forensic Medicine 30: 10-14.
- deMacedo JL, Santos JB (2005) Bacterial and fungal colonization of burn wounds. MemInstOswaldo Cruz 100: 535-539.
- Vostrugina K, Gudaviciene D, Vitkauskiene A (2006) Bacteremias in patients with severe burn trauma. Medicina (Kaunas) 42: 576-579.
- Bagdonas B, Tamelis A, Rimdeika R, Kiudelis M (2004) Analysis of burn patients and the isolated pathogens. Lithuanian Surgery 2: 190-193.
- Al-Akayleh AT (1999) Invasive burn wound infection. Annals of Burns and Fire Disasters 7: 1-3.
- Bahmani N, Ramazanzadeh R (2013) Detection of SHV type Extended-Spectrum B-lactamase and Risk Factors in Pseudomonas aeruginosa Clinical Isolates. Pak J Med Sci 29: 788-792.
- Gulati S, Qureshi A, Srivastava A, Kataria K, Kumar P, et al. (2014) A Prospective Randomized Study to Compare the Effectiveness of Honey Dressing vs. Povidone Iodine Dressing in Chronic Wound Healing. Indian J Surg 76: 193-198.
- Cook N (1998) Methicillin-resistant Staphylococcus aureus versus the burn patient. Burns 24: 91-98.
- Elsayed S, Gregson DB, Lloyd T, Crichton M, Church DL (2003) Utility of Gram stain for the microbiological analysis of burn wound surfaces. Arch Pathol Lab Med 127: 1485-1488.
- Rhbar M, Gra-Agaji R, Hashemi S (2005) Nosocomial blood stream infections in Imam Khomeini Hospital, Urmia, Islamic Republic of Iran, 1999–2001. East Mediterr Health J 11: 478-484.
- Sharma BR, Harish D, Singh VP, Bangar S (2006) Septicemia as a cause of death in burns: an autopsy study. Burns 32: 545-549.
- Gang RK, Sanyal SC, Bang RL, Mokaddas E, Lari AR (2000) Staphylococcal septicaemia in burns. Burns 26: 359-366.
- Wildemauee C, Godard C, Vershragen G, Claeys G, Duyck C, et al. (2004) Ten years phage typing of Belgian clinical methicillin-resistant S. aureus isolates. J Hospital Infection 56: 16-21.
- Edwards-Jones V, Greenwood JE; Manchester Burns Research Group (2003) What's new in burn microbiology? James Laing Memorial Prize Essay 2000. Burns 29: 15-24.
- Heggers JP, McCoy L, Reisner B, Smith M, Edgar P, et al. (1998) Alternate antimicrobial therapy for vancomycin-resistant enterococci burn wound infections. J Burn Care Rehabil 19: 399-403.







