Keywords
|
| EGFR mutation analysis, Molecular diagnosis, ALK gene rearrangement |
Introduction
|
| Lung cancer is the leading cause of cancer-related deaths in the in the world. Histologically, the World Health Organization (WHO) classifies lung cancer into non-small cell lung cancer (NSCLC, 85%) and small-cell lung cancer (15%). Depending upon the stages of the disease, there are four treatment strategies, including surgery, radiation therapy, chemotherapy, and/or targeted therapy. The use of targeted kinase inhibitors is dependent on the targets such as epidermal growth factor receptor (EGFR) or activating chromosomal fusions involving the ALK kinase, which are effective if there is an association of the molecular alterations in the tumors [1]. Therefore, molecular tests are routinely performed to identify mutations in oncogenes in lung cancer, including EGFR and ALK. Molecular tests help in identifying those patients who respond to targeted therapy; and reduce side effects of ineffective treatments. The use of tyrosine kinase inhibitors (TKI), gefitinib or erlotinib, requires confirmation of somatic activating EGFR mutation. |
| ALK gene rearrangements have been reported in 2% to 13% of patients with NSCLC [1]. ALK-rearranged tumors typically lack EGFR and KRAS mutations. Recent studies have demonstrated that lung cancers harboring ALK rearrangements do not respond to EGFR-specific tyrosine kinase inhibitors, but benefit from ALK kinase (i.e., crizotinib). |
| Now-a-days along with the routine histology testing molecular tests have been used frequently in diagnosis. However, some of the molecular techniques like real time, conventional PCR have its limitations including low resolution, poor precision, and turn over time of reporting and cost [2]. Hence, sequencing/pyrosequencing has been developed with widespread application in detecting gene rearrangements, single nucleotide polymorphism (SNP), and loss of heterozygosity (LOH). This manuscript focuses on the importance of the molecular tools and its use in point-of-care testing at an affordable cost [3-5]. |
Materials and Methods
|
| In this study, paraffin-embedded tissue blocks of confirmed NSCLC by two pathologists which had at least 50% of neoplastic cells per sample were collected from 267 consecutive patients (185 males and 82 females; with mean age 56 years (25-84)) during December 2009-March 2015 and referred to the Department of Molecular Biology and Cytogenetics, Apollo Health City, Hyderabad, India, for EGFR mutation testing and ALK-1 gene rearrangement. Informed consent was obtained from all the patients. |
| Fixed samples were processed routinely and finally stained with hematoxylin and eosin (HE). If necessary, additional IHC was performed to verify the microscopic diagnosis. The antibody panel, recommended for the diagnosis of adenocarcinoma by the American and European guidelines, was applied: TTF-1 (Thyroid Transcription Factor-1; 8G7G3/1 IR 056; ready to use - RTU), CK7 (Cytokeratin 7; OU-TL12/30, IR 619, RTU), CK20 (Cytokeratin 20; Ks.20.8, IR 777; RTU), p63). The histological type of NSCLS was diagnosed according to: 2004 WHO classification, 7th edition of the American Joint Committee on Cancer and the latest classification of adenocarcinoma by the International Association for the Study of Lung Cancer/American Thoracic Society/ European Respiratory Society. Paraffin block sections of 5 to 6, “thick” 6 μm sections were deposited in PCR tubes. |
|
EGFR mutation testing
|
| A validation procedure of EGFR mutation testing was done by using DNA isolation QIAamp DNA FFPE Mini Kit (Qiagen). Mutation screening of exons 18, 19, 20 and 21 of the EGFR gene was performed by pyrosequencing. The EGFR mutations in codons 719, 768, 790, 858, and 861, as well as deletions and complex mutations in exon 19 of the human EGFR gene were analysed. The 4 regions are amplified separately by olymerase chain reaction (PCR) and sequenced through the defined region. The amplicon covering codons 768 and 790 is divided into 2 sequencing reactions. Sequences surrounding the defined positions serve as normalization and reference peaks for quantification and quality assessment of the analysis. |
| After PCR using primers targeting exons 18, 19, 20, and 21, the amplicons are immobilized on Streptavidin Sepharose® High Performance beads. Single-stranded DNA is prepared, and the corresponding sequencing primers anneal to the DNA. The samples are then analyzed on the PyroMark Q24 System using a run setup file and a run file. The run was analyzed using the analysis tool integral to the PyroMark Q24 System. |
|
ALK gene rearrangement by FISH analysis
|
| Gene rearrangement per cell was investigated only on histological material by FISH. The LSI ALK (2p23) Spectrum Orange/ALK (2p23) Spectrum Green probe (Vysis, Abbott Laboratories, Illinois and USA) were used in accordance with manufacturer instructions. FISH signals were evaluated under the fluorescence microscope Olympus BX41 (Olympus, Japan) equipped with single filters: DAPI, Spectrum Orange and FITC as well as triple-filter DAPI/FITC/ Spectrum Orange. Images were photographed (camera F-View, Olympus, Japan) and analyzed using the applied spectral imaging software (Olympus, Israel). FISH analysis was independently performed by pathologist and molecular biologist unaware of the clinical and molecular characteristics of patients. A scoring system was adopted at least 80-100 non-overlapping cell nuclei were examined. FISH positive NSCLC were determined for ALK gene rearrangement. |
Results
|
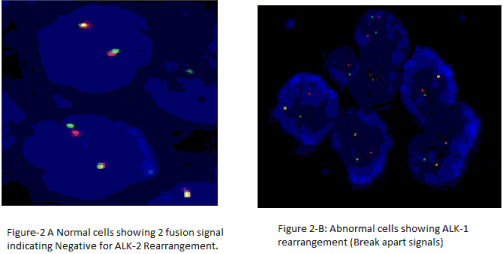
| In this study, fluorescence in situ hybridization (FISH) using a break-apart probe for the ALK gene was performed on formalin fixed paraffin-embedded tissue to determine the incidence of ALK rearrangements and hybridization patterns in 267 patients with a referred diagnosis of non-small cell lung cancer. Among the 267 patients 11 patients (6-Males, 5-Females) were positive for ALK-1 gene rearrangement (Figure 1). Among 267 cases 260 cases were confirmed cases of adenocarcinoma and 7 of the 267 cases were squamous cell carcinoma. |
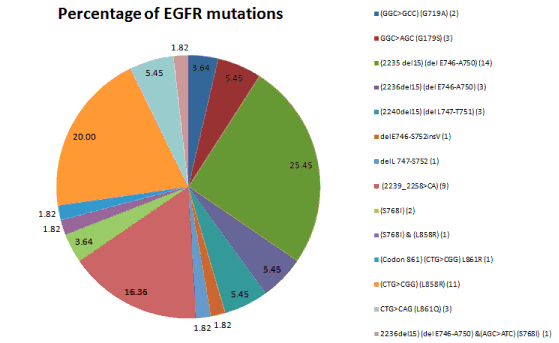
| EGFR mutations were detected in 55 patients (20.59%) but the total number of mutations was 14 as two of the patients had more than one abnormality: Exon 19 (2236del15) (del E746-A750) and Exon 20 (AGC>ATC) (S768I) and Exon 20 (S768I) and Exon 21 (L858R). The most common mutations found were exon 19 (31/55, 56.36%) among the exon 19 deletions (2235 del15) (del E746-A750) were found in (14/31, 45.16%) (2239_2258>CA) (9/31, 29.03%), and second common mutation seen were exon 21 (15/55, 27.27%) among the exon 21 substitutions (CTG>CGG) (L858R) were found in (8/15, 53.33%); mutations in exon 20 (S768I) (3/55, 5.457%) and in exon 18 (5/55, 9.09%) were also found (Figures 2, 3 and Table 1). |
| The EGFR mutation detected were greater in female gender and histologic features more of adenocarcinoma (Figure 1). The influence of gender, non-smokers and histological type on the EGFR mutation incidence was analyzed; the most significant increase of EGFR mutation was found in female group. In our study all females are non-smokers and the EGFR mutations were more common in non-smoker than the smokers (Table 2). |
Discussion
|
| Cancer is diagnosed with morphological parameters along with molecular alternations. Multiple platforms and high throughput techniques have been practiced for development of précised medicine for personalized approach. One such approach is EGFR mutation detection in lung cancer EGFR mutation as an important predictive marker for response to oral tyrosine kinase inhibitors in clinically selected patients. The detection of an EGFR mutation is important for directing targeted therapy with EGFR inhibitors to improve treatment efficacy, reduce adverse effects and cost. Testing for mutations in EGFR is therefore an important step in the treatment-decision. Confirmation of mutations in EGFR and ALK-1 gene by molecular methods would help in treatment for lung cancer. |
| In the present study, EGFR mutation analysis was performed on 267 patients. We found that EGFR mutation in adenocarcinomas than over all (20.59%) and among women than men (58.18% vs. 38.18%), similar to Korean and Chinese data as reported earlier [6-8]. EGFR mutations have been consistently reported to be more common in never-smokers as compared to smokers [7], which our study confirms as well (66.29% vs. 33.70%) (Table 2). Though, the mutation rate varies significantly between never-smokers and smokers across different ethnicities. For e.g., 47% never smokers harbor EGFR mutations compared to 22% among smokers in the Chinese population; among other Asian series, mutation rate among never-smokers have been reported to be as high as 60%; positive compared to 8% smokers [7]. In our study similar observations were found (Table 2). Histopathologically, mutation rates among adenocarcinoma-males were predominantly lower than females, consistent with literature. However, no difference observed between never-smoker adenocarcinoma females and males indicating lack of gender bias among never-smoker patients, possibly due to lower proportion of male non-smokers (258 non-smokers out of 516 males) exists in India compared to female non-smokers (258 non-smokers out of 265 females), unlike in developed countries. This is also consistent with similar studieson never-smoker females of East Asian ethnicity [8,9] wherein rate of mutation varies with clinical stage of never-smoker female patients [10-12]. We could not find EGFR mutations in the squamous cell carcinoma patients (6/267, 2.25%) (Table 2) which could be due to the small population size and to further confirm it a larger study is recommended. However, in other study observed in the west, EGFR mutations were found in squamous cell carcinoma indicating that EGFR mutation test for squamous cell carcinoma need to as well be considered as a routine practice, as recommended for ALK mutations with comparable incidence at around 5% [13]. |
| Our findings of exon 18, 19 and 21 with a mutation rate of 9%, 56% and 27% respectively is similar to that described in Spanish and Japanese populations (Figure 3). In our earlier study, exon 19 mutation was present in 56% cases and exon 21 in 27% of cases, this difference could be because of the smaller sample size of 267 patients [3]. Our results suggest that exon 19 mutations are more common in never- smoker females and males. Of note, some other studies have also observed no association between EGFR mutations and age, gender or smoking history in Chinese patients [14]. |
| In this study, the frequency of ALK gene rearrangements detected using a dual-color break-apart FISH probe was 4.11% of adenocarcinoma cases (Figure 1). This is somewhat similar to earlier studies that generally had smaller number of patients, but close to 3.9% which was observed in two cohorts of 720 and 1121 lung adenocarcinomas from two series in Japan [15,16]. Our results are consistent with previous studies where ALK rearrangements have been largely (but not completely) restricted to adenocarcinomas that lack EGFR or KRAS mutations. |
| Here, we show that the ALK FISH signal patterns may vary from a single split signal to very complex signal patterns. Gains of intact ALK signals as well as typical and variant hybridization patterns for ALK gene rearrangements were observed simultaneously in some samples which could be due to tumor heterogeneity. Although polysomy of chromosome 2 did occur frequently in NSCLC (and was not regarded as evidence of an ALK-specific rearrangement), high-level amplification of the ALK fusion signal was a rare event. |
| In tumors with ALK gene rearrangements lead to activation of ALK kinase, which confirms the sensitivity of NSCLC tumors to ALK inhibitors [17]. Lung cancer patients with ALK rearrangements have been shown to have significant reductions in tumor burden indicating response to ALK inhibitor such as crizotinib. |
| Patients harboring ALK rearrangements do not benefit from treatment with the EGFR-specific tyrosine kinase inhibitors showing that the EGFR inhibition is bypassed. The clinical behavior of NSCLC cases with variant ALK signal patterns is less clear. Amplification of the ALK fusion has been seen in a patient undergoing crizotinib therapy and may be a sign of developing resistance [18]. |
| Several sensitive testing methods have been used for the detection of EGFR mutation analysis, however, pyro sequencing is found to be the accurate, sensitive and appropriate for clinical samples as lung carcinomas are detected at advanced stages. The lung biopsy specimens for diagnosis and molecular analysis are often limited in lung cancers which would have already been exhausted through morphologic and immunophenotypic analysis, Pyro sequencing and FISH technique would help to study gene alternations with little tumor specimen. |
Conclusion
|
| Molecular techniques have been invariably used in many hematological and solid tumor diagnosis and treatment. Among such cancers, lung carcinoma is one such disease where molecular techniques to detect molecular alterations such as EGFR mutation analysis and ALK gene rearrangement are good examples. Molecular techniques such as Pyrosequencing and Fluorescent in-situ hybridization (FISH) have been sensitive techniques in detection of gene alterations in lung carcinomas. The use of such molecular techniques would enables in effective identification of lung cancer patients who would benefit from targeted anti-ALK therapies. |
Acknowledgments
|
| The authors are grateful to patients for sharing the samples and the clinical information and the management of Apollo hospitals, for the financial support. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |








