Key words
|
| |
| Dienogest, Dissolution, Micronization, Air Jet Mill. |
| |
INTRODUCTION
|
| |
| In pharmaceutical products, the particle size of drugs and components may affect the processing and bioavailability[1-4]. A number of compounds that are investigated in pharmaceutical field have low aqueous solubility and fall in class II and IV of the biopharmaceutical classification system. The dissolution rate is one of the limiting factors for attaining good bioavailability. Particle size reduction, leading to increased surface area, is a very promising approach to enhance dissolution rate and, thus, the bioavailability of poorly water- soluble compounds[5- 7]. According to the Noyes- Whitney equation, the rate of dissolution (dC/dt) depends on the effective surface area (A) of the drug particles[8]. The present work attempted to address the problem of slower dissolution of Dienogest from the tablet dosage form by employing micronization techniques as a means of decreasing particle size. Two different formulations of Dienogest, both micronized and nonmicronized were prepared and the dissolution profile was compared with Reference product (Visanne, Mfg By: Bayer Healthcare, Germany). |
| |
MATERIALS AND METHODS
|
| |
| Dienogest (Indo Phyto Chemicals Pvt. Ltd, India), Lactose monohydrate (Friesland Foods, Holland), Microcrystalline Cellulose (FMC Biopolymer, Ireland), Maize starch (Roquett, France), Crospovidone (ISP Technologies), Povidone (ISP Technologies), Talc (Luzenac Italy), Magnesium stearate (Ferro Corporation, USA). All the chemicals were of commercial purity grade. |
| |
MICRONIZATION
|
| |
| Milling of Dienogest was done by using Air Jet Mill (Promas Engineering, Mumbai). Milling performed at primary pressure 4.8 kg/cm2, secondary pressure 4.2 kg/cm2 and Screw feeder speed 5 rpm. |
| |
PARTICLE SIZE ANALYSIS
|
| |
| Particle size analysis of micronised and unmicronised Dienogest was done using Malvern Mastersizer 2000 (Scirocco 2000) that is based on laser diffraction technique - a non-destructive, non-intrusive method, which can be used for size determination of either dry or wet samples. Dienogest of two different particle size distributions was used in two batches F1 (with nonmicronized Dienogest) and F2 (with micronized Dienogest) prepared using Lactose monohydrate, Microcrystalline cellulose, Maize starch as diluent, Povidone as binder, Crospovidone as disintegrant, Talc as glidant and Magnesium stearate as lubricant in the pre-optimized quantities to make a 150 mg tablet containing 2.0 mg Dienogest (Table 2). Dienogest, Lactose Monohydrate and Microcrystalline cellulose were passed through sieve #40. Dry mixing was done in rapid mixer granulator (Ganson, Mumbai) for 10 minutes keeping impeller at slow speed. Povidone binder solution was added over a time of 2 minutes at impeller slow speed. Kneading (Wet mixing) was done at impeller fast and chopper slow speed for 2 minutes. Drying of granules was done using a table top fluidized bed dryer (Retsch GmbH, Germany) at 500C inlet temperature for 45 minutes. Dried granules were milled in Multimill (Ganson, Mumbai) at Slow Speed Knife Forward using 1.5 mm screen and blending was done in Octagonal blender (Ganson, Mumbai) for 10 minutes with addition of Talc, then after add magnesium stearate into Octagonal blender (Ganson, Mumbai) again blend for 5 minutes. The granules of formulations (F1 and F2) were tested for flow properties such as bulk density, tap density, Hausner ratio, compressibility index and angle of repose (Table 3). Compression of blend was done on 12- station single rotary compression machine (CIP, Ahmedabad) using 7.00 mm Round flat beveled edged punches. Compressed tablets of formulations F1 and F2 were subjected to evaluation viz. average weight, thickness, hardness, friability and disintegration time (Table 4). |
| |
DRUG CONTENT
|
| |
| The content of Dienogest in the formulated tablets was determined on HPLC. Content of Dienogest was calculated on the basis of declared content of Dienogest standard using the area of principal peak by single-point standardization technique. |
| |
DISSOLUTION STUDY
|
| |
| In vitro dissolution studies was carried out using USP Type II Paddle (model TDT-08L, Electrolab, India) at 37º ± 0.5ºC and 75 rpm using 1000ml purified water with 0.3% SLS. Samples were withdrawn after 10, 15, 20, 30, 45 and 60 minutes and subjected to HPLC analysis by injecting 50 Nl of blank, standard solution (five injections), and sample solution. The percent drug dissolved was calculated from the peak area. |
| |
RESULTS AND DISCUSSION
|
| |
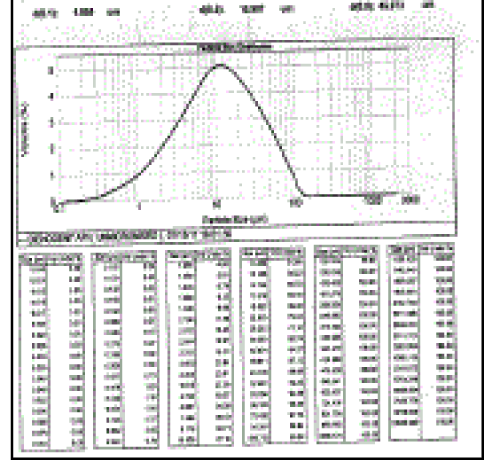
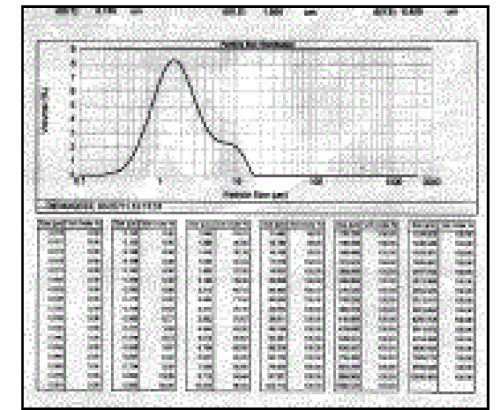
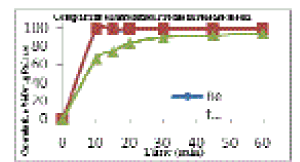
| Particle size analysis (Table 1) of nonmicronized and micronized Dienogest depicted that percentage of fines was found to be highest with Air Jet Mill micronized Dienogest. The appropriate diluents and binder was included in the formulation of wet granulation as shown in Table 2. The granules made using output of Nonmicronized and micronized, i.e., F1 and F2 exhibited flow properties in a narrow range such as bulk density, tap density, Hausner ratio, compressibility index and Angle of repose (Table 3). Two formulations F1 and F2 exhibited nearly alike physical properties such as average weight, thickness, hardness, friability and disintegration time (Table No. 4). However, there was significant difference in percent dissolution observed at 10, 15, 20, 30, 45 and 60 minutes (Table 5). Formulation F1 and F2 Dissolution compared with Reference product (Visnne). The dissolution profile study of formulations F1, F2 and Reference product of Dienogest (Figure 3) exhibit that percentage Dienogest released from Reference product was dissimilar to F1 containing Nonmicronized Dienogest, whereas formulation F2 containing micronized Dienogest provide Similar dissolution Profile. |
| |
CONCLUSION
|
| |
| The finding that Unmicronized Dienogest showed lower dissolution in first 30 minutes provided an impetus to search for an appropriate formulation that can give better dissolution profile. Mechanical methods of micronization are simple, efficient and cost effective way of achieving particle size reduction. The formulation developed using micronized Dienogest produced a higher rate of dissolution than the formulation made with Unmicronized Dienogest. Achievement of increase in dissolution rate to such an extent by micronization suggests that such a formulation may offer an advantage in terms of bioavailability and optimum therapeutic effect. |
| |
ACKNOWLEDGMENT
|
| |
| The authors are thankful to M/s Indo Phyto Chemicals Pvt Ltd, Nainital for providing generous gift sample of Dienogest. |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |









