Keywords
Male infertility; Microscopy; Culture; Semen introduction
Introduction
A semen analysis is vital tool to investigate male infertility [1]. It can also be used to validate a successful vasectomy as to absence of sperm.
Fifty per cent of couples with complaint of infertility have been associated with male factors. Infertility, simply is inability of a sexually matured couple to achieve pregnancy in presence of regular unprotected sexual intimacy of average of three days in a week for a minimum of one year [2].
Infections of the genito-urinary tracts, hormonal imbalance, age factor, stress, environmental pollution, some metabolic disorders among others are contributory to male infertility [3]. Two separate semen specimens at interval of about seven days may be needed to be repeated in three month time are needed [4].
The picture of semen analysis includes as follows; the semen is expected to liquefy within 30 minutes of ejaculation with the volume of about 3(±2.5) ml, sperm count per ml of 20 million and above; morphology which comprises the appearance, size and shape of the sperms, which must be at least 50% normal; motility of the sperm within one hour of ejaculation must be good for at least 50% of the sperms count [5,6].
Infection in semen has been incriminated in 15% cases of male infertility in fertility clinics [7,8]. The infection can be through sexual transmission or urinary tract. The nidus of infection is usually the prostate [9].
Infertility comprises 65% of gynecological consultations in Africa [10]. Male infertility is responsible for an average of 50% infertility globally [10,11]. The most affected areas lie within the central African region referred to as the “infertility belt” of Africa [11].
This study therefore, was aimed at the quantitative and qualitative values of the seminal fluid and the role of microorganisms in male infertility.
Subjects and Method
Study population
After obtaining ethical clearance from the ethics committee of Benue state university teaching hospital, we collected semen samples from three hundred and sixty (360) male subjects. The subjects were attending the Special Treatment Clinics/ Urology/Reproductive clinics of Benue State University Teaching Hospital from January, 2013 to January, 2016. Proper hygiene was ensured in the collection of the samples which was done in one of the private rooms in the laboratory.
Procedures
After liquefaction of the semen; the duration for the liquefaction was charted, the color noted and volume of the semen measured. The semen was mounted on a counting chamber (Improved Neubeuer) where sperm count and sperm motility was read according to WHO standard [1].
Cultures
The following culture media plates were used; chocolate for 10% carbon dioxide incubation and MacConkey and Blood agar for aerobic incubation overnight at 37°C.
The 24 hour growth was examined for size, shape, elevation, hemolysis and other cultural characteristics. Gram staining was done and pure growth was subjected to biochemical testing according to standard [5].
Antibiogram
Antibiotic susceptibility of the isolates was tested by agar diffusion method on dry sensitivity testing agar (DSTA) using oxoid multidisc with standard antibiotics concentrations according to Clinical and Laboratory Standard Institute (CLSI), 2006 guidelines [12].
Analysis of data
The results were analyzed using SPSS 11.0 statistical software; chi-square (x2) was used to compare association between proportions and p-values <0.05 were considered significant at 95.0% confidence level.
Results
A total of 360 seminal fluid samples were collected. The sperm count was as follows: Fifty-one percent of the sperm count was normospermic, 25% mildly oligospermic, 15% moderately oligospermic, 3% severely oligospermic and 6% azoospermic (Figure 1).
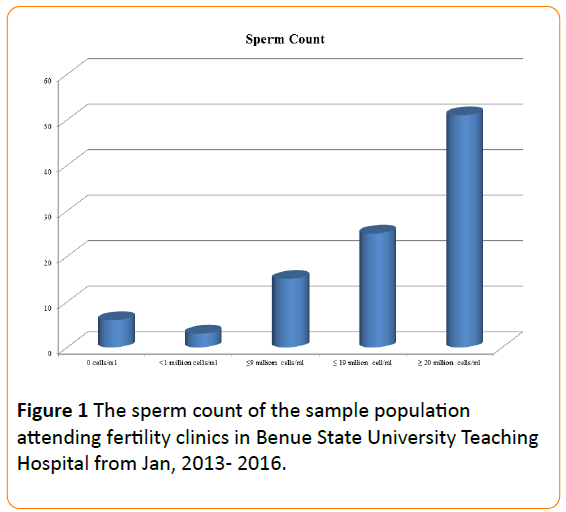
Figure 1: The sperm count of the sample population attending fertility clinics in Benue State University Teaching Hospital from Jan, 2013- 2016.
Forty per cent of the sperm cells were very motile, 20% of moderate progression, 30% sluggish and 10% non-motile (Figure 2). Viability test revealed 9% dead cells. Forty –two per cent (N=150/360) of the seminal fluid samples contained significant number of pus cells (≥2000 cells per ml). Pathogens were isolated in 47% (N=70/150) of this sample. The above criteria were distributed according to the percentage motility groups of sperm cells (Figure 3).
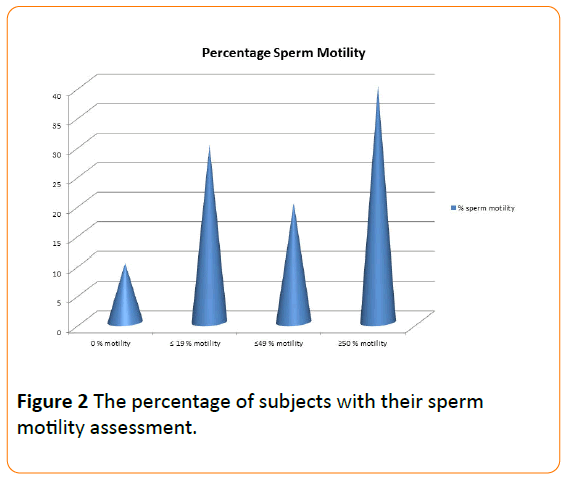
Figure 2: The percentage of subjects with their sperm motility assessment.
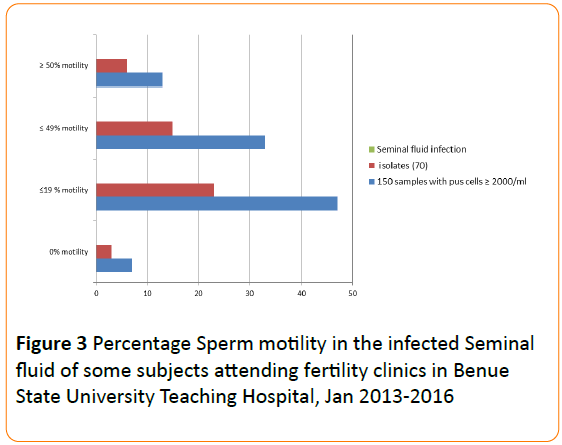
Figure 3: Percentage Sperm motility in the infected Seminal fluid of some subjects attending fertility clinics in Benue State University Teaching Hospital, Jan 2013-2016
Total number of isolates was 70 as follows, S. aureus (53%), S. saprophyticus (10%), E. coli (11.4%), Klebsiella spp (7.1%), S. pneumonia (4.4%), P. aeruginosa (7.1%) and Candida spp (7.1%) (Table 1).
| Isolates |
Number |
Percentage (%) |
| Staphylococcus aureus |
37/70 |
52.9 |
Staphylococcus
saprophyticus |
7/70 |
10 |
| Escherichia coli |
8/70 |
11.4 |
| Klebsiella species |
5/70 |
7.1 |
| Streptococcus pneumoniae |
3/70 |
4.4 |
| Pseudomonas aeruginosa |
5/70 |
7.1 |
| Candidaspecies |
5/70 |
7.1 |
| Total Isolates |
70 |
19 |
| Sterile cultures (of samples with Pus Cells≥2000/ml) |
80 |
22 |
| Total sterile cultures |
290 |
81 |
| Total cultures |
360 |
100 |
Table 1: Microbial Isolates from semen of some subjects attending fertility clinics in Benue State University Teaching Hospital, January, 2013 to January, 2016.
Moxifloxacin (85%), levofloxacin (80%), ceftazidime (80%), ceftriaxone (80%) ciprofoxacin (70%), ofloxacin (68%) and gentamicin (60%) are effectively active across the spectrum of bacteria tested. While penicillin (20%) and tetracycline (10%) are low in activity (Table 2).
| Drugs |
Bacterial |
Isolates |
| |
**S.aureus 37(%) |
S. saprophyticus 7(%) |
E. coli 8(%) |
Klebsiella spp. 5(%) |
P. aeruginosa 5(%) |
S. pneumoniae 3(%) |
| *AMP |
10(27) |
- |
- |
3(60) |
1(20) |
1(33) |
| PEN |
8(22) |
0(0) |
2(25) |
1(20) |
- |
1(33) |
| AMX |
- |
- |
- |
- |
1 (20) |
- |
| SXT |
11(30) |
3(43) |
2(25) |
- |
- |
2(66) |
| TCN |
- |
- |
- |
0(0) |
1(20) |
- |
| GENT |
25(68) |
5(71) |
4(50) |
3(60) |
2(40) |
2(66) |
| ERYTH |
12(32) |
1(14) |
3(38) |
- |
0(0) |
- |
| MOXI |
35(95) |
7(100) |
- |
4(80) |
- |
- |
| CEFTR |
33(89) |
- |
6(75) |
- |
- |
3(100) |
| CEFT |
- |
7(100) |
- |
4(80) |
3(60) |
- |
| CIPRO |
32(87) |
6(86) |
5(63) |
3(60) |
- |
2(66) |
| OFL |
30(81) |
6(86) |
- |
- |
3(60) |
- |
| LEV |
34 (92) |
- |
7(88) |
4(80) |
3(60) |
3(100) |
*AMP=Ampicillin; PEN=Penicillin;AMX=Amoxicillin; MOXI=Moxifloxacin; TCN=Tetracycline; GENT=Gentamicin; ERYTH=Erythromycin; SXT=Trimethoprim-Sulphamethoxazole; CEFTR=Ceftriaxone; CEFT=Ceftazidime; CIPRO=Ciprofloxacin; OFL=Ofloxacin; LEV=Levofloxacin; **S. aureus=Staphylococcus aureus; S.pneumoniae=Streptococcus pneumoniae; E.coli=Escherichia coli; spp=species
Table 2: Antibiotic susceptibility pattern of the isolates from infected semen of the subjects
Discussion
The study shows a 6% azoospermia, 43% oligospermia and 51% normospermic. Some studies in Nigeria showed the following results such as 8.8 % [11] azoospermia in Ogun and 14.9% [13] in Osun both in South West Nigeria; oligospermic cases were reported as 51% [11], 46.5% [13], 45.3% in Ilorin, North-Central Nigeria [14], 60% [11] in Enugu ,Eastern Nigeria are in synchrony with the present study. However, in Tunisia, North Africa was a 13% [15] lower in rate of oligospermia. Azoospermia categorically can be responsible for any couple's infertility, since it is complete sterility, while oligospermia only decreases the chances of fertilization. This condition may result from autoimmune natural sperm agglutinating antibodies in the serum of sub fertile men [10].
Only 40% of the sperm cells were motile within one hour of ejaculation, ten per cent non motile, while remaining 50% were sluggish. Drake et al. [11] documented that bacterial infections could be responsible for decline in sperm motility. In early infection, there may be normal sperm count and sperm motility before the complication sets in. Role of bacterial infection may include change in the chemical milieu of the semen, inflammatory damage of the passage, destruction of the sperm cells and impede their motility. The consequence is therefore, reduced sperm count, teratospermia and asthenospermia. Escherichia coli have been found to decrease sperm motility and cause agglutination [11,16].
The male reproductive tract is essentially sterile except the lower urethra. Hence, greater than 2000 pus cells per ml of semen may be significant for diagnosis of infection [10]. The presence of certain organisms in semen or high number of the organisms is associated with infertility [17]. In the present study, 42% (N=150/360) of the semen samples had significant pus cells (≥2000 cells per ml) and pathogens were isolated in only 47% (N=70/150) of these samples. The fifty- three per cent (N=80/150) rate of sterile culture samples with significant pus cells is a pointer to reckless antibiotic usage in Nigeria [13,18]. This ‘culture-negative semen' challenge can be solved by the introduction of non-culture diagnostics.
The 19% (N=70/360) of the semen contained total number of isolates was as follows, S. aureus (53%), E. coli (11.4%), S. saprophyticus (10%), Klebsiella spp (7.1%), P. aeruginosa (7.1%), Candida spp (7.1%) and S. pneumoniae ( 4.4%) in descending order. The above data are comparable to studies by Orji et al. [16] and Olajubu et al. [11] which identified S. aureus as the commonest isolated bacteria with 37.1% and 51% respectively unlike a study [10] which found E. coli as the most prevalent with 70.4%. The isolation of S. aureus might have resulted from the skin flora of the couples. However, cross- infection might be a factor in some partners “trying outside marriage” [16]. Sex preferences may as well influence the nature and types of isolates in the semen [17,18]. For instance, anal sex may result in the transmission of bacteria which are normal flora of the intestine.
The most active antibiotics tested against the various types of bacterial isolates includes, Moxifloxacin (85%), Levofloxacin (80%), Ceftazidime (80%), Ceftriaxone (80%). However, some older and commonly used antibiotics, Penicillin (20%) and Tetracycline (10%) have been noted to be crying orphans with low activity. Some usually active antibiotics, Ciprofloxacin (70%), Ofloxacin (68%) and Gentamicin (60%) have turned to be moderately effective. The newer generation antibiotics are relatively expensive and are mostly given in hospitals. Therefore, they are not easily reached by the people.
Male infertility has largely been attributed to nonspecific seminal tract infection [17,18]. Alausa and Osoba for infertility [9], discovered that 40% of oligospermia and azoospermia cases presenting to fertility clinics in Lagos Nigeria gave a history of two or more attack of urethritis. Fertility is jeopardized by these infections in several ways by decreasing sperm motility, changing the chemical milieu of the seminal fluid or illicit some inflammatory changes in seminal tract. Bacterial presence may cause testicular damage or the obstruction of the sperm ducts leading to low sperm count. Chronicity of some urinary tract infection is traceable to seminal infection by acting as a reservoir of infection [19].
In exception to infections, other conditions can be incriminated in male infertility. Such conditions like, exposure to heavy metals e.g. lead, zinc and arsenic. Others include; heat, chemical, X-ray exposure, smoking, nutritional deficiencies and use of alcohol, tobacco and caffeine. Also, many recreational and prescription drugs (e.g. nitro furans, cimetidine), some herbal medicines (such as St. John's wort) and specific herbicides and pesticides have been found to be toxic to spermatogenesis. However, the correlations are difficult to develop, as supporting evidence is sketchy [9,18,19].
An automated system example, Computer-Assisted Sperm Analysis (CASA), would have given us a better analysis of sperm motility and other parameters may be a limitation to the study.
Interventions should be focused at prevention since surgical treatment of infertility and Assisted Reproductive Techniques (ARTs) are beyond the reach of many in Africa. Therefore, regular microbiologic screening and investigation for infertility is advised in this sub region.
9941
References
- World Health Organization (2010) WHO Laboratory Manual for the Examination and Processing of Human Semen. (5thEdn), Cambridge University Press, Cambridge, UK.
- Jimoh AA (2002) The Management of Infertility in Africa. Medilorin Journal 1: 3-9.
- Hauser R, Paz G, Botchan A, Yoger L, Yaretz H (2001) Varicocele: Effect on Sperm Functions. Human Reproduction Update 7: 482-485.
- Kenkel S, Rolf C, Nieschlag E (2001) Occupational Risks for Male Fertility: An Analysis of Patients Attending a Tertiary Referral Center. International Journal of Andrology 24: 318-326.
- Jeyendra RS (2001) Semen analysis: method and interpretation. In:Gynaecology and Obstetrics. Sciarra JJ, Lipincot Williams and Wilkins 5: 4-20.
- Budia A, Luis PJ, Broseta E, Tejadios S, Benedicto A, et al. (2006) Value of Semen Culture in the Diagnosis of Chronic Bacterial Prosttatitis : A Simplified Method. Scandinavian Journal of Urology &. Nephrology 40: 326-331.
- Pellati D, Mylonakis I, Bertoloni G, Fiore C, Andrisani A, et al. (2008) Genital Tract Infections and Infertility. European Journal of Obstetrics&GyneacologicReproductiveBiology 140: 3-11.
- Sanocka-Maciejewsky D, Ciupinska M, Kurpisz M (2005) Bacterial Infection and Semen Quality. Journal of Reproductive immunology 67:51-56.
- Ekhaise FO, Richard FR (2008) Common Bacterial Isolates Associated with Semen of Men Complaining of Infertility in University of Benin Teaching Hospital (U.B.T.H), Benin City, Nigeria. World Journal of Medical Sciences 3: 28-33.
- Alo MN, Ugah UI, Elom MO (2013) Semen Culture: A Comparative Analysis between Solid Media and Liquid Media Supplementation . IOSR Journal of Pharmacy and Biological Sciences5: 67-72.
- Festus AO, Deji-Agboola M, Olubunmi AO, Olusoji EJ (2013) Seminal Fluid Characteristics of Men Attending Infertility Clinic of a Teaching Hospital. Open Journal of Medical Microbiology3: 1-4.
- Clinical Laboratory Standard Institute (2007) Performance standard for antimicrobial disk susceptibility tests, Approved standard (9thEdn) Supplement M2 – A9 26(1).
- Kosamat YA, Adebolu TT (2005) Bacteriological study of isolates from seminal fluids of infertile men in Ila and Ifedore local government areas, Osun State, South-Western Nigeria. Journal of Food, Agriculture & Environment 3: 73-74.
- Nwabuisi C, Onile BA (2001) Male Infertility among Sexually Transmitted Disease Clinic Attendees in Ilorin. Nigerian Journal of Medicine 10: 68-71.
- Gdoura R, Keskes-Ammar L, Bouzid F (2001) Chlamydia trachomatis and Male Infertility in Tunisia. European Journal of Contraception and Reproductive Health Care 6: 102-107.
- Orji I, Ezeifeka G, Amadi ES, Okafor F (2007) Role of Enriched media in Bacterial Isolation from Semen and Effect of Microbial Infection on Semen Quality: A Study on 100 Infertile men. Pakistan Journal of Medical Sciences 23: 855-888.
- Cottell E, Harrison RF, McCaffrey M, Walsh T, Mallon E (2000) Microorganisms are commonly found in insignificantquantities in the semen of asymptomatic men. African Journal of Fertility and Sterility 74: 465-470.
- Onemu SO, Ibeh IN (2001) Studies on the Significance of Positive Bacterial Semen Cultures in Male Fertility in Nigeria. International Journal of Fertility and Women’s Medicine46: 210-214.
- Audu BM, Massa AA, Bukar M, Melah GS, Kudi A (2010). Effect of bacterial isolates on the seminal indices of men investigated for infertility in Gombe. Nigerian Journal of Clinical Practice 13: 16-19.








