Abstract
Introduction: The assessment of cancer patients is of great clinical and radiological complexity. Distinguishing between neoplastic tissue infiltration and indirect lesions is key for correct local staging.
Methods: Discussion of extrinsic (metastatic) peripheral neuropathy and muscular (multifidus) denervation cases, mainly based on MRI.
Results: Precise anatomic correlation between vertebral metastases, neural compression/infiltration and selective muscular denervation.
Conclusion: MRI imaging enables highly accurate differentiation between muscular neoplastic infiltration and edemas resulting from secondary peripheral neuropathy.
Keywords
Muscle denervation; Vertebral metastatic lesion; Neoplastic disease
Introduction
The assessment of cancer patients involves a complex clinical context with conditions that may go beyond the neoplastic disease itself (like hypercoagulable states and changes in patients’ immune status induced by chemotherapy, among others). The dissemination of the primary neoplastic disease to other sites may occur through different pathways – such as the hematogenous and lymphatic pathways, as well as by contiguity – and result in direct complications due to structural involvement, such as pathologic fractures caused by metastases in the musculoskeletal system.
There are, nevertheless, other indirect findings with clinical and radiological repercussions that, although similar, are distinguishable from direct neoplastic lesions. Among the possible findings, changes in the trophic condition of the paravertebral musculature, particularly the multifidus muscle, when in the selective form, are usually associated with peripheral neural dysfunction.
Knowledge of local anatomic repairs, typical imaging appearance of vertebral metastases, and inherent complications of the pathological process is imperative for the follow-up of these patients, as it may guide local and systemic therapy, and prevent mistaken interpretations.
Clinical Case Studies
Case 1
A 58-year-old female patient diagnosed with malignant pulmonary neoplasm a year ago, evolving with intense low back pain for two months and progressive worsening. A PET CT scan showed multiple bone lesions, including some in the lumbar vertebrae and a pulmonary mass in the left upper lung field (Figure 1). MR imaging of the skull and spine revealed multiple brain lesions compatible with metastases, as well as lesions predominantly spread in the lumbar vertebrae (Figure 1). The images showed two lesions in the right vertebral pedicles of L2 and L3, extending to the isthmus, facet joints, and transverse processes. There were also findings in the posterior paravertebral musculature (namely the multifidus muscle) that were discontinuous from the bone lesions. Their imaging characteristics differ from those of neoplastic lesions, being compatible with acute/subacute denervation. Spontaneous discontinuity of the fascia and the posterior lumbar aponeuroses resulted in compartment decompression (Figure 2).
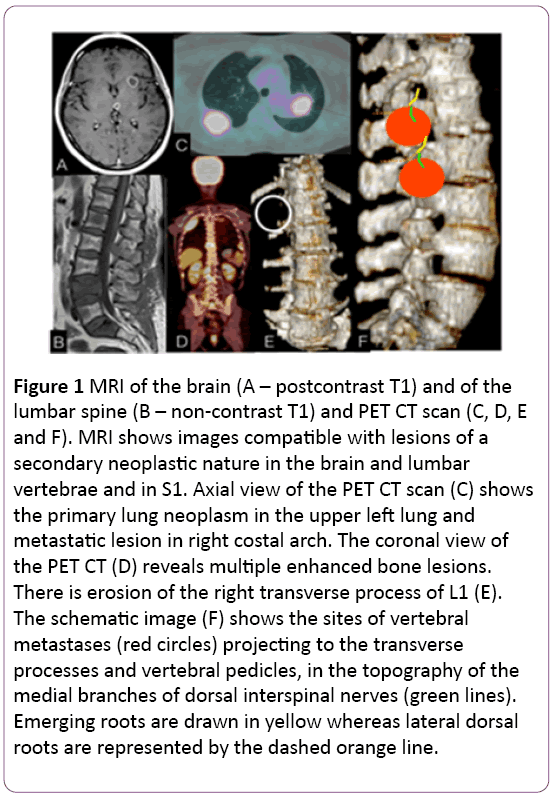
Figure 1: MRI of the brain (A – postcontrast T1) and of the lumbar spine (B – non-contrast T1) and PET CT scan (C, D, E and F). MRI shows images compatible with lesions of a secondary neoplastic nature in the brain and lumbar vertebrae and in S1. Axial view of the PET CT scan (C) shows the primary lung neoplasm in the upper left lung and metastatic lesion in right costal arch. The coronal view of the PET CT (D) reveals multiple enhanced bone lesions. There is erosion of the right transverse process of L1 (E). The schematic image (F) shows the sites of vertebral metastases (red circles) projecting to the transverse processes and vertebral pedicles, in the topography of the medial branches of dorsal interspinal nerves (green lines). Emerging roots are drawn in yellow whereas lateral dorsal roots are represented by the dashed orange line.
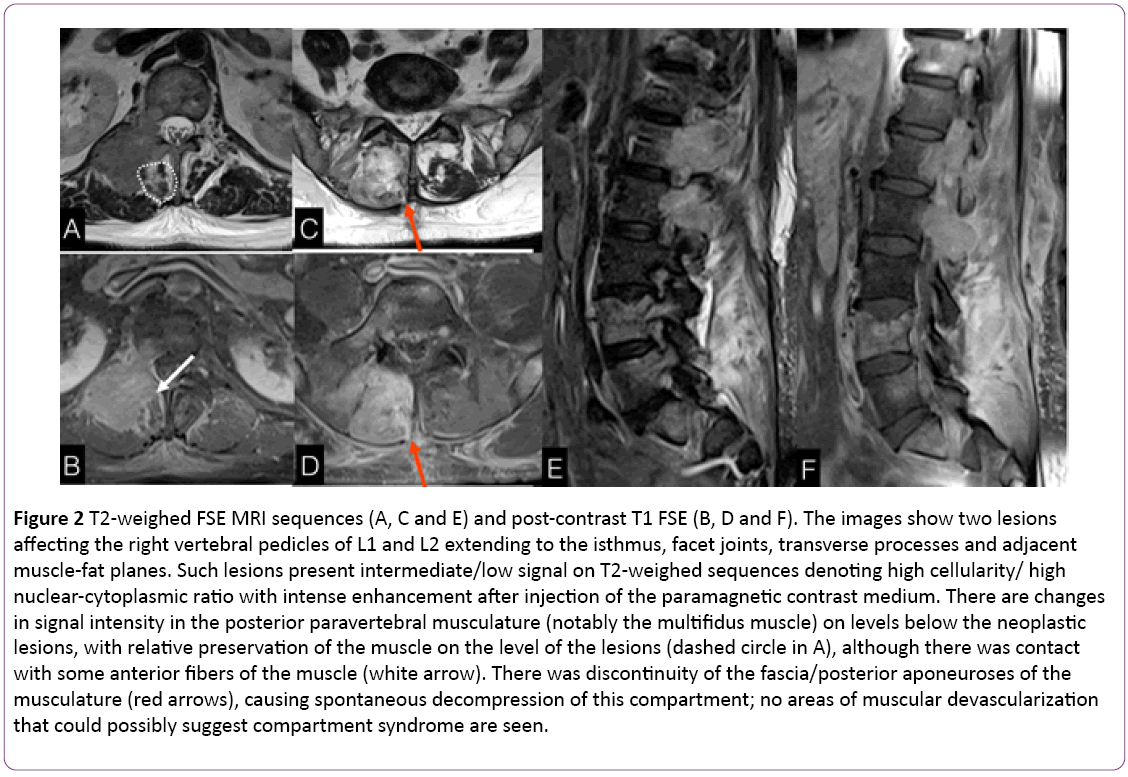
Figure 2: T2-weighed FSE MRI sequences (A, C and E) and post-contrast T1 FSE (B, D and F). The images show two lesions affecting the right vertebral pedicles of L1 and L2 extending to the isthmus, facet joints, transverse processes and adjacent muscle-fat planes. Such lesions present intermediate/low signal on T2-weighed sequences denoting high cellularity/ high nuclear-cytoplasmic ratio with intense enhancement after injection of the paramagnetic contrast medium. There are changes in signal intensity in the posterior paravertebral musculature (notably the multifidus muscle) on levels below the neoplastic lesions, with relative preservation of the muscle on the level of the lesions (dashed circle in A), although there was contact with some anterior fibers of the muscle (white arrow). There was discontinuity of the fascia/posterior aponeuroses of the musculature (red arrows), causing spontaneous decompression of this compartment; no areas of muscular devascularization that could possibly suggest compartment syndrome are seen.
Case 2
A 71-year-old male patient with history of rectum neoplasm subjected to surgical resection followed by chemotherapy and radiation therapy evolved with secondary neoplastic bone lesions two years ago. He has recently complained about low back pain. MRI revealed vast lesions with metastatic characteristics affecting predominantly the left side of the second and third lumbar vertebrae, and overlapping abnormalities in the ipsilateral multifidus muscle discontinuous from the neoplastic lesions and with distinct imaging appearance, suggestive of muscle denervation (Figure 3).
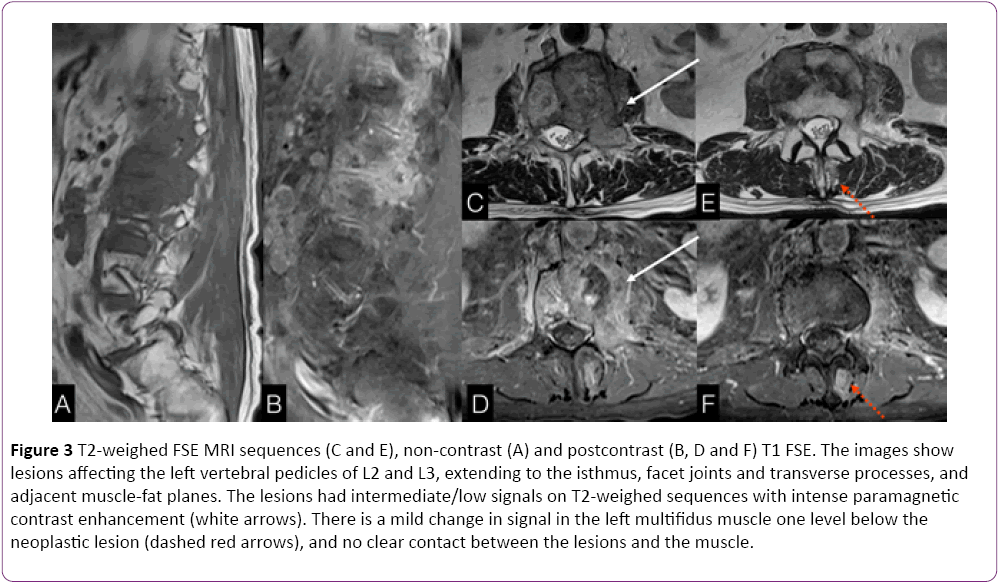
Figure 3: T2-weighed FSE MRI sequences (C and E), non-contrast (A) and postcontrast (B, D and F) T1 FSE. The images show lesions affecting the left vertebral pedicles of L2 and L3, extending to the isthmus, facet joints and transverse processes, and adjacent muscle-fat planes. The lesions had intermediate/low signals on T2-weighed sequences with intense paramagnetic contrast enhancement (white arrows). There is a mild change in signal in the left multifidus muscle one level below the neoplastic lesion (dashed red arrows), and no clear contact between the lesions and the muscle.
Discussion
Vertebral metastatic lesions are the most common neoplasms of the axial skeleton, having as the most frequent primary sites lungs, breasts and prostate [1-3].
The main form of disease dissemination into the vertebral bones is hematogenous, although the lymphatic pathway and tumor infiltration of contiguous structures are also viable possibilities. The vertebral body, and more specifically its posterior contour, is the vertebral segment most commonly infiltrated by neoplasms, followed by the other vertebral body segments, pedicles, and then laminae [4].
Once in the bone marrow, the neoplastic cells promote focal demineralization with trabecular rarefaction and consequent structural fragility through osteoclast activation, which makes the bone susceptible to pathologic collapses, with specific radiologic characteristics that help us distinguish these from benign fractures [5].
Metastatic lesions involving posterior elements, more specifically the isthmus and facet joints, may cause pain resulting either from affected emerging roots, from the possibility of pathologic fractures/misalignment (periosteal distention) or from muscular changes (muscle infiltration by contiguity or secondary denervation of motor neuron branches) [4].
Innervation of the posterior paravertebral musculature, particularly of the multifidus muscles, occurs through medial motor branches originating from the dorsal branches of interspinal nerves, which also give sensitivity to the adjacent vertebral periosteum, facet joint capsule and synovia, as these vertebral and paravertebral structures are functionally related to the interspinal nerve on this level and one level above [6,7]. These branches run inferomedially to the transverse processes (Figure 1).
There are various etiologies that may cause dysfunction of the dorsal medial branches with consequent muscle denervation, such as degenerative conditions (degenerative facet hypertrophy with compression of terminal branches), and neural lesions caused by invasive procedures for treatment of facet arthritis, like radiofrequency ablation [8] and intra/periarticular infiltration [9].
However, neoplastic lesions can promote extrinsic compressions or even neural infiltrations with secondary functional disorders (Figure 1).
Muscle denervation may be caused by intrinsic (primary) or extrinsic peripheral neuropathies. The lower motor neuron syndrome promotes abnormal release of neurotransmitters from the synaptic cleft of motor endplates, leading to intrinsic degeneration of myofilaments, reduction in protein synthesis and, in a few months, loss of 80%-90% of the muscle mass [10].
The diagnosis of this process may be based on clinical history, physical examination (in advanced stages), and electrophysiology studies – ENMG (in earlier stages). Highresolution ultrasound can identify muscular derangement but provides lower spatial resolution. MRI is the imaging method of choice to assess motor endplate disease and consequent denervation. This method also enables diagnosing possible neural impairment in cases of extrinsic lesions [11]. It has advantages when compared to electrophysiology studies as it is a non-invasive method with excellent anatomic resolution and it can provide data that reflects the pathophysiology of the denervation process [12].
On paramagnetic contrast enhanced-MRI studies, the early stages of denervation (acute/subacute) are seen as edema and muscle enhancement (Figures 2 and 3). In advanced stages (chronic denervation) there is muscle atrophy with fatty replacement.
As previously stated, neoplastic lesions in the vertebral pedicles, facet joints and/or transverse processes may cause pain due to periosteal distention, pathologic fractures and, in this specific site, due to infiltration of these dorsal medial branches of the interspinal nerve. In addition to the secondary neuropathic pain, motor dysfunction can also develop. In imaging tests, it is seen as different signals characterizing each stage of the denervation process.
Because of overlapping clinical signs (multifactorial neuralgia) and similar radiological conditions, acute and subacute muscle denervation may be mistaken by neoplastic lesions as both entities present mass effect and are contrast enhancing. Nevertheless, there are nuances, including the site of lesion and signal characteristics on the different MRI sequences that aid in the etiologic differentiation between them, thus preventing overstaging.
Metastatic lesions may share different degrees of certain histologic characteristics (highly aggressive lesions present high cellularity and high nuclear-cytoplasmic ratio). In practice, this translates into lesions with low water content when compared to inflammatory and early denervation processes, both at the cell and at the tissue level (on T2-weighed sequences, metastases have lower signals than acute/subacute denervation).
Anatomic specificities also help to distinguish between both processes. The presence of abnormalities in the multifidus muscle associated with facet/transverse bone metastases one or two levels above the muscular lesion on the same side suggests a close relationship between them (Figure 2).
Having echo-planar-based diffusion imaging available may be helpful as the intrinsic histological characteristics of neoplastic processes described above (high cellularity/high nuclear-cytoplasmic ratio) imply hyperintensity on this sequence [13,14]. These characteristics may also be assessed with quantitative data (apparent diffusion coefficient, ADC) [15].
Conclusion
Accurate cancer staging of advanced cases is imperative to appropriate therapeutic management.
Vertebral metastases may lead to complications with varied clinical and radiological repercussions. Peripheral neural dysfunction resulting from extrinsic compression/tumor infiltration is a possibility and the recognition of the anatomic and functional characteristics is important to try and distinguish direct effects of secondary neoplastic lesions and muscle denervation, reducing false-positive rates in tumor diagnosis and, consequently, improving staging accuracy.
11217
References
- Krishnaney AA, Steinmetz MP, Benzel EC (2004) Biomechanics of metastatic spine cancer. Neurosurg Clin N Am 15: 375–380
- Klimo P Jr, Schmidt MH (2004) Surgical management of spinal metastases. Oncologist 9: 188 –196
- Ries LAG, Melbert D, Krapcho M (2007) SEER Cancer Statistics Review, 1975–2005. National Cancer Institute. Bethesda Md. SEER data submission.
- Georgy BA (2008) Metastatic spinal lesions: state-of-the-art treatment options and future trends. American Journal of Neuroradiology 29: 1605-1611.
- Jung HS, Jee WH, McCauley TR, Ha KY, Choi KH (2003) Discrimination of metastatic from acute osteoporotic compression spinal fractures with MR Imaging. Radiographics 23: 179-187.
- Vancouver BN, Long DM (1979) The anatomy of the so-called ‘‘articular nerves’’ and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg 51:172–177.
- Bogduk N (1983) The innervation of the lumbar spine. Spine 8:286–293.
- Dreyfuss P, Stout A, Aprill C, Pollei S, Johnson B, et al. (2009) The significance of multifidus atrophy after successful radiofrequency neurotomy for low back pain. PM R. 1:719-722.
- Gossner J (2011) The lumbar multifidus muscles are affected by medial branch interventions for facet joint syndrome: Potential problems and proposal of a pericapsular infiltration technique.AJNR Am J Neuroradiol 32: E213.
- Andreisek G, Crook DW, Burg D, Marincek B, Weishaupt D (2006) Peripheral neuropathies of the median, radial, and ulnar nerves: MR imaging features. RadioGraphics 26:1267–1287.
- Su JK, Sung HH, Woo SJ, JaYC, Jae SM, et al. (2011) MR Imaging mapping of skeletal muscle denervation in entrapment and compressive neuropathies. Radiographics 31: 319-332.
- Daniel V (2003) MRI of bone metastases: the choice of the sequence, Cancer Imaging 4: 30–35
- Shah LM, Salzman KL (2011) Imaging of spinal metastatic disease. International journal of surgical oncology.
- Zhou XJ, Leeds NE, McKinnon GC, Kumar AJ (2002) Characterization of benign and metastatic vertebral compression fractures with quantitative diffusion MR imaging. American journal of neuroradiology 23: 165-170.








