Key words
|
| |
| Denture cleansers, blood agar, streptococci, plaque |
| |
INTRODUCTION
|
| |
| The formation of plaque on surfaces of dentures is a common problem among denture wearers often leading to halitosis and gingival inflammation5 with its associated complications like denture stomatitis, inflammatory papillary hyperplasia, and chronic candidiasis.1 The formation of denture plaque by the normal oral flora is facilitated by the presence of debris due to poor hygiene, irregularities in the acrylic resin.6. The use of teeth with more natural contours and the trend towards stippled surfaces tend to provide more recessed area for the accumulation of stains and debris; consequently increasing the patient’s cleaning problems.3 The best approach for controlling these deposits and infections is by implementing preventive measures like maintenance of good oral hygiene, mechanical and chemical cleaning procedures for maintaining the denture. |
| |
| The denture cleansing systems should be safe to both tissues and fabricated material, relatively inexpensive, involve minimal physical effort and must be capable of removing plaque not only from polished surface of the prosthesis, but more importantly from unpolished surfaces.28, 35 Various methods have been reported in prosthodontic literature for cleaning dentures. These have been broadly classified as having mechanical and chemical effects. The former group includes abrasive pastes used in association with brushes and ultrasonic cleaners.18 Effective plaque removal requires a degree of manual dexterity that is often lacking among geriatric, physically handicapped, mentally retarded and non-motivated patients. In such situations use of chemical denture cleansers can be more advantageous.8, 10 |
| |
| A series of laboratory and clinical studies have compared and evaluated the efficacy of different denture cleansing methods using qualitative measurements of the denture plaque i.e. by visual and photographic methods. The studies concluded that these qualitative methods used provided a limited measurement of the effectiveness of denture cleansing methods.18 There were series of studies conducted on in vivo methods to evaluate the efficacy of denture cleansers using microbiologic quantification, i.e. viable counts on selective culture media. The study stated that quantification of microorganisms is one of the most accurate and reliable method to evaluate the effectiveness of denture cleansing agents. The study concluded that use of chemical cleansing agents along with mechanical cleansing, aids in proper maintenance of denture hygiene.10, 14 In addition, series of studies have been conducted on in vitro bacteria – yeast colonization models to assess the efficacy of denture cleansing agents by viable counts of microorganisms. These studies concluded that mechanical cleansing alone does not remove the harbored microorganisms, hence chemical cleansing was found to be adequate for removing adhered microorganisms and provide denture plaque control.12, 17, 25 |
| |
| The previous microbiological studies have focused on patients wearing complete dentures for more than 1 or 2 years and / or patients suffering from denture stomatitis. These studies have reported that the main plaque forming microorganisms were Streptococcus species bacterial colonization followed by secondary Candida species colonization (yeast).9, 13 However studies evaluating the effect of chemical denture cleansers on recently fabricated complete dentures for healthy patients were inadequate. The primary purpose of this study was conducted to compare and evaluate the efficacy of four chemically different immersion types of commercially available denture cleansers on recently fabricated complete dentures in healthy patients, using microbiological quantification method. |
| |
MATERIALS AND METHOD
|
| |
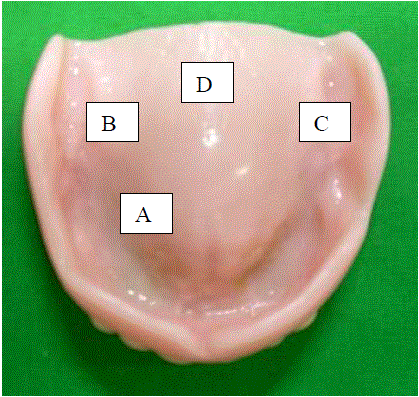
| The materials used in the study were: maxillary complete dentures (Fig 1), Chemical denture cleansing agents: Sodium hypochlorite solution 0.02% [ Diluted from 3% solution](Comet - Novo dent Equipments and Materials Ltd, Mumbai, India), Denture cleansing tablets. (Trisodium phosphate) (Efferdent - PFIZER CONSUMER HEALTH CARE, USA), Denture cleansing powder. (Sodium perborate) (Clinsodent - I.C.P.A. Health Products Ltd, Ankleshwar, India), Chlorhexidine gluconate – 0.2% solution.(Hexidine mouth wash - Group Pharmaceuticals Ltd, Mumbai, India), sterilized cotton swabs (Fig 2), sterile cotton swab holders (Fig 2), nutrient agar powder. (Fig 3) (High Media Instruments Pvt. Ltd, India), Petri Dish. (Fig 4) and bood agar culture media. (Fig 4). |
| |
| The equipment employed for testing included the following: |
| |
| (1) Inoculating loop (High Media Instruments Pvt. Ltd, India),(Fig 5). It was used to streak the cultures obtained into the selected culture media. |
| |
| (2) Optical Microscope (Labomed Vision 2000, N.K. Jain Instruments Pvt. Ltd, India). |
| |
| It was used to identify the growth of microorganisms in culture media. |
| |
| The Methodology for the present study included the following phases: |
| |
|
I) Selection of subjects for the study:
|
| |
| Ten healthy subjects, aged 60 – 70 years, with no signs or symptoms of inflammation or infection of the oral tissues, who had not suffered from any systemic or debilitating disease, who had received maxillary and mandibular complete dentures 3 weeks prior to testing, with dentures exhibiting adequate retention, stability, centric occlusion and surface finish, were selected among the complete denture subjects treated in the department of prosthodontics, Ragas dental college and hospital, Chennai. |
| |
|
II) Collection of test samples:
|
| |
| Chemical denture cleansers used were divided into four groups; Group I – Sodium hypochlorite solution 0.02% , Group II – Trisodium phosphate , |
| |
| Group III – Sodium perborate and Group IV – Chlorhexidine gluconate 0.2%. |
| |
| For the first three weeks, subjects were instructed to use their complete dentures and instructed in cleansing procedures i.e. rinsing with water after every meal, brushing with soft brush using soap and cold water and soaking the dentures in cold water overnight. The subjects were also instructed not to clean their dentures in any manner for 24 hours prior to reporting to the hospital at the end of 3 weeks, thereby providing a substantial, generally reproducible buildup of plaque. During the 3 week recall, the maxillary denture of each subject was removed from the mouth and posterior half of the tissue – bearing surface of the denture was swabbed using sterile cotton swabs at four different sites i.e., A (Anterior), B (Right), C (Left), D (Posterior) (Fig 1) approximately an area of each 1cm x 1cm dimension and to be swabbed for 30 sec. A rotating motion with the swab held firmly and laterally against the denture was used to collect the plaque. Each portion of the tissue – bearing surface at the four different sites was swabbed three times with the same swab to ensure proper adherence of the plaque. These swabs were immediately transferred to commercially available sterile swab holder and placed in refrigerator for no more than 2 hours before plating. |
| |
|
III) Microbiological evaluation:
|
| |
|
a) Preparation of blood agar plates:
|
| |
| 2.8 Gms of commercially available nutrient agar powder was dissolved in 100 ml of distilled water and stirred thoroughly. The mixed solution was sterilized at 15lbs pressure for 20 min in an autoclave. The sterilized solution of nutrient agar obtained was cooled to 500C. Once the nutrient agar was cooled to 500C, 10 ml of sterile defibrinated sheep blood was added and stirred thoroughly.The prepared blood agar was poured into Petri dish (circular plates) and stored in refrigerator. |
| |
|
b) Inoculating the blood agar plates:
|
| |
| Each swab samples of all the 10 dentures, collected from four different sites A, B, C, and D of the tissuebearing surface was cultured on blood agar [selective media for oral streptococcus bacteria and Candida Species.] by streaking the plaque harvested swab on the blood agar media using inoculating loop and incubated aerobically for 24 hours. |
| |
| Each culture plate was divided into two equal halves for streaking the plaque harvested swabs taken from two different sites. In this manner for each denture, 2 plates (i.e. 4 halves) were streaked with swabs from sites A, B, C and D. At the end of 24 hours period, the colonies formed were identified and confirmed as oral streptococcus species bacteria using an optical microscope. The number of colony forming units [CFUs] were then counted visually and transferred to the log base10 units for statistical analysis. |
| |
|
c) Evaluating the effect of chemical denture cleansers on formed microbial colonies:
|
| |
| After initial counting of the microbial colonies, the blood agar culture plates were subjected to four different chemical cleansing agents. Each group of cleanser was allotted a particular culture site to be tested upon as follows. Group I test agent on culture from site A, Group II test agent on culture from site B, Group III test agent on culture from site C and Group IV test agent on culture from site D. 1 – 1.5 ml of the prepared solutions were added to culture from the respective sites and the amount of reduction in colony forming units [CFUs] were counted visually and transferred to the log base10 units. This procedure was carried out for all the 10 culture samples. Each of the above test agents on cultures from above four test sites were treated for 30 min and overnight at room temperature on the streptococcus species colonies formed in the culture plates. In this way the net percentage reduction in the colony forming units [CFUs] before and after treatment with each of the above test agents on cultures from above four sites was calculated, tabulated and subjected to statistical analysis. |
| |
|
COLONY COUNTING
|
| |
| Quantification of the colonies was done by the number of colony forming units per ml (CFUs) which was calculated by the following method: 0.001μl loop was used to inoculate the blood agar plate. The number colonies formed was multiplied by 1000 (103) which was represented as CFU/ml.6 The results obtained were put to statistical analysis to compare and evaluate the efficacy of each denture cleansers used in the study. The percentage reduction in streptococcus species colonies after treatment was analyzed by Wilcoxon’s matched pairs rank test. Reductions between the groups were compared by one way ANOVA followed by Duncan’s multiple range test. During the study double blinding technique was used. The investigator knew which method was being used on each test samples. Samples were then placed in coded sterile tubes. The microbiologist and statistician were unaware of the test agents and method used in the study. |
| |
RESULTS
|
| |
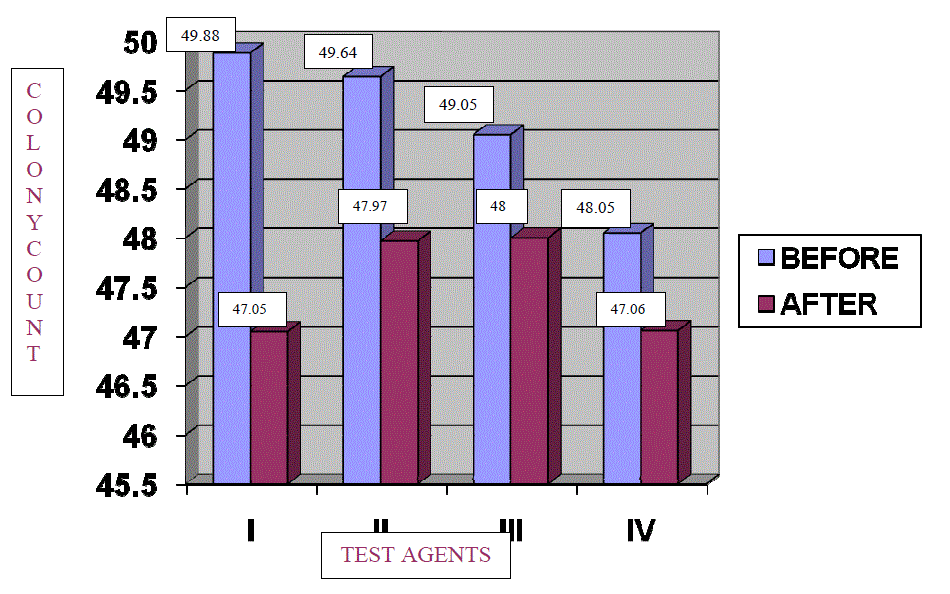
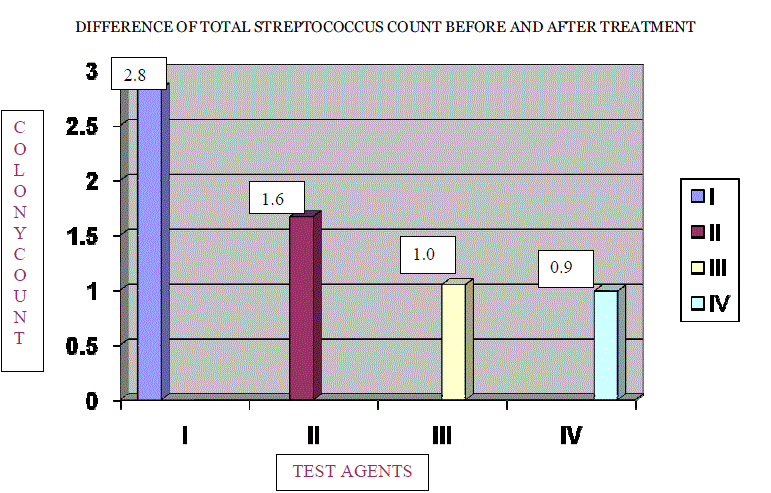
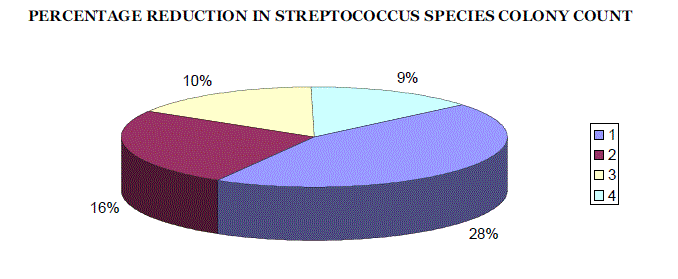
| The percentage reduction in number of colonies in log units for each group was compared by taking pre and post treatment scores. [TABLE NO. 1, 2, 3, and 4] For all the groups the difference of means were statistically significant [TABLE 5], (GRAPH 1 and 2). The percentage reduction in streptococcus species count in log units for Groups I, II, III, and IV was found to be 28%, 16%, 10%, and 9% respectively. The percentage reduction in number of colonies was maximum for Sodium Hypochlorite solution 0.02% followed by Trisodium phosphate, Sodium perborate and Chlorhexidine Gluconate 0.2%. [TABLE 6], (GRAPH 3) |
| |
| The difference data of streptococcus species count in log units in each of the groups I, II, III and IV was subjected to statistical analysis by using ANOVA approach. The computed values are presented in Table – 8. As can be seen from the table of ANOVA, the variance ratio (F = 22.0528) was highly significant at 0.1% probability level. This indicated that the “between samples” variability was substantially more than the “Within samples” variability. This might be taken to imply that the four chemicals were capable of producing considerable differential type of response. The sample data would thus lead us to reject the null hypothesis that the treatments are identical in response. [TABLE 8] Each cleanser i.e. Group I tested on culture A ; Group II tested on culture B; Group III tested on culture C ; Group IV tested on culture D showed no growth of streptococcus colonies in all the cultures with overnight treatment of particular cleansers. [TABLE 7], (FIG NO. 17) There was no yeast colonization in the culture, swabbed from different test sites of the posterior half of tissue – bearing surface of the denture of all the subjects participated in the study. |
| |
DISCUSSION
|
| |
| Edentulism interferes with mastication, speech and esthetics. Providing dentures can overcome the problem of edentulousness and improve the quality of life, but may be potentially harmful when it is not properly cared for. The phenomenon of initial adherence representing the first step in the colonization process is plaque formation that leads from the formation of a thin biofilm, followed by a multilayer, which culminates into denture plaque.24 The process by which dentures accumulate plaque and calculus is similar to the process which takes place on natural teeth. Microbial plaque which forms on the tissue fitting surface of the denture is probably of greatest clinical significance. A variety of soft tissue changes are associated with it. These changes manifest themselves as a series of related symptom complexes which include denture stomatitis, inflammatory hyperplasia and chronic candidiasis. Lack of denture cleanliness is the most common cited etiologic factor for these entities.1, 19, 20, 24, 35 The dentures containing debris cause irritation and subsequent tissue response. Food particles located between denture and palate allow multiplication of bacteria, mainly streptococcus species colonies and yeast, which can cause denture stomatitis and multiple papillomatosis of the palate.13 |
| |
| There is abundant documented evidence showing the relationship between good oral health and denture cleanliness. A significant relationship between poor denture cleanliness and denture stomatitis was first described by Jorgenson EB and Betram in 1970.19 According to Jorgenson EB19 this infection can be best prevented by meticulous oral and denture hygiene. There are various methods of cleaning dentures and there are different reports with conflicting results. Among the cleansing agents used in the present study, Sodium hypochlorite solution 0.02% (Comet), Trisodium phosphate (Efferdent denture cleansing tablets), Sodium perborate (Clinsodent denture cleansing powder) are known to be bacteriocidal9, 13, 19 and Chlorhexidine gluconate 0.2% (Hexidine mouth wash) is known to be bacteriostatic at their respective concentrations.37 These commercially available chemical denture cleansing agents used differ in composition and mechanism of action. |
| |
| Each of the above test agents were tested on four different culture sites obtained from swabbing the posterior half of tissue – bearing surface of the maxillary denture of each subject at four different sites for the period of 30 min (according to manufacturers recommendation) in accordance with the study conducted by Dillis SS. et al9, Drake D. et al11, Moore TC. et al21 and Nikawa H. et al24 These test agents were also tested for an overnight period in accordance with the study conducted by Nikawa H. et al25, Tarbet WJ. et al34, who stated that the efficacy of denture cleansers should be examined under conditions equivalent to a normal overnight cleansing regimen (6 – 8 hours) in addition to recommended by manufacturer. The culture media used in the present study was blood agar culture media which is a selective culture media for the growth of streptococcus species bacteria and yeast.1 The present study identified and confirmed the presence of streptococcus species bacterial colonization in microbial plaque adhered to the posterior half of tissue – bearing surface of the maxillary dentures from ten healthy subjects without the presence of Candida species colonization, which is in accordance with the study conducted by Catalan A. et al6 who stated that denture plaque from healthy patients showed microflora constituted by bacterial cocci organisms without the presence of yeast colonies. |
| |
| Sodium hypochlorite (0.02%) was used in the present study which is in accordance with the study conducted by Moore TC. et al21 who compared the efficiency of several commercially available chemical denture cleansers and found Clorox (Sodium hypochlorite 5.25%) to be more effective than Efferdent denture cleansing tablets. The mean percentage reduction in number of streptococcus colonies in the present study was 2.83 log10 units when Sodium hypochlorite 0.02% was used, which is high compared to a study done by Dillis SS. et al9 who used Efferdent as a denture cleansing agent showing the mean percentage reduction of 2.50 log10 units. Bactericidal action of Efferdent denture cleansing tablets was found to better than Clinsodent denture cleansing powder which may be due to greater effervescence action of Efferdent denture cleansing tablets when compared to Clinsodent denture cleansing powder. Gornitsky M. et al13 compared the efficacy of three different chemical cleansers namely Denture Brite, Polident and Efferdent. The study concluded that the reduction in the number of streptococcus colonies with the Efferdent denture cleansing agent was significantly higher. Dillis SS. et al9 compared the antimicrobial capability of an abrasive paste and Efferdent denture cleanser and concluded that soaking the denture in Efferdent denture cleanser produced greater reduction of microorganisms than did brushing with the paste. Jorgensen EB, Dr. Odont19 found that immersion of dentures daily in a dilute solution of Chlorhexidine gluconate 0.2% caused significant reduction in the amount of denture plaque. The study also reported that long term use of Chlorhexidine gluconate causes heavy discoloration of the acrylic dentures. |
| |
| It is evident from the findings of the present study that, though all the cleansing agents used are effective in reducing the streptococcus bacterial colonies, on statistical comparison with each other they were found to be effective in the following order, Sodium hypochlorite solution (0.02%) (Comet), Trisodium phosphate (Efferdent denture cleansing tablets), Sodium perborate (Clinsodent denture cleansing powder) and Chlorhexidine gluconate (0.2%). |
| |
| Sharp EW. et al32 stated that treatment of dentures with denture cleansers significantly decreased the amount of subsequently formed plaque. Possibly, the potential inhibitory effect of the active agent that persists on the acrylic resin has a better chance of removing the microorganisms which are likely to adhere initially. The significant reduction in the number of microorganisms observed in this study suggests that the use of chemical cleansers is suitable method for cleaning dentures in geriatric patients. This conclusion is supported by the finding of Dillis SS. et al9 that brushing alone with a denture abrasive was less effective than chemical cleanser use for maintaining good denture hygiene.9, 11, 13 , 21, 24, 25 , 26 |
| |
| The present study had certain limitations, microorganisms which were identified and confirmed as Streptococcus species bacterial colonies were not speciated and presence of other bacterial species were not identified from the posterior half of the tissue-bearing surface of the denture in healthy subjects. The study tested the swabbed cultures of recently fabricated maxillary complete denture of healthy subjects who had worn for a period of 1 – 21 days without follow-up period of the subjects after 1 - 2 months. Further studies are needed to determine the efficacy of these four commercially available chemical denture cleansers with newer enzyme cleansers and to speciate the bacterial colonies formed from the microbial plaque adhered to the tissue – surface of the denture in healthy subjects. |
| |
CONCLUSION
|
| |
| The following conclusions were made from the present study: |
| |
| 1. The microbial colonies formed from the cultures obtained from four different sites of the posterior half of the tissue-bearing surface of the maxillary complete denture for all the subjects included in the study was identified and confirmed as streptococcus species bacterial colonies. |
| |
| 2. The mean difference was found to 2.83 for Group – I, 1.67 for Group – II, 1.05 for Group – III and 0.99 for Group – IV showing highly significant results at 0.1% probability level. |
| |
| 3. Among the four test agents used in the present study, Sodium hypochlorite solution 0.02% (Comet) had 28% reduction in colony forming units [CFUs], Trisodium phosphate (Efferdent denture cleansing tablets) had 16% reduction, Sodium perborate (Clinsodent denture cleansing powder) had 10% reduction and Chlorhexidine gluconate 0.2% (Hexidine mouth wash) had 9% reduction when tested for a period of 30min. |
| |
| 4. The percentage reduction in the number of streptococcus species colonies formed was found to be highest with the use of Sodium hypochlorite solution 0.02% (Comet), followed by Trisodium phosphate (Efferdent denture cleansing tablet), Sodium perborate (Clinsodent denture cleansing powder) and Chlorhexidine gluconate 0.2% (Hexidine mouth wash) when used for a period of 30 min. |
| |
| 5. The difference in the percentage reduction of colony forming units [CFUs] between the four test agents for a period of 30 min was statistically significant (p < 0.01). |
| |
| In the present study there was no growth of streptococcus species colonies from the cultures obtained when exposed to the above four test agents for an overnight period. |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
 |
 |
 |
 |
| Table 5 |
Table 6 |
Table 7 |
Table 8 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
 |
 |
 |
| Graph 1 |
Graph 2 |
Graph 2 |
|
| |

















