Serge Diagbouga1, Christelle Nadembèga2, Zékiba Tarnagda1, Arthur Djibougou3, Noellie Henry3, Rosalie Toé Bicaba3, Mathurin Dembélé4, Adjima Combary4, Souba Diandé4, Martial Ouédraogo5, Guy Aurégan4 and Philippe Van de Perre6
1 Research Institute for Health Sciences, Po Box 7192 Ouagadougou, Burkina Faso
2 Biomolecular and Genetic Laboratory (LABIOGENE), University of Ouaga I Prof Joseph Ki Zerbo, Po Box 364 Ouagadougou, Burkina Faso
3 Centre Muraz, 03 Po Box 390 Bobo-Dioulasso, Burkina Faso
4 National Tuberculosis Program, 01 Po Box 6632, Ouagadougou, Burkina Faso
5 CHU Yalgado Ouédraogo, 03 Po Box 7022, Ouagadougou, Burkina Faso
6 UMR 1058, INSERM/University Montpellier/EFS and CHU Montpellier, department of bacteriology and virology, Montpellier, France
*Corresponding Author:
Dr Serge Diagbouga
Research Institute for Health Sciences
Po Box 7192 Ouagadougou, Burkina Faso
Tel: + 226 70231796, +226 75701616
E-mail: diagbouga.serge@gmail.com
Received Date: January 10, 2017; Accepted Date: March 01, 2017; Published Date: March 07, 2017
Citation: Diagbouga S, Nadembèga C, Tarnagda Z, Djibougou A, Henry N, et al. Mycobacterium bovis Prevalence in Humans Does Not Differ Between Regions in Burkina Faso. Arch Clin Microbiol. 2017, 8:1. doi: 10.4172/1989-8436.100035
Copyright: © 2017 Diagbouga S, et al . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Tuberculosis; Mycobacterium bovis prevalence; Anti-tuberculosis drugs; Burkina Faso Sahel; Central; West Regions
Introduction
In humans, tuberculosis is a pulmonary and systemic disease caused by species of the M. tuberculosis complex, mainly Mycobacterium tuberculosis. It spreads from person to person by aerial transmission of droplets with a diameter of 1-5 μm. Several factors determine the probability of transmission. They include (i) infectivity of source patients - smear positive sputums for acid fast bacilli (AFB) or caves on thoracic radiographs being strongly associated with infectivity, (ii) the susceptibility of contact subjects, (iii) the duration of exposure, (iv) the in which exposure occurs - small, poorly ventilated spaces present the highest risks. Tuberculosis is the leading cause of death attributable to a single infectious agent, with 10.4 million new cases and 1.4 million deaths in 2015 [1]. Knowledge of the responsibility of Mycobacterium bovis in human tuberculosis is longstanding [2]. It would occur in parallel with tuberculosis in livestock and a human-to-human transmission of M. bovis, albeit rare, is possible. There are also consistent findings on the relative frequency of the location of tuberculosis due to M. bovis in humans. Where the consumption of unpasteurized milk is frequent M. bovis strains are found in the mesenteric and cervical ganglias. locations were more frequently described in subjects living in close contact with infected cattle [3]. In Europe, before systematic slaughter of infected cattle and pasteurization of milk, human tuberculosis due to M. bovis represented one to 10% of all human tuberculosis cases: 1.5% in France [4] and 10% in England [5]. In 1995, 38 cases of human tuberculosis due to epizootic M. bovis were recorded in France [6]. The localization of tuberculosis was known for 32 cases: 17 (53%) in the lung, 14 (44%) extrapulmonary and one (3%) had a mixed localization. In developing countries, especially in West Africa, tuberculosis called "Sogo-Sogo Gwé" (the "white cough" in the Bambara language), was always dreaded by the populations. In Burkina Faso, epidemiological surveys carried out between 1950 and 1980 showed that high levels of tuberculosis were detected at slaughterhouses. Indeed, the percentage of seizures for tuberculosis at Bobo-Dioulasso slaughterhouse was 19.5% in some period of 1953 [7]. There was also a correlation between the rates of seizures for tuberculosis in slaughterhouses and the rate of positive reactions rates on animals following humanobovine tuberculin injection. In villages where highest rates of positive tuberculin skin test reactions in children were showed, the rates of positive reactions in animals were also very high. Forty years after these major epidemiological surveys data in Burkina Faso, 1996, health statistics and previous years' estimates suggested that there were more cases of human tuberculosis in the Sahel region (Gorom-Gorom and Dori) than in the other regions of the country, particularly those of the center (Ouagadougou) or and the west (Bobo-Dioulasso) regions. One of the hypotheses put forward to explain this situation was the high breeding activity practiced by the populations in the Sahel region which could expose them to M. bovis tuberculosis (by ingestion of unpasteurized milk, or following a closed contact with infected cattle).
The management of patients with M. bovis disease is critical because some strains of M. bovis are known to be less sensitive than M. tuberculosis to anti-tuberculosis drugs [8]: paraaminosalycilic acid, streptomycin, cycloserine and isoniazid; This last antibiotic is among the most important one in the drug regimen of human tuberculosis in Burkina Faso.
The main objective of the study was to analyze the relative frequency of M. bovis among mycobacterial strains isolated from positive culture tuberculosis cases. The second objective was to study the chemosensitivity of all isolated strains to the antituberculosis drugs used by Burkina Faso's National Tuberculosis Program (NTP).
The analysis of drug resistance, against M. bovis in this study, became a therapeutic emergency since the findings of nosocomial transmission of multidrug-resistant tuberculosis among HIV-positive subjects [9-11].
Material and Methods
Type and sites of the study
A prospective study was carried out from January to December 1998 in Sahel (Gorom-Gorom, Dori), Central (Ouagadougou) and Western (Bobo-Dioulasso) regions in Burkina Faso (Figure 1). Tuberculose screnning centers in these regions transfered to Center Muraz, Bobo-Dioulasso the sputum of newly TB infected person with a positive smear.
Figure 1: Map of Burkina Faso showing the three studied regions (Sahel, Center and West).
Description of the study
The implementation of this study required a strong collaboration between Gorom-Gorom Medical Center, Dori Regional Hospital Center (for samples collection from the Sahel region), the NTP Ouagadougou (for samples collection from the Central and Western regions) and the Center Muraz, Bobo- Dioulasso (for bacteriological analyzes). The main stakeholders benefited from theoretical and practical training adapted to their missions: direct microscopy training for laboratory technicians and training on epidemiology, , clinical and bronchial fibroscopy of adults and children for physicians and nurses in charge of patients sorting.
Optimization of laboratory operational procedures
The method of decontamination with sodium hydroxide was used to decontaminate pathological products before AFB culture. The Dubos medium with cetyl pyridinium chloride was used to conserve bacilli in pathological products for delayed mycobacteriological analysis over several days. The sputum sample collected in well identified vials were then transported in a cold cool box (+4°C) to the Centre Muraz mycobacteriology Laboratory in Bobo-Dioulasso.
Isolation of strains of tuberculous mycobacteria
The sputum samples received in the Center Muraz, Mycobacteriology Laboratory, were immediately centrifuged at 3000 rpm for 15 minutes. The pellet was used for smear, then decontaminated by the Petroff method and inoculated three different solid media (Loweinstein Jensen (LJ), Loweinstein Jensen without glycerine and Loweinstein Jensen supplemented with pyruvate to stimulate growth of M. bovis). The cultures incubated at 37°C were examined after 48 hours and then weekly for three months.
A special effort has been made to investigate all M. bovis specimens taking into account its specific morphology and growth characteristics in order to allow its isolation and identification. The growth of M. bovis was slow or incomplete on media usually used for M. tuberculosis [12]. On LJ medium, the colonies of M. bovis grow very slowly (more than a month for isolation). Glycerin has an adverse effect on the growth of M. bovis. Conversely, pyruvate at a concentration of 0.3 to 0.5% stimulated its growth but inhibited the action of isoniazid, cycloserine and thiophene-2-carboxylic acid and decreased the activity of kanamycin and ethambutol [12,13]. On the contrary, pyruvate potentiated the activity of ethionamide [12]. M. bovis did not produce niacin and did not reduce nitrates to nitrites; it was resistant to pyrazinamide, but sensitive to 5 μg of T2H/ml.
Mycobacterium strains identification and antibiograms
The identification of the tuberculosis complex was carried using basic standard criteria: bacilli growth time, colonies appearance and biochemical tests results: niacin test, nitrate reductase, urease. Growth with pyrazinamide or hydrazide of thiophene carboxylate (TCH), or D-cycloserine was also used as an identification criterion.
The antibiograms were carried out using the principle of the proportions method defined by Canetti, Rist and Grosset [14], on a single strain isolate for each patient. The antibiotics used by the National Tuberculosis Program of Burkina Faso were tested: isoniazid (INH), streptomycin (SM), rifampicin (RFP), ethambutol (EMB) and pyrazinamide (PZA). The interpretation took into account the usual resistance criteria, ie 1% for all drugs. Three bacillary dilutions were seeded from the bacillary suspension at 1 mg/mL of 10-1, 10-3, 10-5 bacilli. Each dilution was seeded on 2 control tubes without antibiotics for harvesting the total population and on one tube of each concentration retained for each antibiotic. The tube were incubated at 37°C and examined after 48 hours once a week and then weekly for 3 months.
Quality control and quality assurance
The standard strain H37Rv was used as a reference strain for the internal quality control of LJ culture media and antituberculosis drugs. The National Reference Laboratory of Cotonou, Benin, provided external quality control. A concordance threshold of 90% or greater was established for isoniazid and rifampicin.
Ethical considerations
The tuberculosis national program of Burkina Faso Health department obtained all necessary authorizations for clarification of high screening rate of tuberculosis in the Sahel region of the country.
Statistical analysis
The chi-square (p<0.05) exact test was used to compare tuberculosis proportions in the three regions.
Results
A total of 460 sputum samples were sent to the Centre Muraz's Mycobactetiology Laboratory, from which: 132 specimens from the Sahel region (Gorom-Gorom Medical Center, 80 sputum specimens; and the Regional Hospital Center of Dori, 52 sputum specimens); the Tuberculosis Centers of Ouagadougou and Bobo-Dioulasso sent 186 and 142 specimens respectively. Laboratory results were available for 317 specimens (68%). Table 1 shows the distribution of mycobacterial strains isolated from patients’ sputum according to the region of the country: Sahel (Gorom + Dori), Center (Ouagadougou) and West (Bobo-Dioulasso).
| Cultures |
SAHEL |
CENTER |
WEST |
TOTAL |
| Gorom- Gorom |
Dori |
Ouagadougou |
Bobo-Dioulasso |
|
| M. tuberculosis |
30 |
18 |
109 |
65 |
222 |
| M. bovis |
2 |
0 |
3 |
2 |
7 |
| Non tuberculousmycobacteriae |
0 |
0 |
0 |
2 |
2 |
| Negative cultures |
17 |
15 |
23 |
31 |
86 |
| TOTAL |
49 |
33 |
135 |
100 |
317 |
Table 1: Results of cultures and identification of M. tuberculosis complex in sputum according to the studied region.
Two strains of atypical mycobacteria have been identified among Bobo-Dioulasso patients. No strain of Mycobacterium africanum was identified during this study.
The relative frequency of Mycobacterium bovis is shown in Figure 2. Four percent of strains isolated from Sahel region (Gorom + Dori) were M. bovis; with 6.2% for Gorom-Gorom. M. bovis rates for Central and West regions were 2.6 and 2.9%, respectively, but the differences were not statistically significant (p ≥ 0.05).
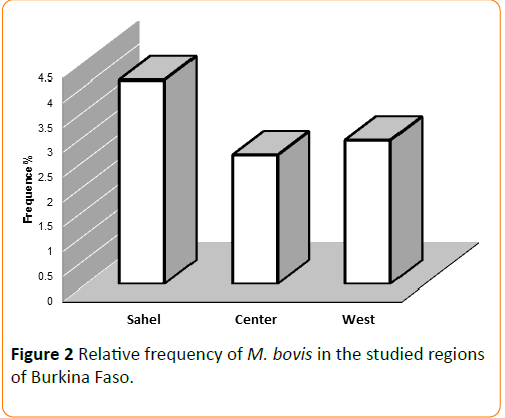
Figure 2: Relative frequency of M. bovis in the studied regions of Burkina Faso.
A total of 45 strains were completely tested for antibiotics (Table 2), from which 31 were susceptible to the main antibiotics (INH, MS, RFP, EMB) and 13 strains showed a single and/or combined resistance; one strain was resistant to both isoniazid and rifampicin.
| |
Ouagadougou |
Bobo-Dioulasso |
Dori |
Gorom-Gorom |
TOTAL |
| INH |
6
2 Single
4 Combined |
4
2 Single
2 Combined |
1
Combined |
0 |
11
4 Single
7 Combined |
| SM |
2
Combined |
3
1 Single
2 Combined |
1
Combined |
0 |
6
1 Single
5 Combined |
| RFP |
3
Combined |
0 |
1
Combined |
0 |
4
Combined |
| EMB |
1
Combined |
0 |
1
Combined |
0 |
2
Combined |
| PZA |
3
Combined |
1
Single |
1
Combined |
0 |
5
1 Single
4 Combined |
INH : isoniazide, SM : streptomycine, RFP : rifampicine, EMB : ethambutol, PZA : pyrazinamide
Single: resistance to one antibiotic, Combined: resistance to at least two antibiotics. 18 strains sensitive to antibiotics (INH, MS, RFP, EMB, PZA), 13 strains sensitive to 4 antibiotics (INH, SM, RFP, EMB), 13 strains with resistance to a single antibiotic or combined |
Table 2: Resistance to the main anti-tuberculosis drugs observed by region.
Discussion
Very few studies in Africa were focused on the ecology of mycobacteria or the prevalence of M. bovis tuberculosis. According to the Tuberculosis Technical Guide of the National Tuberculosis Program of Burkina Faso, the management of tuberculosis requires microscopic examination of AFB sputum. These characteristics are common to the M. tuberculosis complex. Very rarely NTPs have been interested in describing the species involved in human tuberculosis. However, in the years 1996, the NTP in Burkina Faso observed high rates of tuberculosis in the province of Gorom-Gorom compared to the other provinces wished to understand the reasons. Gorom- Gorom is a breeding province, and as consequence, some populations living there are likely to develop human tuberculosis due to M. bovis by ingestion of unpasteurized milk or being in closed contact with infected cattle. In order to determine the frequence of M. bovis in human tuberculosis case, this study attempted to isolate and identify M. bovis strains in culture positive recruited consecutively in Gorom-Gorom and Dori (Sahel region) compared to the central region of Ouagadougou and the western region of Bobo-Dioulasso. Our results showed that the prevalence of M. bovis was 6.2% (2/30) in Gorom- Gorom, 0% (0/18) in Dori, 2.6% (3/109) in Ouagadougou and 2.9% (2/65) in Bobo-Dioulasso.
These results showed that there was a trend toward higher prevalence of M. bovis disease in the Northern part of the country. However the prevalence of M. bovis disease was not statistically significant between the regions (p ≥ 0.05). The fact that the difference is not significant is probably linked to the small sample size and the low power of this study to identify a difference. These prevalences remain low compared to our expectations. And very strangely, in Dori, a city close to Gorom- Gorom with a similar population and environment we did not identify any strain of M. bovis. A low prevalence of M. bovis has been reported by Rey et al., where only one strain of M. bovis (1.8%) was identified from 55 strains of mycobacteria isolated from sputum samples obtained in the Sahel department. The other strains were M. tuberculosis (27/55) and M. africanum (27/55) [15]. Paradoxically, in the same period, the prevalence of bovine tuberculosis (tuberculin test positive) in cattle at Dori (Sahel region) was 6% [16]. The transhumance of livestock practiced by the breeders may play a role in exposure to M. bovis at distance from breeding areas in Burkina. Indeed, the Figure 3 showes the major transhumance routes in the country (from North to West) and between the neighboring countries (from Mali and Northen Burkina Faso to Ivory Coast and Ghana) [17,18].
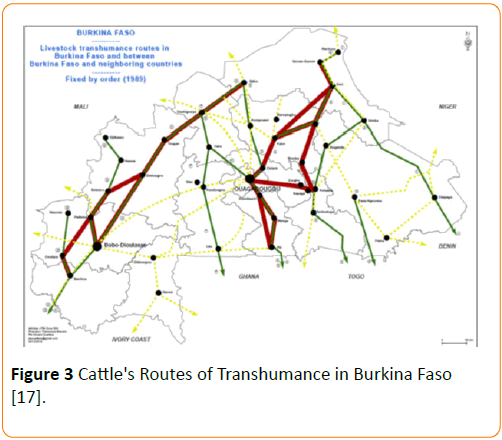
Figure 3: Cattle's Routes of Transhumance in Burkina Faso [17].
The prevalence of tuberculosis due to M. bovis is relatively low in some African countries. Indeed, in Nigeria and Ethiopia, M. bovis prevalence of 0.2% and 0.4% of were respectively found on mycobacterial cultures [19,20]. In Europe and the US, prevalences of 1.4%, 2%, 3% and 7% were found in the Netherlands, France, Ireland and San Diego respectively [21-25].
The transmission of M. bovis from livestock to human is possible by ingestion of contaminated milk or by exposure to diseased animals. A study in Mexico showed a high prevalence of pulmonary latent tuberculosis among workers exposed to infected livestock and increases the risk for those working in non-ventilated areas. Thus, a high prevalence of tuberculosis due to M. bovis in areas with high bovine tuberculosis prevalence would be expected. These results are in accordance with those founded in Ethiopia where between 2000 and 2006, four cases of M. bovis were detected after molecular typing of nearly 1000 isolates and three of these patients (75%) had known regular animal exposure [20]. In China, however, a prevalence of 0.34% of M. bovis was found in a region with a high prevalence of bovine tuberculosis [26].
Inter-human airborne transmission in the contribution of spread of M. bovis was controversial. The predominant idea is that inter-human transmission of tuberculosis due to M. bovis is rare and appears to occur only in people who are particularly susceptible to tuberculosis such as those infected with HIV [9-11,27]. However, previous reports of clusters of cases with social links and molecular epidemiology and evidence from surveys of contact subjects from M. bovis pulmonary tuberculosis patients suggested that inter-human transmission occurs even in non-immunocompromised individuals. Several cases of inter-human transmission of tuberculosis to M. bovis was well documented [28-32].
M. bovis is naturally resistant to pyrazinamide, the molecule of choice in the treatment of human tuberculosis. M. bovis strains can mutate and lead to multi-resistant tuberculosis and these mutants can maintain (original) pathogenicity for humans. Possibly, livestock and other animals may be re-infected by M. bovis bacilli including multidrug resistant strains.
In our study, 31/45 strains of M. tuberculosis isolated were sensitive to the main anti-tuberculosis drugs, 13 strains showed resistance alone and/or combined and one strain was multiresistant to isoniazid and rifampicin.
The main limitations of our study were the lack of a joint survey in cattle to determine the prevalence of bovine tuberculosis in the same period and a study on cow milk, the small sample size, the lack of information on diagnosed tuberculosis patients, including living conditions and their occupational activities (Breeders, shepherds) and the absence of antimicrobial susceptibility testing on M. bovis isolated strains. A previous study undertook by our group on 64 samples milk collected in Bobo-Dioulasso shown a prevalence of mycobacterium of 53% [33].
Conclusion
It seems that there is a relatively "weak" circulation of M. bovis throughout the country, and more in the Sahel region than elsewhere. There is a trend toward a North-South-West prevalence gradient, suggesting persistent exposure of individuals to M. bovis due to breeding and potentially to transhumance activities.
It is necessary that a larger scale study be undertaken throughout the country and over a longer period to include a larger number of patients.
Acknowledgments
We would like to express our deep gratitude to the Regional Director of Health of Dori and to the Chief Medical Officers of the Medical Center of Gorom-Gorom who have succeeded each other in our various passages for helping us to conduct our study. Our thanks also to UNICEF Ouagadougou, who kindly made the coolers available to us. Finally, this study would not be carried out without the funding allocated by the Tuberculosis Mobilizer Fund. Our sincere thanks go to the Health and Development Branch of the French Ministry of Cooperation (in particular to Dr. Christian Marchal and Mr. Jean Marie Bruno.
18435
References
- Schmiedel A (1968) Development and present state of bovine tuberculosis in man. Bull Int Union Tuberc 40: 5-32.
- Robinson P, Morris D, Antic R (1988) Mycobacterium bovis as an occupational hazard in abattoir workers. Aust N Z J Med 18: 701-703.
- Gernez-Rieux C, Gervois M, Tacquet A (1954) [Role of the bovine tubercular bacillus in human pathology]. ActaTubercBelg 45: 357-397.
- Griffith AS (1937) Bovine tuberculosis in man. Tuberculosis 18: 529-543.
- Robert J, Boulahbal F, Trystram D, Truffot-Pernot C, De Benoist AC, et al. (1999) A national survey of human Mycobacterium bovis infection in France. Int J Tuberc Lung Dis 3: 711-714.
- Regnoult MG (1963) [Animal tuberculosis in West African territories of french expression]. Rev Pathol Gen PhysiolClin 63: 1093-1115.
- Bobadilla-del Valle M, Torres-González P, Cervera-Hernández ME, Martínez-Gamboa A, Crabtree-Ramirez B, et al. (2015) Trends of Mycobacterium bovis isolation and first-line anti-tuberculosis drug susceptibility profile: a fifteen-year laboratory-based surveillance. PLoSNegl Trop Dis 9: e0004124.
- Blazquez J, de Los Monteros LE, Samper S, Martin C, Guerrero A, et al. (1997) Genetic characterization of multidrug-resistant Mycobacterium bovis strains from a hospital outbreak involving human immunodeficiency virus-positive patients. J ClinMicrobiol 35: 1390-1393.
- Havlir DV, Barnes PF (1999) Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 340: 367-373.
- Ramos A, Noblejas A, Martin T, Varela A, Daza R, et al. (2004) Prolonged survival of an HIV-infected patient with multidrug-resistant Mycobacterium bovis infection treated with surgical resection. Clin Infect Dis 39: 53–55.
- Simmer P, Stenger S, Richter E, Brown-Elliot R, Wallace R, et al. (2015) Mycobacterium: Laboratory Characteristics of Slowly Growing Mycobacteria. In:Jorgensen J, Pfaller M, Carroll K, Funke G, Landry M, et al. (ed), Manual of Clinical Microbiology. ASM Press pp:570-594.
- Grange JM, Yates MD, de Kantor IN (1996) Emerging and other Communicable Diseases, Surveillance and Control.
- Canetti G, Rist N, Grasset J (1963) Mesure de la sensibilité du bacilletuberculeux aux drogues antibacillairespar la méthode des proportions. Rev TubercPneumol: 217-272.
- Rey J, Villon A, Saliou P, Gidel R (1979) Les rapports entre la tuberculosehumaine et la tuberculose bovine dansunerégiond’élevaged’Afrique de l’Ouest. BoboDioulasso: CESIS.
- Gidel R, Albert J, Rétif M (1969) Enquêtesur la tuberculose bovine au moyen de tests tuberculiniquesdansdiversesrégionsd’Afriqueoccidentale (Haute Volta et Cöte d’Ivoire). Rev Elev Med Vet Pays Trop 22: 337-355.
- Hamro-Drotz D, United Nations Environment Programme, editors (2011) Livelihood security: climate change, migration and conflict in the Sahel. Châtelaine, Geneva: United Nations Environment Programmep:108.
- Food and Agricultural Organization of the United Nation, Economic Community of West African States (2012) The cross-border transhumance in West Africa Proposal for Action Plan.
- Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, et al. (2013) Prevalence of Non-Tuberculous Mycobacterial Infections among Tuberculosis Suspects in Nigeria. PLoS ONE 8: e63170.
- Firdessa R, Berg S, Hailu E, Schelling E, Gumi B, et al. (2013) Mycobacterial Lineages Causing Pulmonary and Extrapulmonary Tuberculosis, Ethiopia. Emerg Infect Dis 19: 460-463.
- Hlavsa MC, Moonan PK, Cowan LS, Navin TR, Kammerer JS, et al. (2008) Human Tuberculosis due to Mycobacterium bovis in the United States, 1995-2005. Clin Infect Dis 47: 168-175.
- LoBue P,Betacourt W, Peter C, Moser K (2003) Epidemiology of Mycobacterium bovis disease in San Diego County, 1994-2000. Int J Tuberc Lung Dis 7: 180-185.
- Majoor CJ, Magis-Escurra C, van Ingen J, Boeree MJ, van Soolingen D (2011) Epidemiology of Mycobacterium bovis Disease in Humans, the Netherlands, 1993-2007. Emerg Infect Dis 17: 457-463.
- Mignard S, Pichat C, Carret G (2006) Mycobacterium bovis infection, Lyon, France. Emerg Infect Dis 12: 1431-1433.
- Ojo O, Sheehan S, Corcoran GD, Okker M, Gover K, et al. (2008) Mycobacterium bovis Strains Causing Smear-Positive Human Tuberculosis, Southwest Ireland. Emerg Infect Dis 14: 1931-1934.
- Chen Y, Chao Y, Deng Q, Liu T, Xiang J, et al. (2009) Potential challenges to the Stop TB Plan for humans in China; cattle maintain M. bovis and M. tuberculosis. Tuberculosis 89: 95-100.
- Guerrero A, Cobo J, Fortün J, Navas E, Quereda C, et al. (1997) Nosocomial transmission of Mycobacterium bovis resistant to 11 drugs in people with advanced HIV-1 infection. The Lancet 350: 1738-1742.
- Evans JT, Smith EG, Banerjee A, Smith RM, Dale J, et al. (2007) Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. The Lancet 369: 1270-1276.
- Sunder S, Lanotte P, Godreuil S, Martin C, Boschiroli ML, et al. (2009) Human-to-Human Transmission of Tuberculosis Caused by Mycobacterium bovis in Immunocompetent Patients. J ClinMicrobiol 47: 1249-1251.
- Thoen C, Lobue P, de Kantor I (2006) The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol 112: 339-345.
- Thoen CO, LoBue PA (2007) Mycobacterium bovis tuberculosis: forgotten, but not gone. The Lancet 369: 1236-1238.
- Thoen CO, LoBue PA, Enarson DA, Kaneene JB, de Kantor I (2009) Tuberculosis: a re-emerging disease in animals and humans. Vet Ital 45: 135-181.
- Vekemans M, Cartoux M, Diagbouga S, Dembélé M, Koné B, et al. (1999) Potential source of human exposure to Mycobacterium bovis in Burkina Faso, in the context of the HIV epidemic. ClinMicrobiol Infect 5: 617-621.








