Keywords
Acetaminophen; Morinda lucida; Hepatotoxicity; Oxidative stress; Phytochemical
Introduction
Acetaminophen, (N-acetyl-p-aminophenol, paracetamol, APAP) is a well-known antipyretic and analgesic, effective at therapeutic doses [1]. At high doses, acetaminophen induces toxicity to the liver, which is usually characterized by chest pain, vomiting, diarrhea, and sometimes shock. Moreover, hepatic failure, myocardial and kidney dysfunctions have been attributed to excessive ingestion of acetaminophen [2,3]. Acetaminophen is metabolized by cytochrome P450 enzymes of the liver and detoxified by glucuronidation as well as sulfation. APAP-induced hepatotoxicity is as a result of the formation of a reactive metabolite N-acetyl-p-amino-benzoquinone imine (NAPQI), which then depletes glutathione (GSH) level [3,4]. Depleted GSH level permits the binding of free NAPQI to other thiol-containing compounds and a variety of cellular proteins, provoking oxidative stress and damage leading to cellular necrosis [5]. Due to dose-dependent toxicity caused by APAP, APAP-induced hepatic damage can be studied in animal models and most mechanisms can be correlated to humans [6]. Several lines of evidence have implicated N-acetylcysteine (NAC) as the best therapeutic option to combating liver failure due to APAP toxicity. However, Clinical studies reveal untoward side effects [7-9].
Plant-derived products have attracted the attention of many researchers today who investigate their medicinal potential for the treatment of various diseases. Currently, a good percentage of the world population relies solely on botanical preparations as medicines to meet their health needs [10]. One of such plants is Morinda lucida. Tests with animals confirm the attributed activity of several traditional medicinal applications of Morinda lucida. Extracts showed anti-inflammatory, antifever and painreducing activity in tests with rats and promoted gastric emptying and intestinal motility [11]. The leaf extract has been reported to possess antimalarial [12,13] and strong oral hypoglycemic activities [14,15].
Since acetaminophen toxicity is still on the rise, and lack of effective medication for hepatic dysfunction has not been effectively tackled, natural remedies such as Morinda lucida with fewer side effects can be considered. We, therefore, evaluated the protective effect of aqueous and propanoic extract of “oruwo” leaves (Morinda lucida) on acetaminopheninduced oxidative damage and hepatic injury in rats.
Materials and Methods
Plant-source and identification
Morinda lucida fresh leaves were obtained from Ikeji Arakeji in Osun state, Nigeria. The plants were taken to the Department of plant biology, Ondo State University of Science and Technology, Okitipupa for identification.
Animals and treatments
Albino rats (Rattus norvegicus) weighing between 91.6 g and 164.3 g were used in this evaluation. These rats were gotten from Ayoola farmhouse in Ilorin, Kwara state, Nigeria. They were kept under standard condition (23 ± 2°C) (55 ± 10% humidity) with 12 h light/dark cycle, standard pellet diet provided as well as free access to water during the experimental period. Importantly, the animals acclimatized to the environment for 2 weeks before the initiation of the experiment. Thirty-six rats were randomly divided into six equal groups as outlined in Table 1.
| Groups |
Dosage regimen for 7 days |
| A (control) |
Distilled water/0.9% Normal saline |
| B |
APAP only (250 mg/kg ) |
| C |
PMO (240 mg/kg) |
| D |
APAP+PMO (240 mg/kg) |
| E |
AMO (240 mg/kg) |
| F |
APAP+AMO (240 mg/kg) |
Table 1: Experimental study design.
Methods
Preparation and extraction of crude plant extract ingredient
Morinda lucida was sundried for three days and was pulverized into powdery form using an industrial blender. One hundred grams of the ground Morinda lucida leaves was measured with an electronic balance and then submerged in propanol/water for 72 hrs on a shaker to ensure maximum extraction. The extract was then filtered with a Whatman filter paper and subjected to rotary evaporation. The extract was then placed in an oven for 22 hours at 61ºC to dry [16].
Phytochemical screening of Morinda lucida leaves
Phytochemical screenings were carried out on the powdered sample using standard procedures to confirm the presence of constituents (alkaloids, anthraquinones, flavonoids, saponins, tannins, cardiac glycosides and phlobatannins) as described by Harborne and Trease et al. [17,18].
Test for saponins: Powdered sample (1 g) was boiled with 10 mL of distilled water in a bottle bath for 10 min. The mixture was filtered while hot and allowed to cool. 5 mL of filtrate was diluted to 10 mL with distilled water and shaken vigorously for 2 min. Two drops of olive oil were added to the solution obtained from diluting 2.5 mL of the filtrate to 10 mL with distilled water (as above), then shaken vigorously for a few minutes. The formation of persistent foam was evidence of the presence of saponins. This demonstrates emulsifying properties.
Test for alkaloids: Sample (1 g) was stirred in 10 mL of concentrated HCl on a steam bath followed by filtration. Filtrate (1 mL) was mixed with two drops of Wagner’s reagent, then two drops of Dragendorff’s reagent were added to another 1 mL of the filtrate, and the mixtures were then observed for turbidity.
Test for tannins: Powdered sample (1 g) was boiled with 20 mL of distilled water in a water bath and was filtered while hot. Cooled filtrate (1 mL) was distilled to 5 mL with distilled water and two to three drops of 10% ferric chloride were added and observed for any formation of precipitates and any color change. A bluish-black or brownish-green precipitate indicated the presence of tannins.
Test for free anthraquinones: Sample (0.5 g) was shaken with 5 mL of chloroform for 10 min, filtered and 5 mL of 10% ammonium solution was added to the filtrate. The mixture was shaken and the presence of a pink, red or violet color in the ammonia phase indicated the presence of free anthraquinones.
Test for cardiac glycoside: Sample (1 g) was extracted with 10 mL of 80% ethanol for 5 min on a water bath. The extract was filtered and diluted with an equal volume of distilled water and two drops of lead acetate solution were added, shaken and filtered after standing for a few minutes. The filtrate was then extracted with aliquots of chloroform, and the extract was dissolved in 2 mL of glacial acetic acid containing one drop of FeCl3 solution in a clean test tube. Concentrated H2SO4 acid (2 mL) was then poured down the side of the tube so as to form a layer below the acetic acid. The formation of a reddish-brown or brown ring at the interface and green color in the acetic layer was taken for a positive result.
Phlobatannins: Deposition of a red precipitate when extracts of the plant were boiled with 1% aqueous HCl was taken as evidence for the presence of phlobatannins.
Preparation of maize and bean cultures
We also assessed the effect of Morinda lucida on maize and Bean seeds germination and on the growth of the germinating seedlings. Maize and bean seeds were cultured in Petri dishes as follows; Cotton wool was placed at the bottom of the Petri dishes. 10 ml of each of the serially diluted solutions was pipette into separate dishes so that the cotton wool was completely wet. Four healthy seeds were well spaced in a circular pattern in each petri dish. 10 ml of distilled water was used as a control culture. About 10 ml of distilled water was added to each culture every day from the second day of culturing so as to replace water loss during evaporation. The experiments were done in duplicates and at room temperature, the culture was observed for 7 days, the roots- and shoot- lengths were measured. The measurements in the test cultures were compared with those of controls to get an index of inhibition of seed germination and seedling growth.
Liver function tests: The liver enzymes including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP). Total bilirubin and total cholesterol were assessed following the manufacturer’s instructions using diagnostic kits obtained from Diamond Diagnostic Cairo, Egypt.
Determination of oxidative stress and antioxidant indices
The liver of each rat was removed, weighed and washed with ice-cold saline and subsequently homogenized in cold potassium phosphate buffer (0.05 M, pH 7.4). Subsequently, the homogenate was centrifuged at 10,000 g for 15 min at 4°C and the supernatant was used for the determination of oxidant/ antioxidant markers. Protein concentration was determined according to the method of Bradford et al. [19], using bovine serum albumin as standard. Catalase (CAT) activity was determined using H2O2 as a substrate according to the method described by Clairborne. GSH was evaluated according to the method described by Jollow et al. [20]. Lipid peroxidation was determined as malondialdehyde levels (MDA) according to the method described by Tahnteng et al. [21].
Concentrations of pro-inflammatory biomarkers
Nitric oxide (NO) level was determined by measuring the testicular nitrite content, the stable end products of NO. Liver nitrites content were obtained using a sodium nitrite curve as standard and expressed as micromolar of nitrites per milligram of protein according to the method described by Green et al. [22]. Myeloperoxidase (MPO) activity was assayed according to the method described by Granell et al. [23]. MPO activity was expressed as micromolar of H2O2 per minute per milligram of protein.
Statistical analysis
All results were presented as mean ± Standard Deviation (SD). Data were analyzed by using Microsoft Excel 2007 (Redmond, Washington, USA) and Graph Pad Prism 5 software. All the data of treatment groups were compared with the control group by using a one-way ANOVA followed by Dunnett ’ s multiple comparison tests. In all the groups, differences were considered statistically significant among groups when p<0.05.
Results
Phytochemical screening
Table 2 shows the qualitative phytochemical analysis of Morinda lucida leaves.
| Tests |
Results |
| Saponin |
Detected |
| Alkaloids |
Detected |
| Anthraquinone |
Not Detected |
| Phlobatanin |
Not Detected |
| Cardiac glycosides |
Detected |
| Tannin |
Detected |
Table 2: Phytochemical results of the propanolic extract of Morinda lucida.
The leaves contained the presence of Alkaloids, cardiac glycosides saponin, and tannins while anthraquinones and phlobatanin are absent or in trace amounts respectively.
Effects of Morinda lucida extracts on maize and bean seedlings
Our results show that Both Maize and Bean seed growths were inhibited. However, Bean-seeds were more sensitive to saponin effects especially the roots compared with Maize seeds (Tables 3-5).
| Maize seedlings |
Germination profile |
| Concentration of propanoic |
Average shoot |
Average Root |
| Extract (%) |
Length (cm) |
Length(cm) |
| Control |
12.76 |
17.5 |
| 2 |
9.25 |
12.1 |
| 1 |
6.29 |
11.75 |
| 0.5 |
9.79 |
12.7 |
| 0.2 |
8.68 |
14.9 |
| 0.1 |
11.44 |
15.8 |
Table 3: Phytotoxic evaluation of propanolic extract of Morinda lucida on maize seedlings.
| Bean seedlings |
Germination profile |
| Concentration of propanoic |
Average shoot |
Average Root |
| Extract (%) |
Length (cm) |
Length (cm) |
| Control |
16.65 |
4.17 |
| 2 |
19.22 |
3.78 |
| 1 |
19.38 |
4.59 |
| 0.5 |
18.41 |
6.89 |
| 0.2 |
20.4 |
6.13 |
| 0.1 |
23.1 |
5.2 |
Table 4: Phytotoxic evaluation of propanoic extract of Morinda lucida on bean seedlings.
| Maize seedlings |
Germination profile |
| Concentration of aqueous |
Average shoot |
Average root |
| Extract (%) |
Length (cm) |
Length (cm) |
| Control |
10.11 |
19.32 |
| 2 |
7.68 |
11.97 |
| 1 |
4.44 |
11.4 |
| 0.5 |
5.18 |
14.54 |
| 0.2 |
5.62 |
15.28 |
| 0.1 |
6.82 |
13.21 |
Table 5: Phytotoxic evaluation of aqueous extract of Morinda lucida on maize seedlings.
Effect of Acetaminophen and M. lucida extracts on liver enzymes (ALT, AST, and ALP), total bilirubin and total cholesterol
Exposure of rats to acetaminophen (250 mg/kg) caused a significant elevation of hepatic serum markers ALT, AST, ALP, total bilirubin and total cholesterol in comparison with the control group (p<0.05). Injection of acetaminophen through the IP route of administration produced evidence of hepatotoxicity. After administration of Morinda lucida extracts, the deleterious effect of acetaminophen were significantly ameliorated in comparison to the acetaminophen group (p<0.05). Importantly, M. lucida extracts modulated changes in total bilirubin level and total cholesterol levels in different groups respectively.
Effect of Morinda lucida extracts on oxidative stress and antioxidant markers in Acetaminophen-Induced toxicity in the liver
Here, we assessed changes on LPO, NO, MPO, SOD, GSH, GPX and CAT activities caused as a result of toxicity (Figure 1). Acetaminophen-induced oxidative stress in liver was in the form of significant elevation (p<0.05) of MPO (Figure 2) NO (Figure 2) and LPO (Figure 2) levels associated with significant (p<0.05) reduction in CAT (Figure 3A), GSH level (Figure 3B), GPX (Figure 3C) SOD (Figure 3D) activities compared with the normal control groups. On co-administration of PMO and AMO with APAP, antioxidant status was improved significantly marked by increased SOD, GPX, and CAT activities (p<0.05) compared to the group treated with acetaminophen. There were no significant differences between propanolic (PMO) and aqueous (AMO) extract treated groups and the associated co-exposed groups with APAP, suggesting that Morinda lucida extract has a potent ameliorating effect when extracted using aqueous or propanol fractions.

Figure 1: Morinda lucida flowering plant.
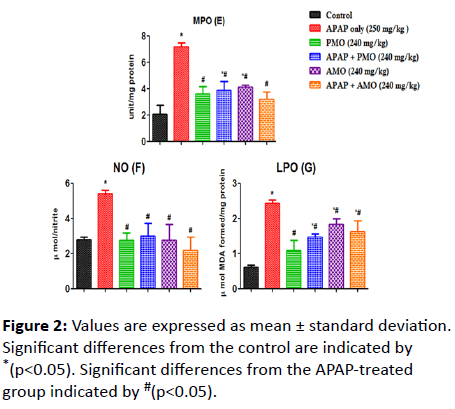
Figure 2: Values are expressed as mean ± standard deviation. Significant differences from the control are indicated by *(p<0.05). Significant differences from the APAP-treated group indicated by #(p<0.05).
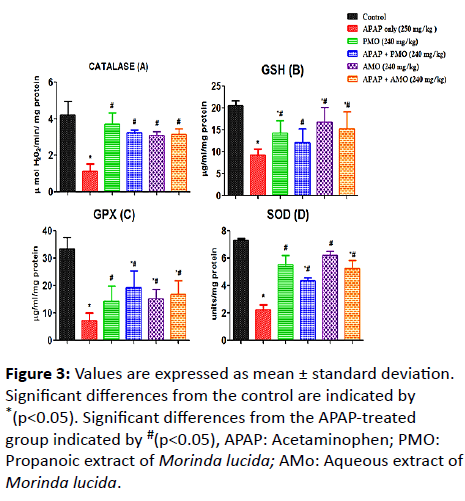
Figure 3: Values are expressed as mean ± standard deviation. Significant differences from the control are indicated by *(p<0.05). Significant differences from the APAP-treated group indicated by #(p<0.05), APAP: Acetaminophen; PMO: Propanoic extract of Morinda lucida; AMo: Aqueous extract of Morinda lucida.
Discussion
The presence of tannins in the leaves of Morinda lucida might confer on the leaves treatment ability for ulcerative wounds and hemorrhoids, leucorrhoea, rhinorrhoea, and diarrhea. Further, Saxena et al. [24] posit that plants used as diuretics, astringents and against stomach disorders and diarrhea essentially contain tannins. These tallies with the findings from Adeyemi et al. [25] The presence of alkaloids in the leaves of Morinda lucida supports the findings by Adeyemi et al. [25], who indicated that the antipyretic and analgesic property of this plant might be due to the presence of alkaloids. Alkaloids containing compounds possess various pharmacological activities including antihypertensive effects, Antiarrhythmic effect, antimalarial and anticancer activity [24]. Fruits and vegetables are important sources of saponins and they have been implicated in the modulation of blood glucose and antimicrobial activity [26]. Our results thus show that extracts (propanoic and aqueous) of leaves of Morinda lucida possess saponin and as such might have a mild effect on blood glucose levels and possibly exhibit antimicrobial activities.
Extracts from plants containing cyanogenic glycosides could be employed as flavoring agents in the pharmaceutical industry, as they are essential components of pharmaceutical formulations [27]. In our study, cardiac glycosides were found in the leaves of Morinda lucida and this finding is in agreement with studies by Achi et al. [28], which reported that its leaves contain a significant amount of glycosides.
Other studies involving the phytochemical screening of Morinda lucida also revealed the presence of anthraquinones and anthraquinones [29], tannins, alkaloids, flavonoids, and glycosides components. However, anthraquinones and Phlobatanin were not detected in this study [30].
Both maize and bean seed growth was inhibited. However, bean-seeds were more sensitive to saponin effects especially the roots compared with Maize Seeds. This might be due to the fact that the Bean is a legume while Maize is a cereal. These findings agree with the results of Moreibise et al. [31]. This study also revealed that the roots of both Maize and Bean seedlings were more inhibited compared with the shoots and also that these inhibitions were concentration-dependent as well [32]. This study has therefore revealed that the shoots, in general, were less sensitive than the roots to Saponin-effects. These findings agree with the result of Oleszek et al. and of Merobise and Fatanso [32-34]. The phytotoxicity of saponin was thought to occur at hormonal and enzymic levels involving inhibitory effects on plant growth hormones like auxins and the gibberellins [35]. It has been reported that saponin retards seed germination by inhibiting endogenous gibberellic acid (GA3) and indole-3-acetic acid (IAA), which are both responsible for seedling elongation [36] since saponins from Olimum gratissium were phytotoxic at the test concentration of 0.1% to 2.0% used, this suggests that the test saponins would be strongly phytotoxic even at lower concentrations when further purified. More so, their application in agriculture could effectively be exploited in weed-control, plant selection, and herbicidal application, in seed preservation and in related micro field experiments.
In the present study, the effect of propanol and aqueous extracts of Morinda lucida leaves on acetaminophen-induced hepatotoxicity in albino rats was evaluated. Hepatotoxicity was of significant increase (p<0.05) with respect to the activities of serum AST and ALT in the group treated with the only acetaminophen when compared with control. This may result from cellular leakage due to peroxidative damage of the membrane.
APAP overdose and toxicity causes the corresponding destruction of hepatocytes and thus resulting in the elevation of serum enzymes [37]. Therefore, measuring the levels of serum hepatic markers such as ALT, AST, ALP, total bilirubin and total cholesterol is vital for the identification of liver damage [38,39]. Bilirubin is a conventional indicator of liver diseases [40]. In the present study, 250 mg/kg acetaminophen caused acute liver injury which was characterized by increased serum activity of AST, ALT ALP and total bilirubin which might imply severe hepatocellular damage caused by leakage of these enzymes into cytoplasmic circulation. However, co-treatment with extracts from Morinda lucida alleviated this effect and caused a reduction in levels of AST, ALT ALP, and total bilirubin, when compared with the APAP-treated group. These biochemical restorations may be due to the inhibitory effects of the plant extract on cytochrome P450 or/and promotion of APAP glucuronidation [41]. Overall, these ameliorative potentials of extracts of Morinda lucida confirms the hepatoprotective effect of the plant.
Oxidative stress is an important mechanism that has been implicated in acetaminophen toxicity. Thus, increased formation of superoxide would result in hydrogen peroxide generation and peroxidation reactions [42]. Acetaminophen-induced liver injury has also been marked by lipid oxidation. Mounting evidence from previous studies [43,44] supports what we found in the present study that acetaminophen-induced toxicity invoked elevated MDA level, depleted GSH level, and reduction in antioxidant activity of SOD, GPX, and CAT respectively. The elevated MDA in liver implies the overwhelming influence of oxidative stress generated by the APAP and likely due to the failure of antioxidant defense mechanisms [45]. Co-treatment with Morinda lucida extracts significantly restored these effects and improved the basal antioxidant status of the cell. At normal conditions, the body defense mechanism against oxidative stress, marked by endogenous antioxidant enzymes, such as GSH, SOD, and CAT prevents cell damage induced by free radicals [46,47]. Thus compounds that ameliorate oxidative stress can cause an improvement on oxidative damage to the liver. Thus, the augmented antioxidant enzymes levels in liver tissues of animals treated with Morinda lucida extracts implies and justifies the antioxidant property of the extract.
Conclusion
Taken together, co-exposure to Morinda lucida extracts confers protection on hepatic lesions and injury induced by an overdose of APAP. Oxidative stress is believed to be the mechanism of toxicity of APAP and therefore, the hepatoprotective effect of Morinda lucida extracts may be associated with a reduction of oxidative stress and significant impact on inflammation. Therefore, further studies may be necessary to investigate and confirm the exact mechanisms of hepatoprotection.
24482
References
- James LP, Mayeux PR, Hinson JA (2003) Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31: 1499-1506.
- Song Z, McClain CJ, Chen T (2004) S-Adenosylmethionine protects against acetaminophen-induced hepatotoxicity in mice. Pharmacology 71: 199-208.
- Kumar V, Abbas AK, Aster JC, Perkins JA (2015) Robbins and Cotran pathologic basis of disease. Philadelphia, PA: Elsevier. pp: 96-104.
- Cristani M, Speciale A, Mancari F, Arcoraci T, Ferrari D, et al. (2016) Protective activity of an anthocyanin rich extract from bilberries and blackcurrants on acute acetaminophen-induced hepatotoxicity in rats. Nat Prod Res 16: 2845-2849.
- Moshaie-Nezhad P, Iman M, Faed MF, Khamesipour A (2018) Hepatoprotective effect of Descurainia sophia seed extract against paracetamol-induced oxidative stress and hepatic damage in mice. J Herbmed Pharmacol 7: 267-272.
- Woolbright BL, Jaeschke H (2017) Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. Journal of Hepatology 66: 836-848.
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH (1988) Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). The New England Journal of Medicine 319: 1557-1562.
- Polson J, Lee WM (2005) AASLD position paper: the management of acute liver failure. Hepatology (Baltimore, Md) 41: 1179-1197.
- Lee KK, Imaizumi N, Chamberland SR, Alder NN, Boelsterli UA (2015) Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology (Baltimore, Md) 61: 326-336.
- Shri JNM (2003) Ginger; It's role in xenobiotic metabolism. ICMR Bulletin 33: 57- 63.
- Oduola T, Bello I, Adeosun G, Ademosun A, Raheem G, et al. (2010) Hepatotoxicity and nephrotoxicity evaluation in Wistar albino rats exposed to Morinda lucida leaf extract. N Am J Med Sci 2: 230-233.
- Makinde JM, Obih PO (1985) Screening of Morinda lucida leaf extract for malarial action on plasmodium berghei in mice. Afr J Med and Med Sc 14: 59-63.
- Tona L, Ngimbi NP, Tsakala M, Mesia K, Cimanga K, et al. (1999) Antimalarial activity of 20 crude extract from nine African medicinal plants used in Kinshasha. Congo J Ethnopharm 68: 193-203.
- Olajide OA, Awe SO, Makinde JM, Morebise O (1999) Evaluation of the anti-diabetic property of Morinda lucida leaves in streptozocin-diabetic Rats. J Pharm Pharmacology 51: 1321-1324.
- Adeneye AA, Agbaje EO (2008) Pharmacological evaluation of oral hypoglycemic and antidiabetic effects of fresh leaves ethanol extract of Morinda lucida benth in normal and alloxan-induced diabetic rats. Afr J Biomed Res 11: 65-71.
- Johari H, Sharifi E, Ansari N, Hosseini M, Amiri F (2003) Effect of hydro alcoholic ginger extracts on the body weight, testis weight and spermatogenesis in male rats undergoing chemotherapy with cyclophosphamide. J Shahid Sadoughi Univ Med Sci 17: 365-374.
- Trease GE, Evans WC (1978) Pharmacology 11th Ed. Bailliere Tindall Ltd, London. pp: 60-75.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Jollow DJ, Mitchell JR, Zampaglione N (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11: 151-169.
- Tahnteng JG, Agboola AO (2012) Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem Toxicol 38: 535-541.
- Green TJ, Sivilotti MLA, Langmann C (2010) When do the aminotransferases rise after acute acetaminophen overdose? Clin Toxicol 48: 787-792.
- Granell S, Gironella M, Bulbena O (2003) Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit Care Med 31: 525-530.
- Saxena M, Saxena J, Nema R, Singh D, Gupta A (2013) Phytochemistry of medicinal plants. JPP 8192: 168-182.
- Adeyemi TOA, Ogboru RO, Idowu OD, Owoeye EA, Isese MO (2014) Phytochemical screening and health potentials of Morinda lucida Benth. IJISR 11: 515-519.
- Igidi OJ, Edene CE (2014) Proximate and phytochemical compositions of Napoleona vogelii hook fruit. Int J Eng Sci 3: 46-51.
- https://pharmareview.files.wordpress.com/2011/10/chemistry-for-pharmacy-students-general-organic-and-natural-product-chemistry.pdf
- Achi NK, Onyeabo C, Ekeleme-Egedigwe CA (2017). Phytochemical, proximate analysis, vitamin and mineral composition of aqueous extract of Ficus capensis leaves in south eastern Nigeria. J Appl Pharm Sci 7: 117-122.
- Akinyemi KO, Mendie UE, Smith ST, Oyefolu AO, Coker AO (2005) Screening of some medicinal plants used in south west Nigerian traditional medicine for anti-Salmonella typhi activity. J Herb Pharmacother 5: 45-60.
- Ajaiyeoba EO, Abiodun OO, Falade MO, Ogbole NO, Ashidi JS, et al. (2006). Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J Ethnopharmacol 112: 470-475.
- Moreibise O, Fafunso MA, Makinde JM (2002) Anti-inflammatory and analgesic property of leaves of Gongronema latifolium, Phytother, Res 16: S75-S77.
- Oleszek, Wieslaw (1993) Allelopathic potential of alfalfa (Medicago sativa) saponins: their relation to antifungal and hemolytic activities. J Chem Ecol 19: 1063-1074
- Waller GR, Jurzysta M, Thorne RLZ (1993) Allelopathic activity of root saponins from alfalfa (Medicago sativa L.) on weeds and wheat, Bot Bull Acad Sin 34: 1-11.
- Igile GO (1995) Phytochemical and biological studies on some constituents of Vernonia amygdalina (composite) leaves. Ph.D thesis Department of Biochemistry, University of Ibadan, Nigeria.
- Waller GR (1989) Biochemical frontiers of allelopathy. Biol Plant 31: 418-447.
- Igile GO, Oleszek W, Jurzysta M, Burda S, Fafunso M, et al. (1994) Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem 42: 2445-2448.
- Alkiyumi SS, Abdullah MA, Alrashdi AS, Salama SM, Abdelwahab SI, et al. (2012) Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules 17: 6146-6155.
- Green TJ, Sivilotti MLA, Langmann C (2010) When do the aminotransferases rise after acute acetaminophen overdose. Clin Toxicol 48: 787-792.
- Freitag AF, Cardia GFE, Da Rocha BA (2015) Hepatoprotective effect of silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evid Based Complementary Altern Med 2015: 1-8.
- Achliya GS, Wadodkar SG, Dorle AK (2004) Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride induced hepatic damage in rats. J Ethnopharmacol 90: 229-32.
- Cavin C, Mace K, Offord EA, Schilter B (2001) Protective effects of coffee diterpenes against aflatoxin B1-induced genotoxicity. Mechanisms in rat and human cells. Food Chem Toxicol 39: 549-556.
- James LP, Mayeux PR, Hinson JA (2003) Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31: 1499-1506
- Dash DK, Yeligar VC, Nayak SS (2007) Evaluation of hepatoprotective and antioxidant activity of Ichnocarpus frutescens (Linn.) R. Br. on paracetamol-induced hepatotoxicity in rats. Trop J Pharm Res 6: 755-765.
- Biswas K, Kumar A, Babaria BA, Prabhu K (2000) Hepatoprotective effect of leaves of Peltophorum pterocarpum against paracetamol induced acute liver damage in rats. J Basic Clin Pharm 1:1.
- Shao HB, Chu LY, Lu ZH, Kang CM (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. IJBS 4: 8-14.
- Prakash J, Gupta SK, Kochupillai V, Singh N, Gupta YK, et al. (2001) Chemopreventive activity of Withania somnifera in experimentally induced fibrosarcoma tumours in Swiss albino mice. Phytotherapy Research 15: 240-244.
- Gini KC, Muraleedhara KG (2010) Hepatoprotective effect of Spirulina lonar on paracetamol induced liver damage in rats. Asian J Exp Biol Sci 1: 614-623.








