Introduction
Yellow fever is a viral hemorrhagic disease transmitted to humans via the bite of infected female mosquitoes. There are an estimated 200,000 cases of yellow fever in the world annually, resulting in 30,000 deaths [1]. Most of these cases occur in the tropical regions of Africa and South America where the presence of infected mosquitoes is most prominent [1]. Yellow fever infections are generally mild and self-limiting, but severe cases can occur, which are characterized by hemorrhagic fever, shock, renal failure, and hepatic failure [2]. Since there are no known treatments for yellow fever other than supportive care, severe cases often result in death.
Yellow fever vaccine is a live, attenuated virus given as a one-time injection with a recommended re-vaccination interval of 10 years [3]. It is a safe and effective vaccine producing only mild systemic adverse effects, such as low-grade fever, headache, and myalgias in 10 to 30% of vaccine recipients [3] However, in select individuals, yellow fever vaccine-associated viscerotropic disease (YEL-AVD) is a rare illness characterized by signs and symptoms very similar to the actual yellow fever disease, but caused by vaccine virus replicating in multiple organs. This often leads to multi-system organ failure and death. As of July 2009, more than 40 confirmed and suspected cases have been reported worldwide [3]. In the United States, the incidence of YEL-AVD is very low, reported as 0.4 cases per 100,000 doses administered [1]. The following is a case of a 77-year-old male who experienced multisystem organ failure and subsequent death shortly after receiving the live, attenuated 17D strain yellow fever vaccine.
Case
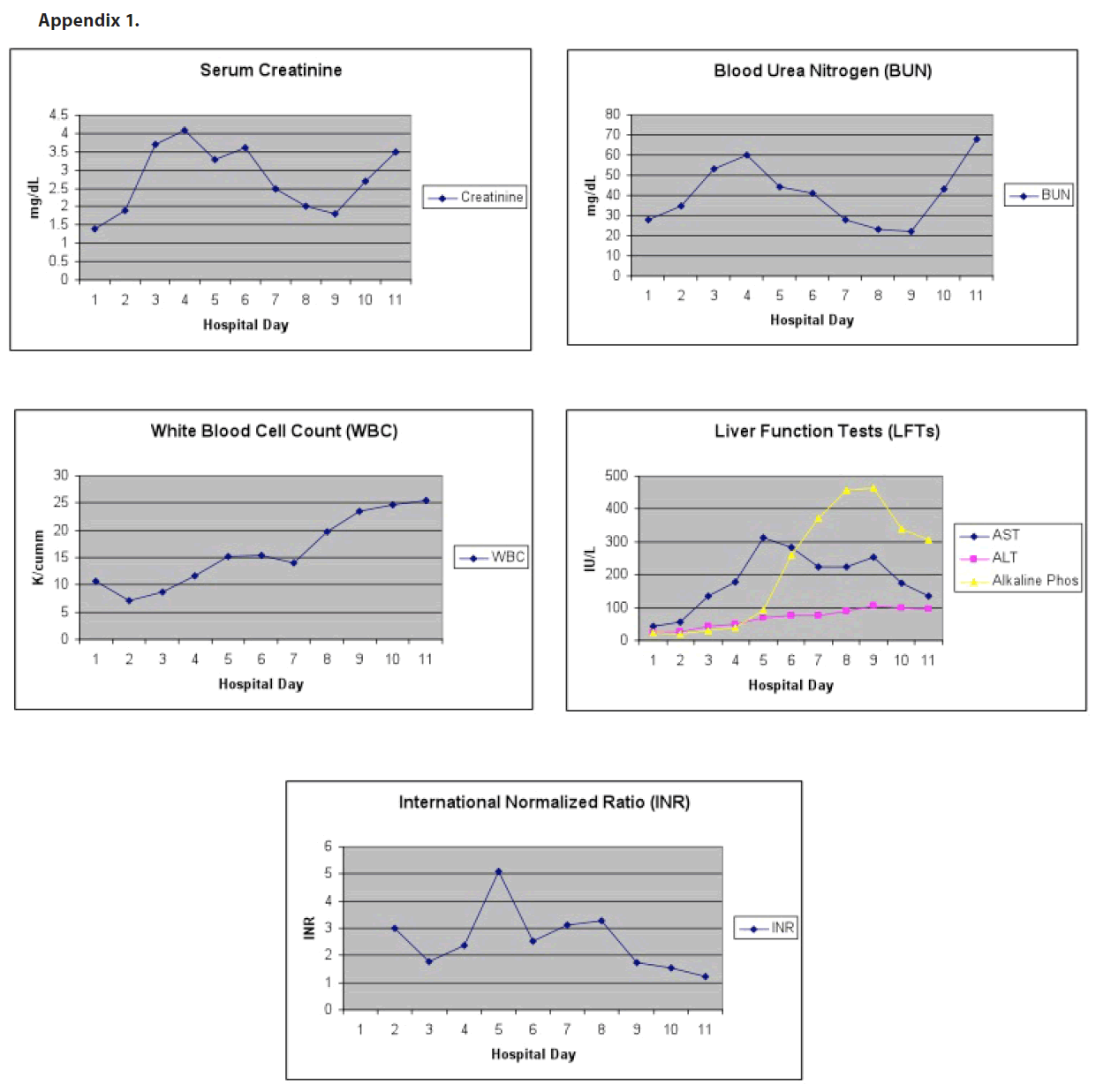
A 77-year-old Caucasian male with a past medical history of hypertension, diabetes mellitus type II, and Factor V Leiden deficiency presented to his primary care physician approximately six days following administration of yellow fever vaccine with complaints of unsteadiness, urinary urgency, and generalized weakness and malaise for the previous two days. The patient received the vaccine in preparation for travel to South America. Physical exam was remarkable only for gait unsteadiness and pale skin color. Multiple labs were drawn, including a comprehensive metabolic panel, complete blood count with differential, prothrombin time and international normalized ratio, thyroid function tests, prostate specific antigen, and urinalysis. The only abnormalities were a slight elevation in serum creatinine from a baseline of 1.0 to 1.4 mg/dL, a urinalysis that revealed moderate blood, and a left shift on complete blood count with greater than 90% neutrophils in the setting of a normal white blood cell count. A diagnosis of urinary tract infection was made and the patient was sent home with a prescription for ciprofloxacin 500 mg twice daily for one week. The following day, the patient presented to the emergency department with complaints of fever, nausea, vomiting, back and abdominal pain, and worsening lethargy. Physical assessment was notable for a temperature of 100.5 degrees. Labs revealed hemoglobin and hemotocrit of 11.7 gms/dL and 35.9% respectively, sodium of 127 mmol/L, potassium of 3.3 mmol/L, serum creatinine of 1.4 mg/dL, and neutrophils of 97%. An infectious process was suspected at this time, and the patient was admitted to the hospital’s general medicine service for further treatment. Upon admission, blood and urine cultures were obtained and empiric broad-spectrum antibiotic coverage was initiated with vancomycin, ceftriaxone, ampicillin, and acyclovir. Over the next 24 hours, the patient was consistently febrile, with temperatures in excess of 103 degrees. He quickly progressed to hemodynamic instability and sepsis syndrome exhibited by tachypnea, tachycardia, hypotension, and mental status changes. He was admitted to the intensive care unit the next day, intubated, and placed on cardiopulmonary support with a working diagnosis of sepsis syndrome of unknown etiology. In order to rule out an infectious process in the central nervous system, a successful lumbar puncture was performed at this time, which was negative. Doxycycline was added to his antibiotic regimen as well as norepinephrine and vasopressin for pressure support. He received continuous intravenous fluids with normal saline for volume resuscitation. The patient began to experience oliguric renal failure requiring continuous veno-venous hemofiltration (CVVH) beginning on hospital day 4 with a serum creatinine of 3.3 mg/dL with a peak serum creatinine of 4.5 mg/ dL eleven days later. Acute hepatitis as evidenced by elevated transaminases was noted beginning on hospital day three and continued throughout hospital course (peak AST of 312 IU/L on hospital day six and peak ALT of 106 IU/L on hospital day 10). Interestingly, total bilirubin throughout hospital course remained within normal limits. On hospital day 5, coagulopathy was noted with an International Normalized Ratio of 5.1, which was unresponsiveness to fresh frozen plasma. The patient’s neurological status declined to a point where he no longer responded to painful stimuli. The patient also experienced acute muscle injury as evidenced by a creatine kinase of 4334 IU/L. CT scans, MRI images, and chest X-rays were obtained and did not elucidate any signs of infection or causes of the patient’s multi-system organ failure. The patient was tested for Ehrlichia, leptospira, Rocky Mountain spotted fever, herpes simplex virus, cytomegalovirus, and human immunodeficiency virus, which were all negative. At this point, yellow fever vaccine-associated viscerotropic disease (YEL-AVD) was suspected. On this same day, cultures and viral PCRs were sent to the Centers for Disease Control and Prevention (CDC) for analysis, which came back as negative for infection and detection of yellow fever virus, respectively. A serum dilution-plaque reduction neutralization test (PRNT) to detect yellow fever neutralizing antibodies was greater than 160 on the day of admission and climbed to greater than 10,000 on hospital day 5. The patient continued to require cardiopulmonary support despite broad spectrum antibiotic, antiviral, and antifungal coverage. He required constant volume resuscitation, which resulted in diffuse anasarca with a weight gain of greater than 55 kilograms. After more than a week of cardiovascular support, permission was granted by the family to withdraw care, and the patient passed away. Autopsy was offered to the patient’s family, but was declined.
Discussion
The live, attenuated yellow fever vaccine was first introduced in the 1930s and has protected millions of susceptible people from contracting the illness. However, when the Vaccine Adverse Events Reporting (VAERS) system was established by the CDC and the United States Food and Drug Administration (FDA) in 1990, rare cases of multi-system organ failure shortly following administration of the vaccine began to appear. First described in the literature in 2001, this condition has since been termed yellow fever vaccine-associated viscerotropic disease (YEL-AVD). YEL-AVD occurs as a result of an atypical, widespread inflammatory response upon administration of the vaccine. Initial symptoms of the disease usually occur two to five days following administration of the vaccine and include fever, nausea, vomiting, and jaundice. Signs of multi-organ failure appear quickly, most notably elevated hepatic enzymes, respiratory failure, blood dyscrasias, and renal failure. In some cases, the attenuated virus can be found in body tissues. Fatality rates with YEL-AVD, even with aggressive treatment, are approximately 53% based on currently available literature [1]. The specific factors that increase a patient’s risk for developing YEL-AVD are not fully known, making it difficult to predict possible reactions.
Previously published reports indicate that advanced age may be a risk factor for developing YEL-AVD. Martin and colleagues reported four cases of YEL-AVD in patients greater than 63 years of age [3]. In each of these cases, the patients presented within five days of receiving the vaccine with major complaints of fever, myalgias, and confusion, similar to the presentation of our patient. Vaccine-like yellow fever virus was isolated in three out of the four patients in their report. Of the four patients described, three of them died. Another similar case was reported by Chan and colleagues in a 56-year-old male [4].
While the case presented in this paper is a geriatric patient, it is important to note that older patients are not the only population at risk for developing YEL-AVD. Gerasimon and Lowry reported a case of an otherwise healthy, 22-year-old female who presented to an emergency department with a four day history of vomiting and diarrhea [5]. Records indicate that she had received the yellow fever vaccine six days prior as standard protocol for military deployment. She rapidly decompensated while hospitalized, and was transferred to the intensive care unit for respiratory failure. Four days after being admitted to the hospital, she went into cardiac arrest and died. Several similar case reports describing YEL-AVD with subsequent death in young patients have been published, with ages ranging from 4 years of age to 26 years of age [6-9].
The patient presented in this case report fits the criteria for YEL-AVD. It is important to note that he had no known drug or food allergies that could have contributed to the described events. Since an autopsy was not performed, a major limitation of confirming YEL-AVD as the true diagnosis was the unavailability of organ tissue for biopsy to confirm presence of the attenuated virus. However, based on the patient’s presentation and hospital course, it is strongly believed that he succumbed to the rare and serious effects associated with receiving the yellow fever vaccine.
Conclusions
Current recommendations from the CDC state that anyone nine months of age or older traveling to or living in areas with risk of yellow fever transmission, especially in South America and Africa, should be vaccinated with yellow fever vaccine to reduce the risk of contracting the illness [1]. However, because severe adverse events are possible following vaccination, physicians should administer the vaccine only to persons truly at risk of exposure to yellow fever.
Appendix 1
276
References
- Yellow fever (2009) World Health Organization. Available : https:// www.who.int/mediacentre/factsheets/fs100/en/. Accessed 18 Mar 2010.
- Yellow fever fact sheet (2007) Centers for Disease Control and Prevention. Available : https://www.cdc.gov/ncidod/dvbid/yellowfever/ YF_FactSheet.html. Accessed 18 Mar 2010.
- Gershman M, Schroeder B, Staples JE (2010) Chapter 2: The pre-travel consultation, travel-related vaccine-preventable diseases. In: Travelers’ Health – Yellow Book. Available: https://wwwnc.cdc.gov/travel/yellow-book/2010/chapter-2/yellow-fever.aspx Accessed 18 Mar 2010.
- Martin M, Tsai TF, Cropp B, Chang GJ, Holmes DA, et al (2001) Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet 358: 98-104.
- Chan RC, Penney DJ, Little D, Carter IW, Roberts JA, Rawlinson WD (2001) Hepatitis and death following vaccination with 17D-204 yellow fever vaccine. Lancet 358: 121-122.
- Gerasimon G, Lowry K (2005) Rare case of fatal yellow fever vaccine-associated viscerotropic disease. Southern Med Journ 98(6): 653-656.
- Struchiner CJ, Luz PM, Dourado I, Sato HK, AguiarSG, et al (2004) Risk of fatal adverse events associated with 17DD yellow fever vaccine. Epidemiol Infect 132: 939-946.
- VasconcelosPFC, Luna EJ, Galler R, Silva LJ, Coimbra TL, et al (2001) Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet 358: 91-97.
- Doblas A, Domingo C, Bae HG, Bohorquez CL, Ory F, et al (2006) Yellow fever vaccine-associated viscerotropic disease and death in Spain. Journ of Clinical Virology :156-158.
- BelsherJL, Gay P, Brinton M, DellaValla J, Ridenour R, et al (2007) Fatal multiorgan failure due to yellow fever vaccine-associated viscerotropic disease. Vaccine 25: 8480-8485.






