Keywords
BiV pacing; Multi-site pacing with two RV leads; Cardiac resynchronisation; Heart failure; Nonresponders
Introduction
Cardiac resynchronization therapy (CRT) is known to reduce morbidity and mortality in severely symptomatic heart failure (HF) patients (NYHA III/IV) with evidence of left ventricular (LV) dyssynchrony [1,2]. Beyond that, this therapy has also recently been proven to be of value in patients with less symptomatic HF (NYHA II) [3]. Efforts to demonstrate the benefits of CRT in wider patient populations continues with variable success including patients with systolic dyssynchrony despite a narrow QRS [4] complex; non HF-indicated patients with bradycardia indications [5] and HF patients with a non left bundle branch block (LBBB) etiology [6]. Despite the advances in CRT and significant research evaluating predictors of CRT response, a substantial percentage of patients still fail to respond to the therapy [7] leading to considerable efforts to evaluate whether the delivery of CRT through optimized lead placement can be tailored to improve outcome. Areas of investigation include targeted left ventricular (LV) lead placement [8], direct His bundle pacing [9] and stimulation of multiple LV sites (“multisite pacing”) using either multiple LV leads [10,11] or multipolar LV leads [12]. Despite acknowledging that right ventricular (RV) apical pacing alone may be detrimental [13], there are limited data evaluating the impact of multiple, concomitant RV pacing sites in combination with LV lead placement. The TriV Resynchronization in Paced Heart Failure with an ICD Indication (or “TriV HF ICD”) trial was designed to demonstrate the feasibility of biventricular pacing in CRT patients comparing different combinations - acute and chronic-of two RV leads and one LV lead.
Methods
Study population
The TriV HF ICD Trial was designed as a single center, randomized feasibility study to prospectively test the acute and chronic hemodynamic impact of three CRT pacing configurations using two RV leads instead of one. Data were collected during implantation, pre-hospital discharge and after 3, 6 and 12 months. The major inclusion criteria was a standard indication for CRT-D (congestive HF despite optimal medical treatment; left ventricular ejection fraction (LVEF) ≤ 35%; NYHA Class III-IV; QRS widths ≥ 120 ms, and PQ ≥ 200 ms or 2nd/3rd degree AV block). Rationale for the latter inclusion criterion was to select those patients most likely to require a high degree of true biventricular pacing. Major exclusion criteria were; any pacemaker indication without the need for implantable cardioverter defibrillator (ICD) back up; previous artificial aortic or tricuspid valve replacement; any indication for revascularization; recent (within the last 3 months) heart surgery or myocardial infarction; hypertrophic obstructive cardiomyopathy and severe kidney disease. Ethical Committee approval was obtained prior to commencement of the trial and all patients were required to provide written informed consent.
Baseline assessment
After patients consented they were included in the trial prior to a baseline clinical assessment including a 6 minute hall walk (6MHW), blood draw for baseline markers including Btype Natriuretic Peptide (BNP), completion of the Minnesota Living with Heart Failure (MLWHF) questionnaire, 12-lead electrocardiogram (ECG) and echocardiogram. Patients also underwent cardiopulmonary exercise (CPX) testing to evaluate peak oxygen transport using a bicycle ergometer protocol and continuous respiratory gas analysis. Peak oxygen consumption (peak VO2) was defined as the mean of values obtained during maximal exercise, starting with 20 W and adding incremental workload of 10 W per minute.
Echocardiographic assessment
Prior to implantation, before pre-hospital discharge and after 12 months follow up, echocardiographic examinations [Vivid™ 7; General Electric; USA] were performed in the randomized configuration to comprehensively assess LV function (LV ejection fraction [LVEF]), left ventricular end diastolic diameter [LVEDD], end systolic diameter [LVESD], mitral regurgitation and cardiac dyssynchrony [aortic and pulmonary pre-ejection delay and 2D-strain measurements of the LV]). 2D-strain recordings and a score method [14] served to quantify dyssynchrony [15].
Implant procedure and pacing protocol
Commercially available, CE marked leads and CRT-D devices were used for the trial. All patients were implanted with a right atrial (RA) pacing lead preferably located in the RA appendage and an RV ICD lead placed in the RV apex. A second active fixation RV lead was implanted using a 3-dimensionally curved lead stylet in the septum in vicinity of the His to avoid a possible variety of different septal lead positions: However direct His-pacing was not the goal and excluded by highoutput- pacing the lead cathodal and anodal with the result of only typical RV septal pacing (broad LBBB) QRS morphology in the electrocardiogramm (ECG) and without QRS normalization. The His area was identified by using a steerable 10 pole EP catheter (Inquiry™; St. Jude Medical; USA).
Avoiding apical positions the LV lead was placed through the coronary sinus in a posterolateral or lateral coronary vein. The aim of lead placement was to reach a long electrical distance of at least 100 ms (using the sense markers of the ICD) between LV and RV leads. An example of the 4-lead configuration is shown in Figure 1. All leads were tested uni and bipolar for sensing, impedance and cathodal threshold and the RV His lead also for anodal threshold.
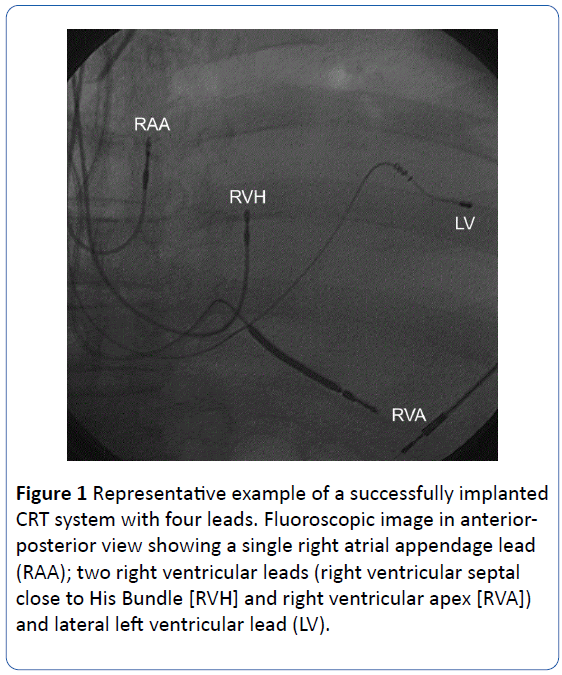
Figure 1: Representative example of a successfully implanted CRT system with four leads. Fluoroscopic image in anteriorposterior view showing a single right atrial appendage lead (RAA); two right ventricular leads (right ventricular septal close to His Bundle [RVH] and right ventricular apex [RVA]) and lateral left ventricular lead (LV).
Pacing configurations
All leads were connected to a standard, commercially available CRT-D device (Promote™ or Atlas™ , St Jude Medical, USA). The LV and RV His leads were connected to the LV port of the CRT-D device using a bipolar (“cathodal-anodalspitting”) Y adapter (VIS 16; Dr. Osypka GmbH; Germany). The tip of the LV lead was connected via the Y-adaptor to the tip electrode (cathode) and the tip of RV His lead was connected to the ring electrode (anode) of the CRT-D LV port. RV Apex and RA leads were connected as standard. Programming of “LV bipolar” leads to cathodal LV pacing and anodal RV pacing (with a higher but since endocardial acceptable threshold). Programming of “LV tip unipolar” results in cathodal LV pacing without RV His pacing. Accordingly three separate pacing configurations were programmed and evaluated in all patients: “BiV Apex” = LV + RV Apex (programmed mode: BiV with LV tip pacing configuration); “BiV His” = LV + RV His (programmed mode: LV only with LV bipolar configuration); and “TriV”=LV +RV Apex+RV His (programmed mode: BiV with LV bipolar configuration). During implantation all three biventricular pacing configurations were hemodynamically tested for acute response. During follow-up beside routine lead testing anodal threshold of RV His lead was performed and effective pacing in the three configurations was again confirmed by a 12-channel ECG. Figure 2 shows a representative electrocardiogramm of one patient.
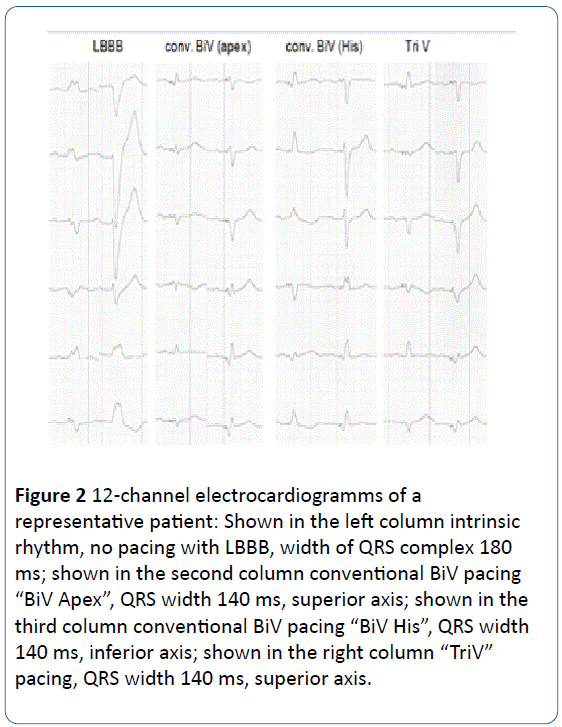
Figure 2: 12-channel electrocardiogramms of a representative patient: Shown in the left column intrinsic rhythm, no pacing with LBBB, width of QRS complex 180 ms; shown in the second column conventional BiV pacing “BiV Apex”, QRS width 140 ms, superior axis; shown in the third column conventional BiV pacing “BiV His”, QRS width 140 ms, inferior axis; shown in the right column “TriV” pacing, QRS width 140 ms, superior axis.
Acute perioperative study
The endpoint of the acute perioperative study was acute hemodynamic changes in LV pressure over time (LV dP/dtmax). Measurements were obtained using a pressure wire™ (Certus™; St. Jude Medical; USA) inserted into the LV via the femoral artery. Intrinsic rhythm measurements were performed, as well as measurements all three pacing configurations using a DDD pacing mode. Pacing was applied at a base rate of 60 beats/min with AV delays between 60 and 200 ms applied in increments of 20 ms, which provided 25 dP/dtmax measurements per patient. In the event that the full 25 dP/dtmax measurements could not be attained, the patient was excluded from the acute analysis. Data were checked and corrected for abnormal events (eg. artefacts, ectopic beats, non-capture) which were excluded from analysis. LV dP/dtmax measurements were averaged over the last 10 stimulated beats and compared to the non-stimulated (intrinsic) beats. Positive acute hemodynamic response for each test configuration was defined as increase in LV dP/dtmax of at least 10% versus intrinsic rhythm.
Randomization
Chronic biventricular pacing configuration programming was determined by randomization. Patients were randomized in a 1:1:1 fashion to one of the three biventricular pacing configurations independent of the acute testing results. The devices were programmed in accordance with randomization group for the chronic phase of the study (after acute hemodynamic measurements until end of follow up).
Chronic study outcome
The primary objective of the study was demonstration of a clinical improvement as indicated by the Packer Heart Failure Clinical Composite Response [16] score (primary endpoint) after 12 months of CRT with randomized pacing configuration.
Briefly, the Packer score classifies each patient as improved, unchanged or worsened using criteria of major clinical events (death or heart failure hospitalizations), NYHA class progression and global self-assessment.
Secondary analyses evaluated during the chronic study phase included changes in CPX capacity; BNP levels, 6MHW changes over time; Quality of Life (MLWHF); ECG assessment; changes in Echo parameters (LVEF, LVEDD, LVESD, mitral insufficiency, degree of dyssynchrony); complications, morbidity and mortality.
Statistical and Data Analysis
Baseline demographics and clinical variables, including medical history, co-morbidities, and NYHA functional class as well as measurements of acute changes in dP/dtmax in the 3 test configurations compared to intrinsic rhythm are presented. Continuous variables are expressed as mean ± standard deviation and were compared using Analysis of Variance (ANOVA). If the assumption for ANOVA was violated, the equivalent non-parametric Kruskal-Wallis test was used. In cases where these comparisons were significant, paired comparisons were carried out and p-values were adjusted using the Bonferroni method. Categorical variables were expressed as frequency and percentage and compared using either the Chi-square test or Fisher’s exact test where applicable. Furthermore, repeated ordinal or continuous measurements within subjects were compared using the nonparametric Friedman’s test while repeated categorical measurements within subjects were compared using the McNemar test.
Results
Between November 2007 and October 2008, 39 patients (85% male, average age 68 ± 7 yrs) were consented, randomized and underwent de novo CRT-D device implantation (87% primary prevention) or upgrade (5 patients). In all patients four leads were implanted according to the study protocol with possible and reliable anodal septum pacing in the para His area during implant testing and followup. Thirteen patients were randomized to each of the three pacing groups (n=39). There were no significant differences in baseline characteristics between groups (despite a trend to a greater proportion of ischemic patients in the TriV group). The majority of patients were NYHA class III (average 3.1 ± 0.5), mean LVEF of 27 ± 7%, average QRS width was 171 ± 24 ms. (87% with left bundle branch block (LBBB), 13% right bundle branch block (RBBB) plus left anterior hemiblock). The major baseline patient characteristics, HF medications at the time of implantation, a brief cardiac history and underlying reasons for HF are summarized in Table 1. All patients were in sinus rhythm at the time of implantation and LV dP/dtmax was tested according to the protocol in all three biventricular configurations in all patients. Due to artefacts or incomplete measurements (defined at least one missing value of the expected 25 dP/dtmax measures for different pacing configurations per patient) five patients were excluded from the acute dP/dtmax analysis (but still included in the chronic phase).
| Baseline Characteristic |
All Patients |
BiV Apex |
BiV His |
TriV |
p |
| N |
39 |
13 |
13 |
13 |
|
| Age [Years] |
68 ± 7 |
69 ± 7 |
66 ± 8 |
70 ± 6 |
0.253 |
| Male [n (%)] |
33(85%) |
11(85%) |
10(77%) |
12(92%) |
0.855 |
| Cardiac disease [n (%)] |
0.323 |
| Ischemic |
26(67%) |
8(62%) |
7(54%) |
11(85%) |
| Non-ischemic |
13(33%) |
5(38%) |
6(46%) |
2(15%) |
| ICD Indication [n (%)] |
0.588 |
| Primary prevention |
34(87%) |
12(92%) |
12(92%) |
10(77%) |
| Secondary prevention |
5(13%) |
1(8%) |
1(8%) |
3(23%) |
| QRS width (ms) |
171 ± 24 |
164 ± 28 |
178 ± 20 |
170 ± 25 |
0.344 |
| Bundle Branch Block [n (%)] |
>0.999 |
| LBBB |
34(87%) |
12(92%) |
11(85%) |
11(85%) |
| RBBB+LAHB |
5(13%) |
1(8%) |
2(15%) |
2(15%) |
| NYHA Class [n (%)] |
0.917 |
| II |
2(5%) |
0(0%) |
1(8%) |
1(8%) |
| III |
30(77%) |
11(85%) |
9(69%) |
10(80%) |
| IV |
7(18%) |
2(15%) |
3(23%) |
2(15%) |
| B-natriuretic peptide [pg/ml] |
862 ± 1155 |
937 ± 736 |
903 ± 1579 |
745 ± 1079 |
0.236 |
| 6 min hall walk distance [m] |
292 ± 135 |
282 ± 135 |
270 ± 141 |
322 ± 134 |
0.482 |
| Peak VO2 [l/min] |
15 ± 4 |
14 ± 4 |
16 ± 3 |
16 ± 4 |
0.29 |
| MLWHF score |
36 ± 20 |
42 ± 23 |
33 ± 18 |
33 ± 19 |
0.526 |
| LV ejection fraction [%] |
27 ± 7 |
27 ± 7 |
27 ± 8 |
26 ± 8 |
0.987 |
| LV end diastolic diameter [mm] |
64 ± 10 |
64 ± 7 |
64 ± 12 |
65 ± 12 |
0.783 |
| LV end systolic diameter [mm] |
56 ± 11 |
53 ± 9 |
56 ± 13 |
58 ± 13 |
0.823 |
| Medication [n (%)] |
| ACE or ARB |
35(90%) |
11(85%) |
13(100%) |
11(85%) |
0.519 |
| ß-Blocker |
30(77%) |
11(85%) |
9(69%) |
10(77%) |
0.89 |
| Diuretic |
32(82%) |
12(92%) |
12(92%) |
8(62%) |
0.17 |
| Aldosterone Antagonist |
23(59%) |
6(46%) |
10(77%) |
7(54%) |
0.355 |
ICD: Implantable Cardioverter Defibrillator; LBBB: Left Bundle Branch Block; RBBB: Right Bundle Branch Block; LAHB: Left Anterior Hemi Block; NYHA: New York Heart Association; MLWHF: Minnesota Living with Heart Failure; LV: Left Ventricle; ACE: Angiotensin Converting Enzyme; ARB: Angiotensin Receptor Blocker
Table 1: Baseline characteristics.
Of the 34 patients with complete acute measurements, 24(71%) demonstrated a positive response in the BiV Apex configuration, 27(79%) in BiV His configuration and 24(71%) in TriV configuration, respectively (p=0.486).
Twenty-nine (85%) patients had at least one configuration in which LV dP/dtmax was increased by more than 10% compared to intrinsic rhythm. Table 2 documents the key outcome measures of LV dP/dtmax responses for different pacing configurations. Each configuration had a significantly improved LV dP/dtmax as compared to intrinsic rhythm (Figure 3).
| LV dP/dtmax improvement vs. intrinsic |
Best configuration (n=34) |
BiV Apex |
BiV His |
TriV |
| |
|
(n=34) |
(n=34) |
(n=34) |
| ≤10% |
14.70% |
29.40% |
20.60% |
29.40% |
| 11-20% |
23.50% |
14.70% |
26.50% |
11.80% |
| 21-30% |
11.80% |
11.80% |
23.50% |
23.50% |
| >30% |
50.00% |
44.10% |
29.40% |
35.30% |
| LV: Left Ventricle; Comparison between CRT configurations: p=0.915 |
Table 2: Acute improvements in LV dP/dtmax (different CRT pacing configuration vs. intrinsic rhythm).
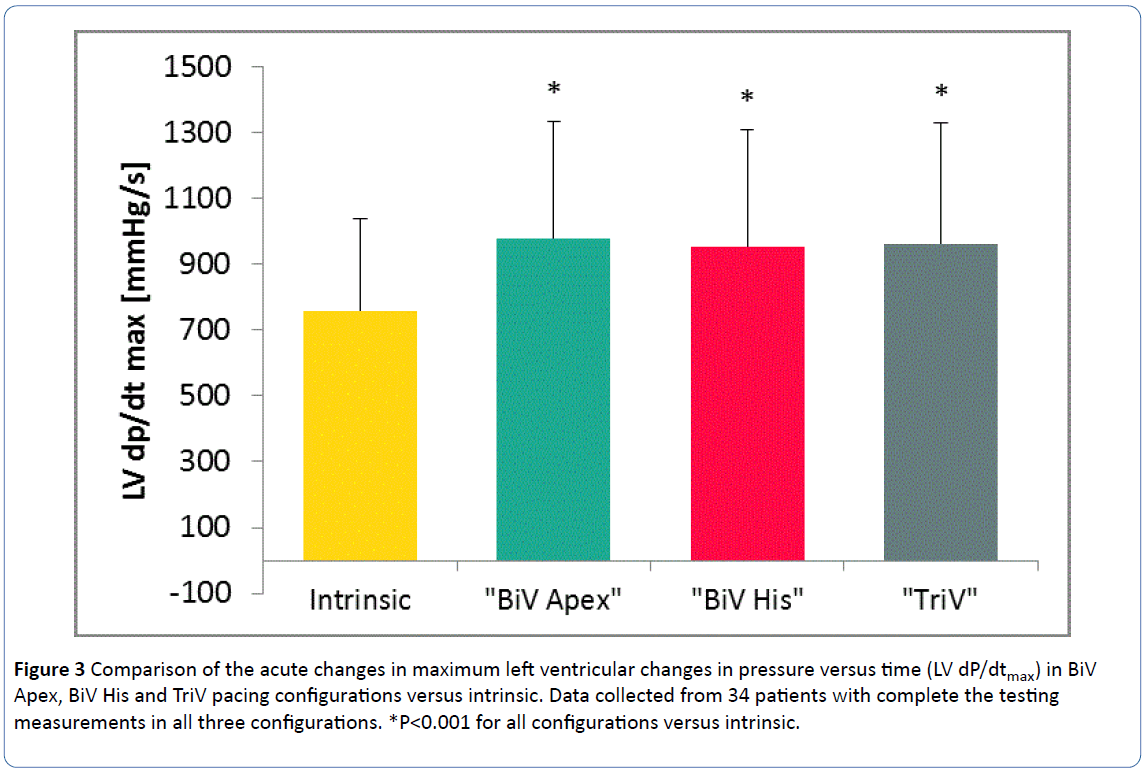
Figure 3: Comparison of the acute changes in maximum left ventricular changes in pressure versus time (LV dP/dtmax) in BiV Apex, BiV His and TriV pacing configurations versus intrinsic. Data collected from 34 patients with complete the testing measurements in all three configurations. *P<0.001 for all configurations versus intrinsic.
There was a non-significant trend for the best vector to be BiV Apex (best in 47% of the patients), versus BiV His (best in 21%), and TriV (best in 32%; p=0.185). Independent of best acute vector, patients were chronically programmed to the vector assigned via randomization.
Of note, in only 10 patients (29%) the randomized pacing configuration matched the best vector as determined by the acute dP/dtmax measurement. The majority of patients who were programmed chronically to their best vector were in the BiV Apex cohort (n=6 versus n=3 BiV His and only n=1 TriV).
Impact of CRT pacing mode on acute LV dyssynchrony
Prior to pre-hospital discharge, echo examination was performed to identify the impact of the three different pacing configurations on global ventricular and LV dyssynchrony within each patient. Significant improvements were observed for all tested CRT pacing modes versus intrinsic rhythm for Delta PED; z-ratio and 2D-score (Table 3). There were no significant differences in the dyssynchrony measurements between the pacing groups.
| |
Intrinsic rhythm |
BiV Apex |
BiV His |
TriV |
p# |
| Delta PED [ms] |
39 ± 36 |
17 ± 17* |
21 ± 15* |
22 ± 17* |
0.82 |
| |
(n=39) |
(n=35) |
(n=35) |
(n=35) |
|
| Z-ratio |
0.75 ± 0.10 |
0.82 ± 0.09* |
0.84 ± 0.10* |
0.85 ± 0.09* |
0.15 |
| |
(n=37) |
(n=37) |
(n=37) |
(n=38) |
|
| 2D-score |
11.0 ± 4.0 |
7.1 ± 3.3* |
8.2 ± 3.5* |
7.9 ± 4.2* |
0.08 |
| |
(n=33) |
(n=35) |
(n=35) |
(n=34) |
|
| PED: Pre Ejection Delay; *p<0.05 vs. intrinsic rhythm; # comparison between pacing configurations. |
Table 3: Acute LV dyssynchrony parameters for different pacing configurations.
Long-term clinical outcome measurements
Two of the 39 randomized patients had missing data at the 12-month follow-up, meaning a total of 37 patients were eligible for determination of the Packer Heart Failure Composite Response endpoint.
Overall, 27(73%) patients were classified as improved at 12 months, 6(16%) worsened and 4(11%) were unchanged. Data for patients with each pacing configuration are shown in Figure 4.
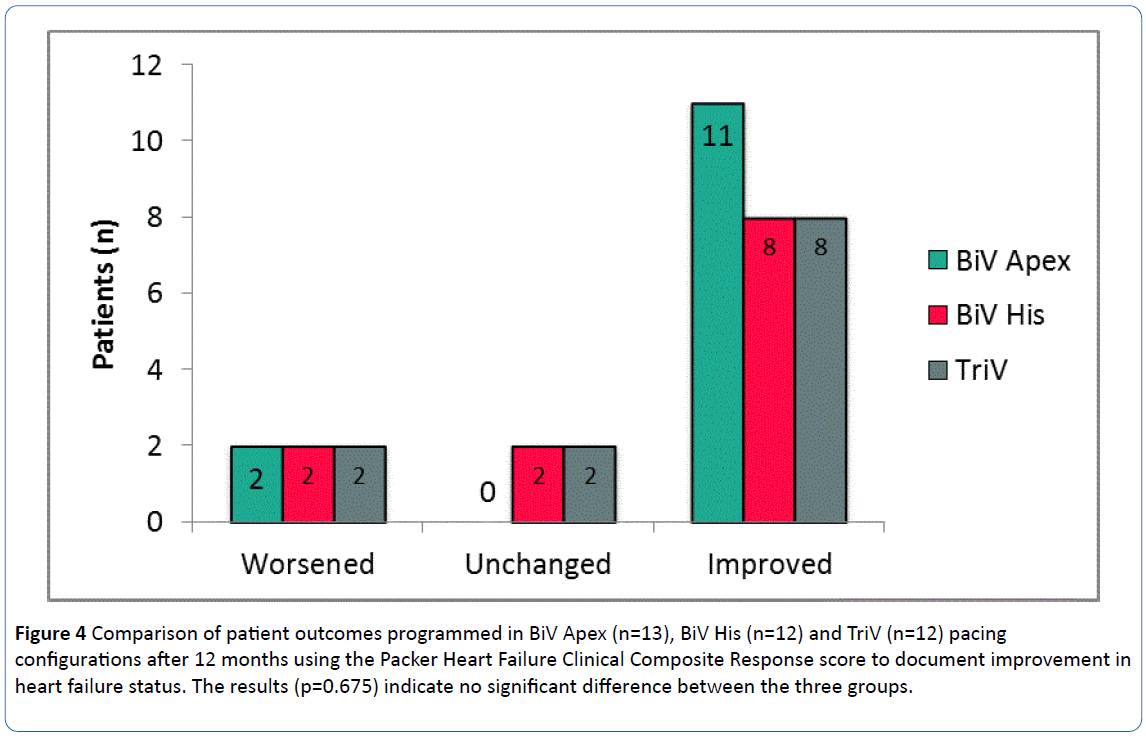
Figure 4: Comparison of patient outcomes programmed in BiV Apex (n=13), BiV His (n=12) and TriV (n=12) pacing configurations after 12 months using the Packer Heart Failure Clinical Composite Response score to document improvement in heart failure status. The results (p=0.675) indicate no significant difference between the three groups.
There were no significant differences in each pacing group with respect to the Packer endpoint (p=0.675) however there was a non-significant trend towards a higher percentage of patients in the BiV Apex group being classified as improved (85% vs. 67% in both BiV His and TriV) which may be reflective of the fact that more patients in the BiV Apex group were programmed to their “best” vector.
During follow up six patients (16%; 2 in each cohort)-all with coronary artery disease-did not sufficiently respond to CRT and had a worsened outcome compared to baseline: Three of the six patients had an LV aneurysm including one patient who was finally treated with a left heart assist device and only one of the patients was programmed to their best vector as determined by acute dP/dtmax testing. Three further patients who had responded to CRT during hemodynamic testing died during follow-up: one was later randomly assigned to the best, another one to the worst tested stimulation vector and the third did not have sufficient data to complete baseline testing. The documented reasons for death were: cerebral infection two months post implant (n=1); septic pulmonary infection and multi organ failure eight months post implant (n=1), and arrhythmic death due to failed ICD therapy one day postimplant (n=1).
At the 12 month follow-up assessment, there was additional evidence of long term benefit for patients in all three pacing configurations, with significant improvements versus baseline in QRS width, 6MHW distance, BNP levels, LVEF, mitral valve regurgitation, CPX workload and peak VO2, NYHA class and Quality of Life. In the paired comparison for the overall study population, there was a reduction in average QRS duration from 171 ± 24 ms to 144 ± 16 ms (p<0.001); an improvement in 6MHW distance from 292 ± 135 m to 405 ± 102 m (p<0.001) and a substantial reduction in BNP from 862 ± 1155 pg/ml to 267 ± 397 pg/ml (p<0.001).
LVEF improved from 27 ± 7% at baseline to 40 ± 12% at 12 months (p<0.001) and mean mitral valve regurgitation grade improved from 1.03 ± 0.72 to 0.56 ± 0.50 (p=0.003). CPX workload improvement (75 ± 26 W at baseline versus 89 ± 26 W at 12 months, p=0.003) was also observed and peak VO2 improved from 15.3 ± 3.6 l/min at baseline to 19.0 ± 5.0 l/min at 12 months (p=0.005).
NYHA class decreased from 3.1 ± 0.5 to 1.7 ± 0.7 (p<0.001) and MLWHF scores improved from 34 ± 19 at baseline to 24 ± 21 at 12 months (p=0.006). There were no significant differences in the outcome measures between patients within the three pacing groups (Figure 5).
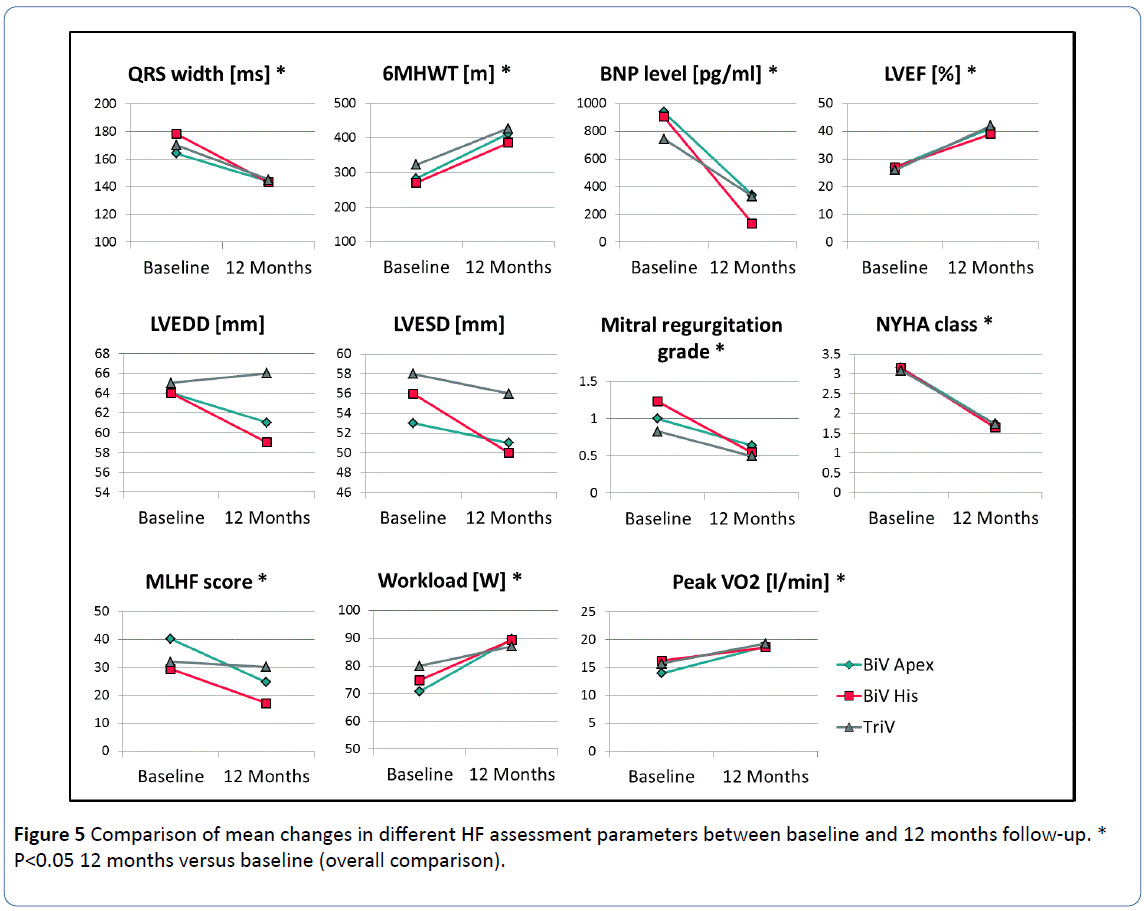
Figure 5: Comparison of mean changes in different HF assessment parameters between baseline and 12 months follow-up. *P<0.05 12 months versus baseline (overall comparison).
There was no significant differences in the outcome measures between pacing groups, indicating that no one group demonstrated incremental benefit over any other.
6MWHT: 6 Minute Hall Walk Test; BNP: B-Naturetic Peptide; LVEF: Left Ventricular Ejection Fraction; LVEDD: Left Ventricular End Diastolic Diameter; LVESD: Left Ventricular End Systolic Diameter; NYHA: New York Heart Association; MLHF: Minnesota Living With Heart Failure.
Despite improvements in the majority of key functional outcomes, there was limited effect in other paired parameters that did not reach significance, including LVEDD (64 ± 10 mm at baseline versus 62 ± 16 mm at 12 months, p=0.062) and LVESD (56 ± 11 mm vs. 52 ± 15 mm at 12 months, p=0.103).
Complications and Device-related Events
During implantation one coronary sinus dissection and pneumothorax was seen respectively. During follow up one ventricular tachycardia ablation and one device related infection leading to device explant and re-implant were reported. In six patients lead related complications occurred and could be corrected by programming or lead revision. Of note, no problems related to the Y-adaptor or the RV His lead were observed.
Discussion
Despite the well documented clinical benefit of CRT in specific HF patient groups, approximately one third of patients remain “non-responders”. The reasons for this lack of response to CRT are not well understood, yet. Currently research on this topic is ongoing, including evaluation of different CRT therapy configurations. Areas of interest include multisite pacing with the use of multiple leads for triple-site pacing, endocardial pacing and multipolar electrodes [8-12,17]. The TriV HF ICD trial was designed to demonstrate the feasibility, safety and effectiveness of three CRT pacing configurations incorporating different RV pacing sites. Since the beginning of CRT anodal stimulation is well known and reported as a possible problem in programming of varying VV delays [18]. Splitting of cathode and anode by Y-adaptor and placing them in different ventricles was the key trick-recently also reported by Yoshida [19] to realize and investigate several pacing configurations with currently available CRT devices in the same patient but has the prize of losing the possibilty of programming different VV intervals (it remains zero).
Our study demonstrates that CRT with one LV and two RV pacing leads is feasible and offers the clinician another option to provide therapy for patients not sufficiently responding to conventional CRT with a single apical RV lead. In our study, approximately 70% of patients showed increased acute LV dP/ dtmax in each configuration (79% response rate in the BiV His configuration and 71% response rate in both BiV Apex and TriV), meaning that there is not difference between RV septal and apical pacing sites in terms of hemodynamical response provided that the LV lead is implanted in an optimal area. More recently the septal CRT study [20] found comparable results and described a non-inferiority of mid-septal location as compared to conventional apical location of the right ventricular lead in CRT patients. But our study remarkably further demonstrates the fact that the total percentage of patients with a significant acute CRT response could be further increased to 85% by simply choosing the best intraoperative, optimized pacing configuration from the three options in each individual patient. A possible cause for this finding could be a better resynchronisation effect by pacing with various BiV- or TriV- configurations obviously important in very dilated hearts which exhibits mostly greater heterogeneity of dyssynchrony and concordantly. In another study [19] patients with larger LV end-diastolic volume prior to CRT were those who profited from Tri-V.
In addition, and despite the fact that the majority (71%) of patients in our study were not chronically programmed to the vector that was demonstrated acutely to be most effective, more than 70% of the patients had an improved chronic outcome as indicated by the 12 month Packer’s Heart Failure Composite score.
Other, longer term determinants of improved chronic outcome such as narrowed QRS width, improved 6MHW distance, improved BNP levels, LVEF, CPX workload, peak VO2, Quality of Life as well as an overall reduction in NYHA class were demonstrated.
Importantly for the feasibility of this therapy concept, the rate of complications related to the more complex implantation procedure was similar to those of standard CRT-D implantations [21]. No RV His lead- or Y-adaptor-related adverse event was observed.
Of note, neither the acute measurements at implant (LV dP/dtmax), nor the echocardiographic measurements at predischarge or the 12 month follow-up visit provided any evidence of additional benefit in the TriV configuration compared to BiV Apex or BiV His configurations. Echo dyssynchrony measurements (compared with no pacing) improved significantly in all three pacing configurations without any significant difference between the groups. Despite the lack of difference between groups, the overall response rates (70-80%) are in line or higher than those previously published [22-24]. It should also be considered that the chronic response rate was potentially an underestimation of the true effectiveness of the three pacing vectors considering that such a small number of patients were programmed chronically to their most effective acute vector. In contrast to these results significant acute beneficially effects (LV dp/dt) of Tri-V pacing were reported by Yoshida et al. [19] but with a third ventricle electrode implanted higher up in the RV outflow tract (RVOT). More recently Rogers et al. [11] and Anselme et al. [25] demonstrated that Tri-V pacing was also associated with significant improvements in clinical and echocardiographic parameters compared with conventional Bi- V pacing. In both studies the third additional ventricular lead was implanted septal-but higher than in our study - above the level of His bundle or in the RVOT or as a second LV lead in antero-lateral position. Overall the response rate in our study suggests that there may be benefit to having the option of multiple alternative vectors during acute dP/dtmax testing since it may improve the likelihood of being able to provide the best CRT-response for each individual patient.
While for the majority of outcome measures a significant, chronic improvement was demonstrated in all pacing configurations including change in QRS width, 6MHW distance, LVEF, CPX workload, BNP level, NYHA class and Quality of Life, measures of LV diameter showed only a nonsignificant trend towards improvement. Such inconsistent outcomes is not unique in heart failure trials and is likely related to the well documented variability in the responses under investigation coupled with the small sample size of each randomized group within the study. Furthermore the much higher percentage of ischemic patients in the TriV group (85% vs. 61% BiV Apex and 54% BiV His) potentially combined with the fact that only one patient was chronically programmed to their “best” pacing vector, may explain the reduced improvement in MLWHF Quality of Life scores in this group versus both the other groups.
Study Limitations
This single center study was designed as a feasibility trial however although every patient was hemodynamically examined in a complex manner during implantation in all three CRT stimulation configurations a major limitation of the study remains the relatively small number of patients that were tested and randomized. Moreover it should be noted that the study was not designed to specifically evaluate dP/dtmax guided CRT therapy since patients were required to remain in their randomization-assigned pacing mode throughout follow-up, independent of the acute hemodynamic test results. Therefore it may be, that the patient outcomes do not reflect the possible full benefit of the therapy. Furthermore, the follow-up period in the present study is limited to 12 months without further data related to longer-term outcomes of the patients. And of course the used Y-adaptor and the anodal stimulation is only an auxiliary tool to be able to realize different biventricular or triple ventricular pacing configurations with commercially available devices. Moreover effective pacing of a septal area close to His could not completely exclude direct His pacing during follow-up since higher output pacing was neccessary in some patients. In the lead configuration utilized in our study it’s not possible to advance the LV activation by VV interval programming, which might further improve CRT response in many cases.
Conclusion
The TriV HF ICD study was designed to demonstrate the feasibility of three CRT pacing configurations with two RV leads. The data presented support that concomitant multisite pacing at the RV apex and septum nearby His bundle is feasible and effective. Choosing the best configuration of the three available allowed 85% of patients to have ≥1 configuration with a significant acute dP/dtmax rise and so may provide an option to improve a patients’ probability of responding to therapy. Further studies are needed to understand which patient has to be implanted in which CRT configuration to gain the best benefit from CRT.
Acknowledgements
The TriV HF ICD study was supported by an unrestricted grant from St Jude Medical GmbH, Germany.
11150
References
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, et al. (2004) Comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350: 2140-2150.
- Cleland J, Daubert JC, Erdmann E, Freemantle N, Gras D, et al. (2005) Cardiac resynchronization-heart failure (CARE-HF) study investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352: 1539-1549.
- Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, et al. (2009) Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am CollCardiol 54: 1837-1846.
- Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, et al. (2013) Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 369: 1395-1405.
- Funck RC, Blanc JJ, Mueller HH, Schade-Brittinger C, Bailleul C, et al. (2006) Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the biventricular pacing for atrioventricular block to prevent cardiac desynchronization (BioPace)’study. Europace 8: 629-635.
- Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, et al. (2010) Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 363: 2385-2395.
- Ypenburg C, van Bommel RJ, Borleffs CJW, Bleeker GB, Boersma E, et al.(2009) Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am CollCardiol 53: 483-490.
- Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O'Halloran D, et al. (2012) Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am CollCardiol 59: 1509-1518.
- Barba-Pichardo R, Sanchez AM, Fernandez-Gomez JM, Morina-Vazquez P, Venegas-Gamero J, et al. (2013) Ventricular resynchronization therapy by direct His-bundle pacing using an internal cardioverter defibrillator. Europace 15: 83-88.
- Leclercq C, Gadler F, Kranig W, Ellery S, Gras D, et al. (2008) A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am CollCardiol 51: 1455-1462.
- Rogers DP, Lambiase PD, Lowe MD, Chow AW (2012) A randomized doubleâ€ÂÂÂÃÂblind crossover trial of triventricular versus biventricular pacing in heart failure. Eur J Heart Fail 14: 495-505.
- Rinaldi CA, Leclercq C, Kranig W, Kacet S, Betts T, et al. (2014) Improvement in acute contractility and hemodynamics with multipoint pacing via a left ventricular quadripolar pacing lead. J Interv Card Electrophysiol 40: 75-80.
- Wilkoff B, Cook J, Epstein A, Greene H, Hallstrom A, et al. (2002) Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 288: 3115-3123.
- Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, et al. (2004) Two-dimensional strain–a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am SocEchocardiogr 17: 1021-1029.
- Kowalski M, Kranig W, Ludorff G, Wolff E, Azem T (2008) Echokardiographischequantifizierung der linksventrikulärenasynchroniemittelseines 2D-strain asynchronie-scores.
- Packer M (2001) Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 7: 176-182.
- Sohal M, Chen Z, Sammut E, Jackson T, Behar J, et al. (2014) New developments in the delivery of cardiac resynchronization therapy: targeted lead placement, multi-site and endocardial pacing. Expert Rev Med Devices 11: 295-304.
- Barold SS, Herweg B, Giudici M (2005) Electrocardiographic follow-up of biventricular pacemakers. Ann Noninvasive Electrocardiol 10: 231-255.
- Yoshida K, Seo Y, Yamasaki H, Tanoue K, Murakoshi N, et al. (2007) Effect of triangle ventricular pacing on haemodynamics and dyssynchrony in patients with advanced heart failure: a comparison study with conventional bi-ventricular pacing therapy. Eur Heart J 28: 2610-2619.
- Leclercq C, Sadoul N, Mont L, Defaye P, Osca J, et al. (2016) Comparison of right ventricular septal pacing and right ventricular apical pacing in patients receiving cardiac resynchronization therapy defibrillators: the SEPTAL CRT Study. Eur Heart J 37: 473-483.
- Ghani A, Delnoy P, Misier AR, Smit J, Adiyaman A, et al. (2014) Incidence of lead dislodgement, malfunction and perforation during the first year following device implantation. Neth Heart J 22: 286-291.
- Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, et al. (2009) Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am CollCardiol 53: 765-773.
- Yu CM, Hayes DL (2013) Cardiac resynchronization therapy: state of the art 2013. Eur Heart J 34: 1396-1403.
- Birnie DH, Tang AS (2006) The problem of non-response to cardiac resynchronization therapy. CurrOpinCardiol 21: 20-26.
- Anselme F, Bordachar P, Pasquie JL, Klug D, Leclercq C, et al. (2016) Safety, feasibility, and outcome results of cardiac resynchronization with triple-site ventricular stimulation compared to conventional cardiac resynchronization. Heart Rhythm 13: 183-189.











