Antonella Cotoia1*, Gilda Cinnella1, Giorgina Specchia2, Salvatore Grasso3, Nadia Rossana Fede1, Paolo Luigi Pugliese1, Francesca Massenzio4, Francesco Sollitto5, Domenico Loizzi5, Arcangelo Liso4, Giuseppe Capotorto5, Lucia Mirabella1, Michele Loizzi6, Michele Dambrosio1 and Paolo Pelosi7
Department of Anaesthesia and Intensive Care, University of Foggia, Foggia, Italy
Department of Haematology, University of Bari, Bari, Italy
Department of Anaesthesia and Intensive Care, University of Bari, Bari, Italy
Department of Haematology, University of Foggia, Foggia, Italy
Department of Thoracic Surgery, University of Foggia, Foggia, Italy
Department of Thoracic Surgery, University of Bari, Bari, Italy
Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy
Corresponding Author:
Antonella Cotoia
Department of Anaesthesia and Intensive Care
University of Foggia, Foggia, Italy
Tel: 390881732387
E-mail: antonella.cotoia@unifg.it
Received date: December 08, 2015; Accepted date: December 23, 2015; Published date: December 30, 2015
Citation: Cotoia A. Mobilization of Haematopoietic Stem Cells CD34+ in Patients undergoing Elective Lung Resection: Observational Pilot Study. Arch Med. 2015, 8:1.
Keywords
Haematopoietic stem cells; Blood cells; Bone marrow cells
Introduction
Haematopoietic stem cells (HSC) are the precursors of all peripheral blood cells, and can differentiate into all hematological cellular lineages, being self-sufficient thanks to a pool of resting stem cells in the bone marrow that proliferate in response to neuro-humoral signals. The HSC surface membrane exhibits antigens that allow them to be identified as CD34+ [1,2]. In humans, the CD34+ cell surface marker has been shown to characterize cells with a haematopoietic activity which is present on approximately 0.5-3% of bone marrow and <1% of peripheral blood mononuclear cells [2].
CD34+ cells represent a heterogeneous fraction of enriched endothelial/hematopoietic progenitors in peripheral blood that differentiate into endothelial cells and mesenchymal cells [3-5]. The potential of human HSC to migrate into solid organs in response to stress signals in order to repair non-haematopoietic tissues such as myocardium and liver has been demonstrated in experimental and clinical studies [4,6,7]. Moreover, musculoskeletal trauma may cause a systemic increase of circulating CD34+ haematopoietic progenitor cells through bone marrow cells expansion and subsequent release into circulation [8].
As regards lung injury, a study in mice demonstrated that HSC contribute to cell lineages from the lung alveolar epithelium and that lung injury alone is sufficient to promote circulating stem cell migration [9]. However, few data are available on the time course of HSC migration following lung surgical trauma.
Purpose of this study was thus to test if lung surgical trauma in patients undergoing elective lung resection evokes an increase of HSC, and the time course. The secondary aim was to evaluate factors predictive of HSC variations over time in such patients.
Materials and Methods
Patients and protocol
After obtaining approval from the O.O.R.R. hospital ethics committee of Foggia (n.66, 03/02/2012) and written informed consent from each patient, the study was performed in patients scheduled consecutively to undergo elective lung parenchymal resection. Inclusion criteria were age>18 years, ASA physical status II and III. Patients with clinical signs of systemic inflammation (white blood cells (WBC)>10,000 cells/μl), and/or preexisting lung or cardiac disease, or pathologic lung function were excluded from the study.
Pre-operative spirometry was carried out in all patients undergoing elective lung parenchymal resection for evaluation of forced expiratory volume in 1 second-forced vital capacity ratio (FEV1/FVC).
Upon their arrival in the operating room, patients were premedicated with midazolam 0.03-0.04 mg/kg, standard monitoring devices [electrocardiogram and pulse oximeter (Intellivue MP40 monitor, Philips, Boeblingen, Germany)] were applied and the radial artery was cannulated (Radial Artery Catheterization Set, Arrow International, Reading, PA) for continuous monitoring of blood pressure. Normal saline was administered to patients intravenously at 8ml/kg before the anaesthesia induction and at 5 ml/kg/h during the maintenance of anaesthesia. Propofol 2 mg/ kg, fentanyl 3 mcg/kg and succinylcholine 1 mg/kg were used for anaesthesia induction. Afterward the trachea and the left bronchus were intubated with a left double lumen endotracheal tube of appropriate size (Rushelit Rush AG Lab, Waibling, Germany). Correct double lumen endotracheal tube position was checked by fiber optic bronchoscopy and confirmed after positioning patients in lateral decubitus. At one lung ventilation (OLV) time, the lumen of the non ventilated lung was left open to the atmosphere.
Anaesthesia was maintained with an infusion of propofol 5 mg/kg/h, remifentanil 0.1-0.2 mcg/kg/min and cisatracurium 1.5 mcg/kg/min and bi-spectral index (BIS) (Aspect A-2000 ®; Aspect Medical System, Newton, MA) was used for monitoring anaesthesia depth and for modulating the infusion rate of propofol to maintain a BIS value between 40 and 60. The patients were ventilated through a Servo Ventilator 900C (Siemens- Elema AB, Berlin, Germany) with a square flow waveform with a tidal volume (Vt) of 8 ml/kg, respiratory rate of 12 breath/min, inspiratory time of 33% and an inspiratory pause of 20%. The inspiratory oxygen fraction was set at 50% and increased as needed to maintain the SaO2 ≥95%. During OLV, Vt was reduced to 6 ml/Kg, while the other parameters were unvaried.
Blood heparinized venous samples for WBC count, circulating haematopoietic cells and blood count were recorded before surgery (T0) and at four time points postoperatively: 24 (T24h) and 72 (T72h) hours and 5 (T5d) and 7 (T7d) days after surgery.
Postoperative complications such as fever > 38°C, systemic infection (defined as WBC rate>10,000 cells/μl) were recorded.
CD34+ isolation
CD34+ cells were detected by a modified ISHAGE (International Society of Hematotherapy and Graft Engineering) method [10]. In the present study, we modified the basic ISHAGE method based on a four-parameter flow cytometry (CD45 FITC/CD34 PE staining, side and forward angle light scatter) that allow to generate an absolute CD34+ count by incorporating the leukocyte count from an automated analyser (two-platform method), with the addition of a known number of Flow-Count fluorospheres. To reduce errors inherent to sample washing/centrifugation, we implemented ammonium chloride lysis, no-wash no-fix sample processing. These modifications convert the basic protocol into a single-platform method to determine the absolute CD34+ count directly from a flow cytometer, and form the basis of the Stem-Kit (Coulter/Immunotech.). Quantification of CD34+ cells was performed by flow Cytometry (COULTER EPICS XL-MCL, Instrumentation Laboratory). Briefly, peripheral blood samples were collected aseptically into sterile evacuated blood collection tubes with anticoagulant EDTA, and stored at room temperature (18-25°C) until staining within 24 hours. Prior to sample preparation, blood cells (WBC) were counted. If the WBC rate was >30 × 109 WBC cells/L, the sample was discarded. Specific cell surface staining was accomplished by incubating duplicate samples of a biological specimen with the two colour CD45-FITC/ CD34-PE reagent. An additional test of the same sample is stained with the CD45-FITC/ IsoClonic™ Control-PE reagent to check the non-specific binding of the CD34-PE monoclonal antibody. 7-AAD Viability Dye, a nucleic acid dye that binds to accessible base pairs, is used to distinguish between viable and nonviable cells. Each specimen sample was divided into three parts: 45/34/7- AAD *1, 45/34/7AAD *2, 45/CTRL/7AAD.
In the first and second samples, 20 μL of CD45-FITC/CD34-PE Reagent were added; and 20 μL of CD45-FITC/IsoClonic Control- PE Reagent was pipetted into the 45/CTRL/7AAD tube. In all tubes 20 μL of 7-AAD Viability Dye were pipetted. Using a positive displacement pipette, 100 μL of the patient sample were pipetted into the tubes that were immediately vortexed for 5 seconds and incubated at room temperature (18-25°C) for 20 minutes, protected from light. After incubation, 2 ml of prepared 1 × NH4Cl Lysing Solution were added to each tube, which was vortexed immediately for 5 seconds. The tubes were then incubated at room temperature for 10 minutes, protected from light. Prior to acquisition by Cytometry COULTER EPICS XL-MCL, 100 μL of Stem-Count Fluorospheres were added into each of the three tubes, and the tubes were immediately vortexed for 5 seconds. Each fluorosphere contained a fluorochrome with a length of fluorescence ranging between 525 to 700 nm when excited at 488 nm [7].
Statistical analysis
The sample size calculation was performed based on a study published by Laing et al. [11], which described the number of CD34+ cells in patients with isolated, low-energy, closed tibial fractures. A total of 16 patients (8 patients per group) were needed to obtain a desired statistical power of 0.9 (anticipated effect size (Cohen's d):2.9; probability level for a two-tailed hypothesis (p):0.001). Data are reported as mean ± standard deviation (SD) or 95% confidence limits (CL) as appropriate. Cluster analysis was used to categorize patients:firstly a hierarchical agglomerative clustering [12] was performed by complete-linkage clustering to evaluate how many “natural” clusters that could be labelled in a meaningful manner were formed by our patients; afterwards the k-mean clustering method [13] was applied on the basis of this number of clusters so as to assign observations to each cluster. Cluster analysis was conducted using the CD34+ count recorded at T0, T24h, T72h, T5d and T7d. Subsequently, intra- and intergroup comparison between the two clusters in terms of CD34+, monocytes and WBC count over time was performed by means of repeated measures two-ways analysis of variance (ANOVA). Finally, one-way ANOVA or Chi-square test as appropriate were used to evaluate whether clusters were significantly different in terms of baseline characteristics (age, sex, FEV1/FVC, ASA score, duration of surgery, postoperative presence of fever), in order to identify factors predictive of haematopoietic stem cells variations over time in such patients. The test of Tukey was used for post hoc analysis. A p value <0.05 was considered significant.
Statistics was performed using Statsoft.Inc (2011) STATISTCA (data analysis software system, version 10, www.statsoft.com, Padova, Italy).
Results
75 patients undergoing elective surgical lung resection were considered for possible inclusion. We excluded 20 patients because they did not meet inclusion criteria. Consequently, 55 participants were recruited to the study and underwent CD34+ sampling: 2 patients were lost to follow-up due to their transfer to another hospital, in 2 patients data were missing. Therefore, the final statistical analysis was performed on 51 patients. The enrolment flow diagram is reported in Figure 1.
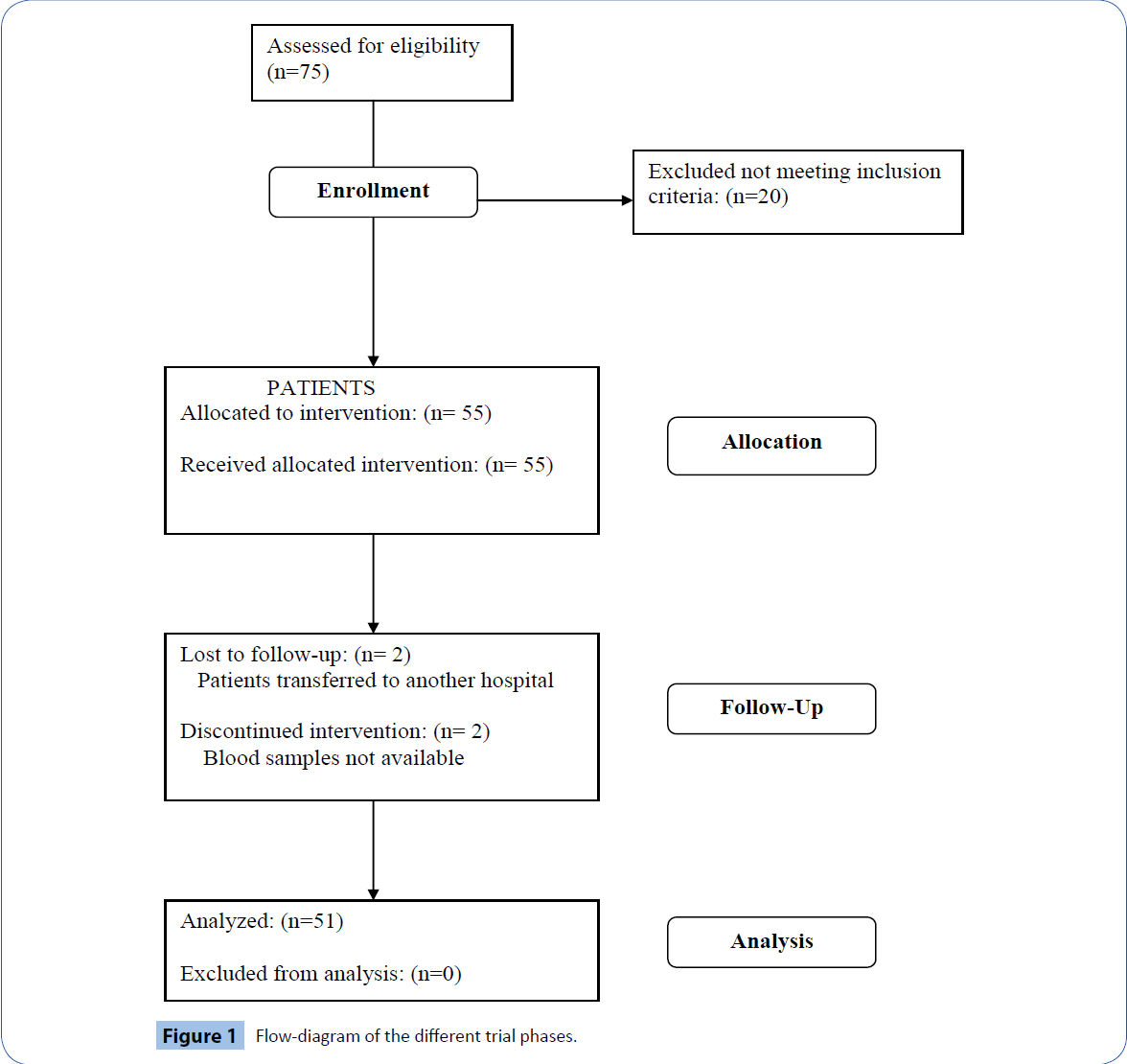
Figure 1: Flow-diagram of the different trial phases.
The hierarchical clustering analysis identified two clusters based on the identification of the CD34+ count time course and the k-mean cluster analysis allowed us to identify members of each cluster for the subsequent ANOVA analysis, that confirmed a significant difference in the mean CD34+ time course between the two clusters (Figures 2 and 3) : in cluster #1 (14 patients), CD34+ decreased by 46% on T24h after surgery (p=0.0023 vs T0) and then increased constantly, reaching 230% on T7d (p=0.00016 vs all the other time points); in cluster 2 (37 patients), CD34+ remained substantially stable throughout the study. Intragroup comparison showed that patients in cluster 1 had higher baseline values of CD34+ (p=0.0023 vs cluster 2 on T0). The two clusters were different in terms of demographic and baseline blood cells count: patients in cluster 1 were younger (58 ± 4 years vs 66 ± 1 years in cluster 2, p=0.03), and had higher T0 baseline values of WBC, and CD34+/monocytes count (p=0.00016 respectively (Table 1).
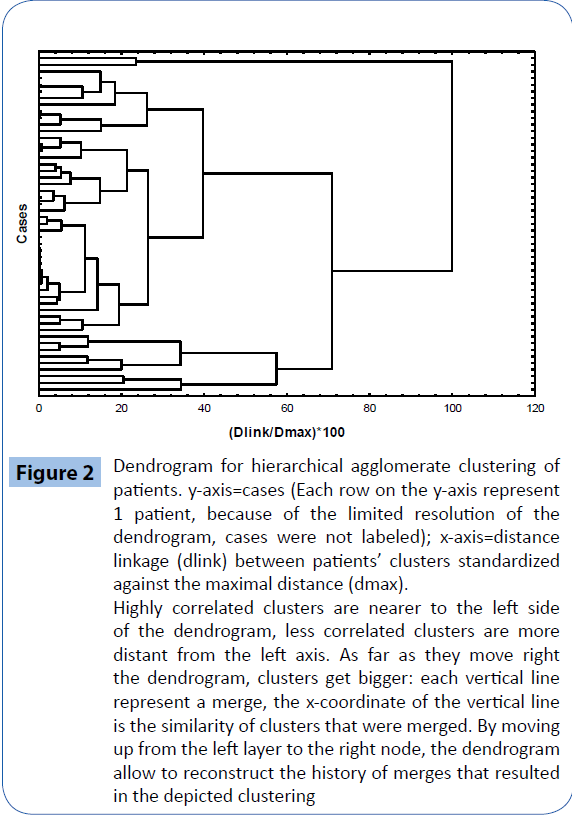
Figure 2: Dendrogram for hierarchical agglomerate clustering of patients. y-axis=cases (Each row on the y-axis represent 1 patient, because of the limited resolution of the dendrogram, cases were not labeled); x-axis=distance linkage (dlink) between patients’ clusters standardized against the maximal distance (dmax). Highly correlated clusters are nearer to the left side of the dendrogram, less correlated clusters are more distant from the left axis. As far as they move right the dendrogram, clusters get bigger: each vertical line represent a merge, the x-coordinate of the vertical line is the similarity of clusters that were merged. By moving up from the left layer to the right node, the dendrogram allow to reconstruct the history of merges that resulted in the depicted clustering
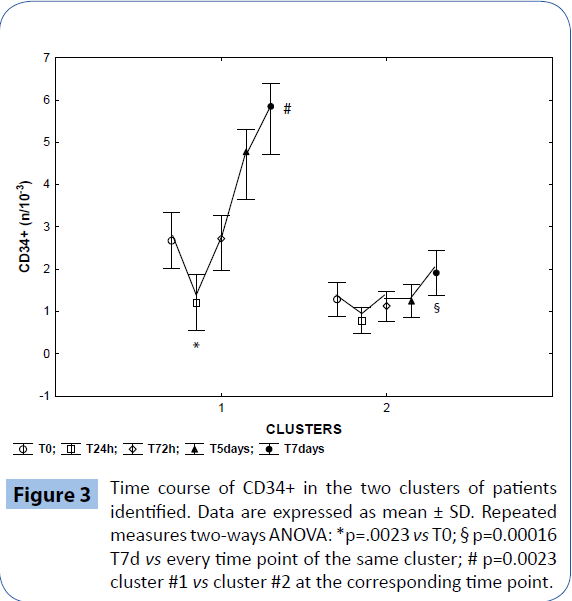
Figure 3: Time course of CD34+ in the two clusters of patients identified. Data are expressed as mean ± SD. Repeated measures two-ways ANOVA: *p=.0023 vs T0; § p=0.00016 T7d vs every time point of the same cluster; # p=0.0023 cluster #1 vs cluster #2 at the corresponding time point.
| |
Cluster 1 |
Cluster 2 |
|
| (N 14) |
(N 37) |
| Age (years) |
58 ± 4.2 |
66 ± 1.5 |
p<0.003 |
| Sex (M:F) |
4-Oct |
28/9 |
NS |
| FEV1/FVC |
95.7 |
76.6 |
p<0.02 |
| Surgery duration (min) |
167 ± 23 |
183 ± 13 |
NS |
| ASA score (II:III) |
8-Jun |
19/18 |
NS |
| Fever N (%) |
8 (55%) |
12 (32%) |
NS |
| White Blood Cells (cells/µl) |
7327 ± 867 |
5864 ± 269 |
P<0.03 |
| Monocytes (cells/µl) |
569 ± 85 |
477 ± 36 |
NS |
| CD34+ (cells/µl) |
2.6 ± 0.39 |
1.20 ± 0.09 |
p<0.0001 |
| CD34+/Mono |
0.203 ± 0.033 |
0.098 ± 0.078 |
p<0.0001 |
ASA=American Society of Anesthesiologists physical status; CD34+=haematopoietic cells exhibiting the CD34 cell surface marker; FEV1/FVC=forced expiratory volume in 1 sec to forced vital capacity ratio.
Table 1: Baseline characteristics of the two clusters of patients.
Pre-operative FEV1/FVC was significantly higher in cluster 1 (95.7 vs 76.6, p=0.02).
The mean WBC time course did not vary statistically in the two clusters. Circulating CD34+cells were more numerous in cluster 1 patients at every time points but T24h. No differences regarding the remaining demographic parameters were found between the two clusters.
Discussion
The main results of the present study are a) peripheral blood CD34+ are mobilized from the bone marrow in patients undergoing lung resection; b) a basal value of CD34+> 2 cells/ml, together with patients age, seem to be predictive factors for CD34+ stem cells mobilization; c) the CD34+ time course was characterized by a sharp consumption of circulating cells in the first 24 h after lung resection, followed by a net increase from 72 h until 7 days after the parenchymal injury.
Before discussing our results, some preliminary considerations on stem cells physiopathology are required. Up to now, it has been recognized that HSC can home to and/or participate in repairing of missing or damaged lung tissue [13-15]. The HSC compartment is phenotypically heterogeneous: many different surface markers have been studied to identify HSC from mouse bone marrow, but a consensus as to which markers are consistently expressed on these cells remain unclear, since there are populations of HSC that are positive and negative for CD34 expression [16-20]. It may be that the expression of CD34 is reversible and related to cell cycle activation [21].
Circulating CD34+ progenitor cells gained importance in the field of regenerative medicine due to their potential to home in injury sites and differentiate into cells of endothelial and osteogenic lineages [22-27].
Our data show a significant increase of peripheral blood CD34+ cells in patients undergoing lung resection. Based on our results we speculate that the differences in postoperative CD34+ cells release could be related to patients’ bone marrow ‘functional reserve’: indeed, patients showing, after a reduction at T24h, a progressive increase in circulating CD34+ cells had a number of circulating CD34+ cells significantly higher at baseline. Very likely, this can be explained by the groups different age distribution, younger patients without respiratory function impairment belonging to cluster 1: mounting evidence supports the idea that in tissue-specific stem cell compartments, aging is invariably accompanied by a reduced capacity to adequately maintain normal homeostatic tissue maintenance and regenerative response after injury [24,28]. In recent years, many theories have been proposed to justify the stem cell decline with aging, related to cell proliferation, differentiation, cell cycle phases, alterations in gene expression and cytokine and growth factor production [13,29]. The observation of a reduced cellularity of bone marrow in the elderly, including CD34+ bone marrow cells support the hypothesis of the human haematopoietic system decrease in advancing age [30,31].
Furthermore, Fadini et al. postulated an age-dependent CD34+ exhaustion between patients and control and demostrated that circulating CD34+ decrease is related to disease severity, especially in patients with restrictive lung disease vs chronic obstructive pulmonary disease (COPD) or controls (respectively FEV1/FVC ratio 78.59 vs 48.31 or 71). The oxigen therapy, reflecting severity of hypoxia in COPD, could lead a compensatory effect of hypoxia associated with higher number endothelial progenitor cells mobilization in COPD [32].
In our study the baseline FEV1/FVC ratio was statistically lower in cluster 2 (76.6 vs 95.66 in cluster 1, p=0.02) although the patients were free from clinical and instrumental evidence of chronic lung disease. We postulate that the decreased FEV1/FVC ratio is agerelated.
In our study the baseline FEV1/FVC ratio was statistically lower in cluster 2 (76.6 vs 95.66 in cluster 1, p=0.02) although the patients were free from clinical and instrumental evidence of chronic lung disease. We postulate that the decreased FEV1/FVC ratio is agerelated. in predicting the optimal timing of peripheral blood CD34+ stem cell collections by apheresis have been described in literature, especially in the hematological field [33-35].
The present study has some limitations: a) firstly, the study was conducted in otherwise healthy patients undergoing elective surgery and none of them showed postoperative complications; more data are needed to study the CD34+ time course in patients with co-existing cardiopulmonary diseases and/or postoperative complications; b) there was a certain lack of homogeneity in the surgical procedures among our patients, that must be taken into account when interpreting our data; c) we could not calculate postoperative FEV1/FVC ratio.
Conclusions
Our observational pilot study showed that peripheral blood CD34+ are mobilized from the bone marrow in patients undergoing lung resection and their count seems to be correlated with WBC and CD34+ basal values and with patients age. The data do not allow to attribute this phenomenon to the surgical resection per se or the anesthesia or the lung disease and future work will be required to obtain this information.
Advances in stem cell research have explored many therapeutic avenues [36,37] and a better understanding of the mechanisms of mobilization may promote major future suggestion, and provide a means of direct and therefore more predictable, and higheryield mobilization.
Acknowledgements
We thank Dr Patricia RM Rocco, Laboratory of Pulmonary Investigation, Carlos Chagas Filho Institute, Federal University of Rio de Janeiro, Rio de Janeiro for the critical revision of the manuscript and the kindly suggestions.
8204
References
- De Silvestro G,Vicarioto M, Donadel C, Menegazzo M, Marson P, et al. (2004) Mobilization of peripheral blood hematopoietic stem cells following liver resection surgery.Hepatogastroenterology 51: 805-810.
- Civin CI, Strauss LC, Brovall C, FacklerMJ, Schwartz JF, et al. (1984) Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol133: 157-165.
- He S,Nakada D, Morrison SJ (2009) Mechanisms of stem cell self-renewal.Annu Rev Cell DevBiol 25: 377-406.
- Orlic D,Kajstura J, Chimenti S, Limana F, Jakoniuk I, et al. (2001) Mobilized bone marrow cells repair the infarcted heart, improving function and survival.ProcNatlAcadSci U S A 98: 10344-10349.
- Cottler-Fox MH,Lapidot T, Petit I, Kollet O, DiPersioJF, et al. (2003) Stem cell mobilization.Hematology Am SocHematolEducProgram .
- Mark AL, Sun Z, Warren DS, Lonze BE, Knabel MK, et al. (2010) Stem cell mobilization is life saving in an animal model of acute liver failure.Ann Surg 252: 591-596.
- Orlic D,Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, et al. (2001) Bone marrow cells regenerate infarcted myocardium.Nature 410: 701-705.
- Kuroda R, Matsumoto T, Kawakami Y, Fukui T, Mifune Y, et al. (2014) Clinical Impact of Circulating CD34-Positive Cells on Bone Regeneration and Healing.
- Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, et al. (2004) Cells derived from the circulation contribute to the repair of lung injury. Am J RespirCrit Care Med. 170: 1158-1163.
- Sutherland DR, Keating A, Nayar R, Anania S, Stewart AK (1994) Sensitive detection and enumeration of CD34+ cells in peripheral and cord blood by flow cytometry.ExpHematol 22: 1003-1010.
- Laing AJ, Dillon JP, Condon ET, Street JT, Wang JH, et al. (2007) Mobilization of endothelial precursor cells: systemic vascular response to musculoskeletal trauma.J Orthop Res 25: 44-50.
- Krause DS,Theise ND, Collector MI, Henegariu O, Hwang S, et al. (2001) Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell.Cell 105: 369-377.
- Rossi DJ, Jamieson CH, Weissman IL (2008) Stems cells and the pathways to aging and cancer.Cell 132: 681-696.
- Abreu SC,Antunes MA, Pelosi P, Morales MM, Rocco PR (2011) Mechanisms of cellular therapy in respiratory diseases.Intensive Care Med 37: 1421-1431.
- Rojas M,Xu J, Woods CR, Mora AL, Spears W, et al. (2005) Bone marrow-derived mesenchymal stem cells in repair of the injured lung.Am J Respir Cell MolBiol 33: 145-152.
- Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M (1998) Human bone marrow CD34- cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells.ExpHematol 26: 353-360.
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo.J Exp Med 183: 1797-1806.
- Bhatia M, Bonnet D, Murdoch B, GanOI, Dick JE (1998) A newly discovered class of human hematopoietic cells with SCID-repopulating activity.Nat Med 4: 1038-1045.
- Krause DS, Ito T, FacklerMJ, Smith OM, Collector MI, et al. (1994) Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells.Blood 84: 691-701.
- Morel F,SzilvassySJ, Travis M, Chen B, Galy A (1996) Primitive hematopoietic cells in murine bone marrow express the CD34 antigen.Blood 88: 3774-3784.
- Sato T, Laver JH, Ogawa M (1999) Reversible expression of CD34 by murine hematopoietic stem cells.Blood 94: 2548-2554.
- Krause DS,FacklerMJ, Civin CI, May WS (1996) CD34: structure, biology, and clinical utility.Blood 87: 1-13.
- Salati S,Zini R, Bianchi E, Testa A, Mavilio F, et al. (2008) Role of CD34 antigen in myeloid differentiation of human hematopoietic progenitor cells.Stem Cells 26: 950-959.
- Wu Y, Zhao RC, Tredget EE (2010) Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration.Stem Cells 28: 905-915.
- RatajczakMZ,Wysoczynski M, Reca R, Wan W, Zuba-SurmaEK, et al. (2008) A pivotal role of activation of complement cascade (CC) in mobilization of hematopoietic stem/progenitor cells (HSPC).AdvExp Med Biol 632: 47-60.
- Ritz U, Spies V,Mehling I,Gruszka D,Rommens PM, et al. (2014) Mobilization of CD34+-progenitor cells in patients with severe trauma.PLoS One 9: e97369.
- Maruyama S, Taguchi A, Iwashima S, Ozaki T, Yasuda K, et al. (2008) Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients.Kidney Int 74: 1603-1609.
- Rossi DJ,Bryder D, Weissman IL (2007) Hematopoietic stem cell aging: mechanism and consequence.ExpGerontol 42: 385-390.
- Yahata T,Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, et al. (2011) Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood 118: 2941-2950.
- Ogawa T, Kitagawa M, Hirokawa K (2000) Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages.Mech Ageing Dev 117: 57-68.
- Beerman I, Maloney WJ, Weissmann IL, Rossi DJ (2010) Stem cells and the aging hematopoietic system. CurrOpinImmunol 22: 500-506.
- Fadini GP,Schiavon M, Cantini M, Baesso I, Facco M, et al. (2006) Circulating progenitor cells are reduced in patients with severe lung disease.Stem Cells 24: 1806-1813.
- Pastore D,Mestice A, Perrone T, Gaudio F, Delia M, et al. (2008) Subsets of CD34+ and early engraftment kinetics in allogeneic peripheral SCT for AML.Bone Marrow Transplant 41: 977-981.
- BoulasselMR(2008) Associations among white blood cells, CD34+ cells and GM-CFU in predicting the optimal timing of peripheral blood stem cell collections by apheresis. J Assoc Physicians India 56: 96-98.
- Specchia G,Pastore D, Mestice A, Liso A, Carluccio P, et al. (2006) Early and long-term engraftment after autologous peripheral stem cell transplantation in acute myeloid leukemia patients.ActaHaematol 116: 229-237.
- Masterson C,Jerkic M, Curley GF, Laffey JG (2015) Mesenchymal stromal cell therapies: potential and pitfalls for ARDS. Minerva Anestesiol 81: 179-194.
- Lee JW, Rocco PR, Pelosi P (2015) Mesenchymal stem cell therapy for acute respiratory distress syndrome: a light at the end of the tunnel?Anesthesiology 122: 238-240.









