Review Article - (2023) Volume 15, Issue 1
Nanoemulgel: A Novel Approach for Topical Delivery System
Dr. Ram Babu Sharma*,
Gaurav Kumar,
Hitesh Thakur and
Dr. Sakshi Tomar
Himalayan Institute of Pharmacy, Kala Amb Distt. Sirmaur (H.P.)-173030, India
*Correspondence:
Dr. Ram Babu Sharma, Himalayan Institute of Pharmacy, Kala Amb Distt. Sirmaur (H.P.)-173030,
India,
Tel: + 7018379926,
Email:
Received: 30-Dec-2022, Manuscript No. Ijddr-23-13354;
Editor assigned: 09-Jan-2023, Pre QC No. Ijddr-23-13354;
Reviewed: 23-Jan-2023, QC No. Ijddr-23-13354;
Revised: 27-Jan-2023, Manuscript No. Ijddr-23-13354;
Published:
31-Jan-2023, DOI: 10.36648-0975-9344-15.1-988
Abstract
The incorporation of a nanoemulsions system integrated into the hydrogel matrix
affects better skin penetration. Nanoemulgels are known as the formulation
of nanoemulsion based on hydrogel. Nanoemulgel improves the stability of a
nanoemulsion formulation by lowering surface and interfacial tension, which
increases the aqueous phase viscosity. Because the system has a higher viscosity
than the nanoemulsion system, nanoemulgel is also known as hydrogel-thickened
nanoemulsions. Hydrophobic medication delivery using nanoemulgel is extremely
effective. With greater drug loading due to improved solubilizing efficiency,
improved bioavailability due to superior permeability, and the ability to control
drug release, it is an efficient alternative delivery technique for the treatment of
many disorders. Nanogels protect biomolecules like enzymes and genetic material
from destruction, while their macromolecular features let tiny molecules circulate
longer and serve as a handy platform for combining therapeutic compounds.
Nanoemulgel use has increased in recent years as a result of the preparation's
improved acceptability among patients due to its non-greasy, convenient Spread
ability, easy application, and good therapeutic and safety profile. Nanoemulgel
has a strong potential of being the primary topical delivery route for lipophilic
medications in the future, despite several challenges.
Keywords
Nanoemulgel; Spreadability; Nanoemulsions
INTRODUCTION
Emulsions have been used in drug delivery systems from the
beginning of time. Because of their inability to swell in other
solid dosage forms, our forefathers were always forced to utilize
emulsion to deliver medications to the elderly and children. By
that time, various revolutions in the emulsion had been made
to improve the preparation in terms of safety, efficacy, patient
compliance, and side effects. The emulsion preparation is now
utilized topically by transforming it into gel formulations, in
addition to being used orally [1-10]. The novel emulsion-gel
combination idea has been developed to make the Due to its wide
acceptability as a topical preparation for both medication and
cosmetic purposes around the world; nanoemulgel has recently
drawn the attention of various scientists to develop nanoemulgel
preparations. Nanoemulgel preparation induces its effect for
a longer period as the entire system acts like a drug reservoir
and allows the drug to release in a very controlled manner. The
releasing mechanism is influenced by the crosslink density and
the type of the network polymer chains [11]. The tendency of a
drug to diffuse out of the vehicle and pass through the barrier
influences its ability to enter the skin and release therapeutic
molecules (Figure 1).
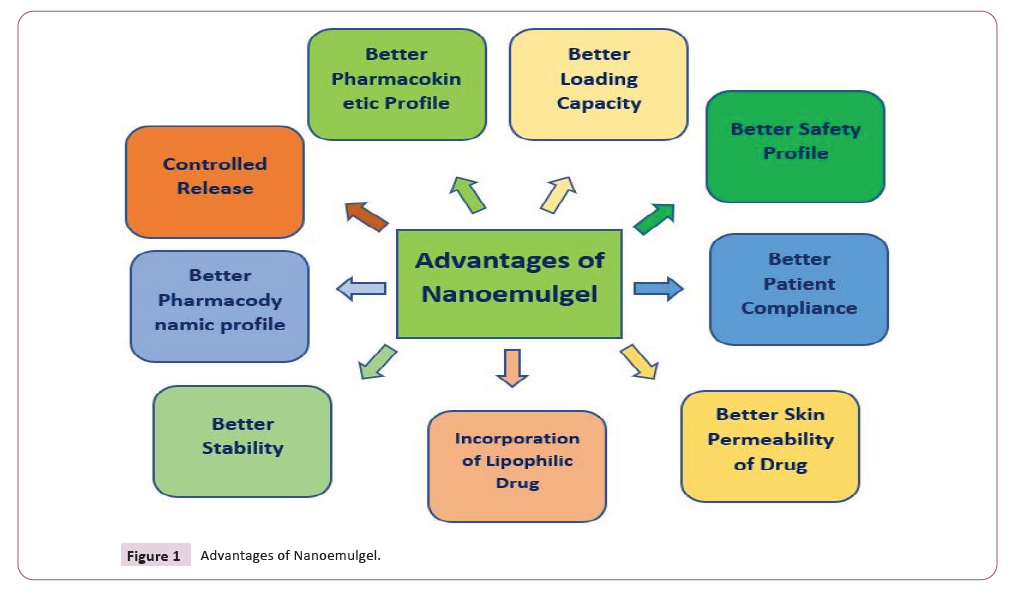
Figure 1: Advantages of Nanoemulgel.
From the inner phase to the outer phase topical administration
systems act as drug reservoirs, affecting drug release from the
inner phase to the outside phase and, eventually, onto the skin.
The releasing mechanism is influenced by the crosslink density
and the type of the network polymer chains [12]. The tendency of
a drug to diffuse out of the vehicle and pass through the barrier
influences its ability to enter the skin and release therapeutic
molecules. The therapeutic effect of the drug is produced by
liberating the drug in droplet form from the gel network and then
reaching the stratum corneum and penetrating it and reaching
into the systemic circulation [13].
The method of preparation of nanoemulgel is as simple as
emulsion like water in oil and oil in water emulsion with a gel
basis. Nanoemulgels are appealing possibilities for drug delivery because they have a dual nature, namely a nanoscale emulsion
and a gel base, both combined in a single formulation. The oil
droplets will then reach the stratum corneum of the epidermis,
skipping the hydrophilic phase of nanoemulsions and transporting
the medicine molecules directly to the stratum corneum [14].
The nanoemulgel has several benefits over other topical
formulations that have been studied, including It is preferable to
avoid first-pass metabolism. Acceptance is uncomplicated for the
patient and perfectly safe to self-medicate. Medication can easily
be discontinued. It is well tolerated by the skin's environment
and proven, well-controlled, and long-lasting medication
administration technique [15].
Many scientists gave their reviews about the
nanoemulgel by using different methods and
drugs:
Raemdonck Koen et al., (2009) have reported that as
multifunctional polymer-based drug delivery methods, nanosized
hydrogels (nanogels) have gotten a lot of interest. Both drug
encapsulation and drug release reveal their flexibility. Nanogels
may be made to allow for the encapsulation of a wide range
of bioactive substances. Nanogels may be tailored to sense
and respond to environmental changes by optimizing their
chemical composition, size, and shape to enable spatial and
stimuli-controlled drug release in vivo. The goal of this paper is
to highlight recent breakthroughs in the interaction between
biology and nanomedicine, with a focus on nanogels as drugdelivery
vehicles [16].
Choudhury Hira., et al (2017) reported that Nanoemulgel, as a new transdermal delivery method, has been shown to provide
unexpected benefits for lipophilic medicines when compared
to previous formulations. Because of the lipophilic character
of newer medications created in this age, they have low oral
bioavailability, unpredictable absorption, and pharmacokinetic
variability. As a result, this unique transdermal delivery technique
is superior to traditional oral and topical medication delivery
systems in preventing such disruptions. These nanoemulgels are
essentially oil-in-water nanoemulsions that have been gelled
with the addition of a gelling agent. This formulation's gel phase
is nongreasy, which improves user compliance and stabilizes
the formulation by lowering surface and interfacial tension. At
the same time, it can be directed more precisely to the site of
action, avoiding first-pass metabolism and relieving the user
of gastric/systemic incompatibilities. This brief review focuses
on nanoemulgel as a superior topical drug delivery technology,
covering component screening, formulation procedure, and
current pharmacokinetic and pharmacodynamics advances in
research investigations conducted by experts throughout the
world. As a result, after this study, nanoemulgel may be a more
effective and efficient drug delivery technique for the topical
system [17-20] (Figure 2).
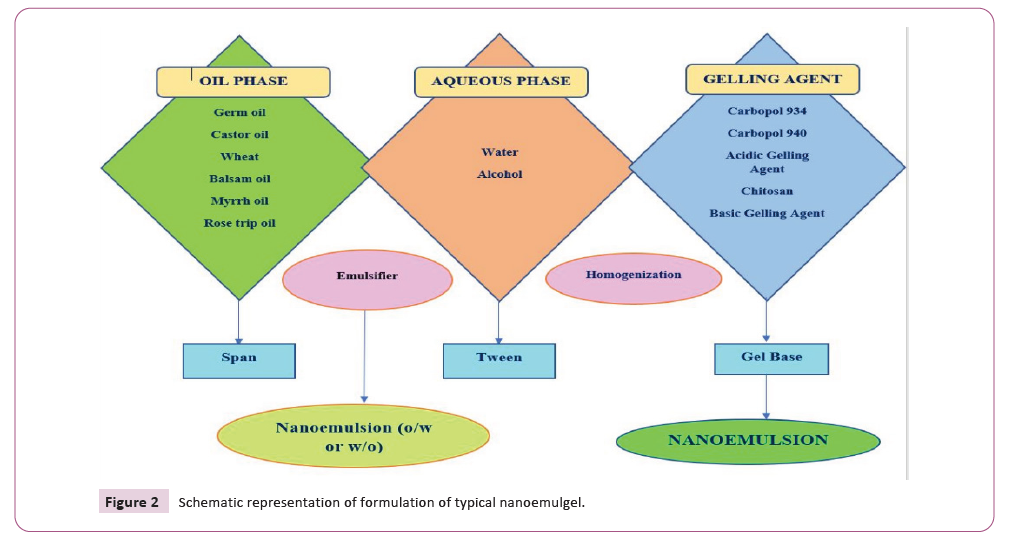
Figure 2: Schematic representation of formulation of typical nanoemulgel.
Kumar Anand et al., (2019) reported that many recently
licensed medications nanoemulgel formulations are being
effectively employed in various fields of health care, redefining
the importance of topical administration above other methods.
However, toxicological analyses of the ingredients employed
in such formulations must be considered, in addition to other
changes in the existing state of the delivery system (44).
Figure 1 Advantages of Nanoemulgel.
Morsy A. Mohamed et al., (2019) reported that Tissue repair and
wound healing are intricate processes including inflammation,
granulation, and tissue remodelling. It was discovered that
several statins, particularly atorvastatin (ATR), could promote
wound healing. The goal of this study was to develop and test
a topical application of ATR-based nanoemulgel for wound
healing. The physical appearance, rheological behavior, in vitro
drug release, and ex vivo drug permeation of the produced
formulations (ATR gel, ATR emulgel, and ATR nanoemulgel) were
all assessed. In wound-induced rats, the in vivo wound healing
impact was assessed. The physical characteristics of the produced
ATR gel formulations were satisfactory and similar. Drug release
characteristics from gel, emulgel, and nanoemulgel were all
different [18, 19].
Harshitha V et al., (2020) reported that Nanoemulsions are
a non-equilibrium, optically transparent, thermodynamically
stable, metastable dispersion of nano-sized particles with
established surface tension produced by certain shears, made
up of appropriate oil and a specific mixture of surfactants and
co-surfactants, and capable of dissolving large amounts of
hydrophobic drugs. Homogenizers, low-energy emulsification,
and phase temperature inversion approaches can all be used
to achieve the nanoemulsion mechanism. Nanoemulgel is
often referred to as hydrogel-thickened nanoemulsion (HTN)
since it has a higher viscosity than nanoemulsion. Nanoemulgel
improves the stability of nanoemulsion formulations by lowering
surface and interfacial tension, resulting in higher aqueous phase
viscosity [20].
Aithal GC et al., (2020) have reported that Nanoemulgels are
good candidates for drug delivery because they have a dual
nature, namely, a nanoscale emulsion and a gel foundation,
both integrated into a single formulation. The active moiety is protected by the nanoemulsion component of the nanoemulgel,
which prevents enzymatic degradation and certain processes
like hydrolysis. The gel base gives the emulsion thermodynamic
stability by raising the aqueous phase's viscosity and lowering
interfacial and surface tension. Nanoemulgels have rheological
properties that make them ideal for topical and other kinds of
delivery, such as dental delivery since they improve patient
acceptability. Because the globule size is present in the nano
form, using penetration enhancers can improve the formulation's
efficiency by increasing permeability and diffusibility. According to
reports, several commercially available topical dosage forms have
a poor spreading coefficient when compared to nanoemulgels,
focusing on the use of nanoemulgels in dermatology, despite
opening the way for numerous other disciplines has not been fully
utilized. With an overview of a few illustrations supporting the
case, this detailed analysis illustrates the merits of nanoemulgel
as a viable carrier for medication delivery [21-25].
Mohammed S. Algahtani et al., (2020) reported in their review
that a retinyl palmitate-containing nano-emulgel system was
successfully produced for topical distribution using a low-energy
emulsification approach. This study found that nanoencapsulation
of nutraceutical, cosmeceutical, and pharmaceutical goods
results in improved UV and storage stability as well as increased
skin permeability following topical administration, despite
poor biopharmaceutical performance and chemical/photoinstability.
This increase in outcomes can be explained by the
nanoemulsion system's improved solubilization ability, as well as
the nano dimension of the encapsulating delivery vehicle, which
favors a more permeable distribution of retinyl palmitate into
the skin via several epidermal mechanisms/routes. Controlling
HLB of the oil phase and vertexing duration in the preparation
of a nanoemulsion with a droplet dimension of 50 nm using low-energy emulsification techniques are critical aspects for
topical delivery of hydrophobic nutraceuticals, cosmetics, and
pharmaceuticals into the skin, according to the findings of this
study [26,27].
Sreeharsha Nagaraja et al., (2021) transcutaneous medication
penetration through the keratinized stratum corneum is a
significant barrier and problem for topical administration.
Furthermore, the existing available skin cancer therapy has
severe negative effects. As a result, skin-permeable and suitable
formulations are essential. Using the therapeutic qualities of
herbal components allows for the development of non-toxic,
non-irritating, and suitable formulations. Self-nano-emulsifying
drug delivery systems containing chrysin have been successfully
created and tested for use in cancer therapies, particularly
skin cancer. The physicochemical analysis revealed that the
formulations' mean droplet size was nanoscale, with limited size
distribution and adequate thermodynamic stability [28]. The
nanoemulgels mechanical qualities, as evaluated by the forcetime
relationship and mechanical texture features, were ideal
for their quick and simple application to the skin surface. In
vivo experiments showed that the nano-emulsified formulation
dramatically increased chrysin transcutaneous penetration and
skin deposition, indicating that it might be used as a topical
treatment. After being converted into nanoemulgel form, chrysin
demonstrated an improved therapeutic response in cytotoxicity
assays. The findings indicate that the developed self-emulsifying
drug delivery system is safe and biocompatible and that it will
significantly lower total dosage and chrysin consumption. By
taking into consideration the increased physicochemical qualities,
the findings of this study might lead to a slew of new uses for
the chrysin self-emulsifying drug delivery system, such as oral,
nasal, and rectal distribution, giving herbal nutraceuticals a new
lease of life. The formulation is a flexible platform technology that
may be tweaked to include a range of hydrophobic, drug-loaded
lipid nanocomplexes that enable localized therapeutic agent
delivery at the afflicted spot. The present platform technology for
skin illnesses has unique benefits such as versatility, longer skin
preservation, and the avoidance of systemic penetration [29, 30].
Advanced Technologies used in Nanoemulgel:
Topical Application of a Nanoemulgel from a Self-
Nanoemulsifying Concentrate: The gel was made by dispersing
the self-nano emulsifying preconcentrate in water containing the
gelling ingredient. Pluronic® F127 was dissolved in cold water
(20% w/w). By adding a -nano emulsifying preconcentrate (10
percent v/w) containing chrysin 100 mg/mL to a transparent
Pluronic® F127 solution at 10 °C, a 1 percent w/w chrysin
concentration was produced. The mixture was sonicated for 5
minutes in an ultrasonic water bath to remove the trapped air.
For comparison, a gel with a chrysin dispersion (1 percent w/w)
was created by entirely dispersing the same amount of chrysin in
Pluronic® F127 gel.
Droplet size, polydispersity index, electron microscopy, and
viscosity were all used to characterize the chrysin nanoemulgel for
topical application. The gel sample was diluted with water (1:100)
and the droplet size was determined using the same approach as
the nano-emulsifying drug delivery system. As previously noted, the nanoemulgel was photographed in cryo-mode for SEM. To
investigate the impacts on size and size distribution, the droplet
size of the nanoemulgel was examined over three months. Chrysin
analysis using RP-HPLC, for accuracy, precision, specificity, and
solution stability, the RP-HPLC technique for determining chrysin
content was verified. The technique was determined to be
specific, as evidenced by the lack of any interfering peaks during
the analyte's retention period [31-35].
Nanosized Nasal Emulgel of Resveratrol: The goal of this study is
to create a nasal nano-emulgel for resveratrol using Carbopol 934
and poloxamer 407 as gelling agents. Further characterization
of the chosen system yielded the best nano-emulsion(57).
With slight changes, the cold approach was used to make
nasal mucoadhesive nasal nanoemulgel. To avoid air bubbles,
Carbopol 934 was gently added to the developed optimal nanoemulsion
and blended at a constant slow stirring rate, and then
the mixture was chilled overnight to allow complete swelling.
Following that, poloxamer 407 was added and mixed slowly to
get a clean dispersion. Finally, triethanolamine was added to
neutralize the dispersion, and the gel was kept at 4°C until the
investigation was completed. FTIR was used to characterize the
produced mucoadhesive nasal nano-emulgel. The IR spectra of
a mucoadhesive nasal nano-emulgel physical combination, The
RES and each component's spectra were then compared to the
RES spectra [36-40].
Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: In the further study of Thymoquinone loaded topical nanoemulgel
for wound healing, it has been observed that the oil phase
and Smix phase (combination of surfactant and co-surfactant)
for the synthesis of thymoquinone loaded nanoemulsions
are determined using the results of the solubility study and
emulsification efficiency inquiry (TQM-NE). TQM-NE was created
using a high-energy ultrasonication process. The coarse emulsion
was made by mixing 5 percent w/w (50 mg/g) of TQM in the
oil phase and Smix through the vortex mixture, then adding
the aqueous phase while continuously vertexing for 1 minute.
The ultrasonically agitated coarse emulsion phase was further
ultrasonically agitated in a water bath for a separate time interval
(3, 5, and 10 minutes) at a 40 percent ultrasonication amplitude.
To find the best TQM-NE formulation, researchers created
and tested eighteen formulations with various compositions.
Thermodynamic stability, droplet size distribution, polydispersity
index (PDI), zeta potential, viscosity, and drug concentration of
TQM-NE formulations were all tested in triplicate. In the Drug
Content analysis, the content of TQM in the improved TQM-NE
formulations was measured by diluting 100 L of TQM-NE 1000
times with methanol and measuring the TQM content using a UVvisible
spectrophotometer at max at 254 nm [41-45].
Methylcellulose-Based Nanoemulgel Loaded with Nigella
Sativa Oil for Oral Health Management: As a gelling agent,
high-viscosity methylcellulose E461 was utilized in this work.
It dissolves in cold liquids to generate a transparent, viscous
solution or gel that is naturally non-toxic and non-allergenic.
The dental formulation was created in three steps, with minor
adjustments, utilizing procedures from the literature. The dental
nanoemulgel formulation was optimized using the response
surface methodology (RSM) of Box–Behnken statistical design
with a quadratic model with 17 runs. With the use of columns,
cubes (standard error of design), and 3D graphs, the impacts of
formulation elements and variables, such as water (A), oil (B),
and gelling agent (C), were seen on the two responses of the
formulation, pH (R1) and viscosity (R2). The statistical analysis of
answers was done using ANOVA [46].
Because of the favorable and practical properties for
topical distribution of NSO, the produced NSO nanoemulgel
demonstrated high promise for the treatment of periodontal
disorders. The addition of NSO to a nanoemulgel formulation will
enhance patient compliance by making it easier to apply while also
improving effectiveness. The nanoemulgels cost-effectiveness
and improved mucoadhesiveness are two additional benefits
that make them an appealing alternative to traditional topical
formulations. The nanosized NSO droplets are predicted to assist
sustain tighter mucosal contact, allowing for more surface area
for NSO penetration and higher medication concentration in the
target region [47].
The impact of different emulsifiers and gelling agents on the
globule size, stability, drug release, viscosity, and pH of the
formulation can also be investigated. NSO can also be mixed with
other natural or synthetic antimicrobial agents, and the resulting
nanoemulgel formulations can be utilized for preclinical and
clinical testing. More preclinical and clinical research is needed
to determine the efficacy of this formulation in the treatment of
periodontal diseases [48].
Novel Formulation of Fusidic Acid Incorporated into a Myrrhoil-
based Nanoemulgel for the Enhancement of Skin Bacterial
Infection Treatment: The BBD technique was used to create and
optimize several nanoemulsions made with myrrh essential oil.
FA-NEG was created using the optimized nanoemulsion and a
hydrogel basis. The FA-NEG that was created has physical qualities
that were suitable for topical application. Following application
to the skin, it demonstrated improved permeability and no
irritation. When compared to commercial Fusidic acid, FA-NEG
and the blank nanoemulgel had a lot more antibacterial activity.
The study found that myrrh essential oil and Fusidic acid have a
strong antibacterial effect and that their actions are synergistic.
Fusidic acid and myrrh essential oil nanoemulgel systems might
be potential nanocarriers for imparting antibacterial effects via
topical application. Our long-term aim is to investigate the effect
of the formulation's action on animal wounds infected with
various bacteria and compare healing rates to those given by
commercial Fusidic acid solutions [49-50].
Techno-bio functionality of Mangostin extract-loaded virgin
coconut oil nanoemulgel: Ultrasonication effectively generated
nanoemulsions loaded with Mangostin extracts made from
mangosteen peel extracts recovered by VCO, combined VCOPG,
and PG in the dispersed phase containing mixed surfactants
(Tween20/Span20) with an HLB value of 15.1 On the nanoscale,
the resulting nanoemulsions were globular and evenly dispersed,
with an average droplet size of less than 100 nm. The particles'
zeta potentials exerted the greatest negative charge, indicating a
steady dispersion. All nanoemulsions generated with a surfactant
with an HLB value of 15.1 remained stable after numerous freezethaw cycles. Furthermore, as compared to their bulk extracts, the
nanoemulsions' smaller droplet sizes showed higher antioxidant
and antibacterial properties [51, 52-60].
Scope of Nanoemulgel for Topical
Delivery
In the topical delivery system, Nanoemulgel plays an important
role. The various scopes of nanoemulgel for the topical delivery
system are as follows:
Because of its greater absorption capabilities, enhanced
pharmacokinetic profile, and therefore higher therapeutic
effectiveness, topical nanoemulsion gel can be regarded as a
preferable alternative to traditional lipophilic drug formulations.
One of the main reasons for the nanoemulgel formulation's
increased patient acceptance when compared to other topical
administration alternatives is its lower stickiness and superior
spreading qualities [61, 62-70].
Topical Nanoemulgels are a more effective and convenient
method of medication administration. Patient compliance is
higher thanks to the gel and non-greasy qualities, and the lack
of an oily foundation allows for greater medication release
when compared to other formulations. With the incorporation
of Nanoemulsion into the gel matrix, problems like creaming
and phase separation that are linked with traditional emulsions
are overcome, as is increased spared ability. In some topical
conditions, a nanoemulsion-loaded gel is more beneficial [71, 72- 80]. Nanoemulsion-Gel-based formulations might be a better and
more dependable way to deliver hydrophobic medications in the
future. Many drugs used to treat skin infections are hydrophobic
in nature, and these treatments can be delivered successfully
as Nanoemulgels, in which the drug is integrated into the Nano
emulsion’s oil phase and subsequently merged with the gel basis.
Despite a few roadblocks, nanoemulgel has a good chance of
becoming the focal point for the topical delivery of lipophilic
medicines in the future [81, 82].
Nanoemulgel has been discovered to be an excellent vehicle for
the delivery of hydrophobic drugs. It's a potent alternative delivery
method in the treatment of numerous illnesses, with high drug
loading due to increased solubilizing effectiveness, enhanced
bioavailability due to better permeability, and the capacity to
modulate drug release. The use of nanoemulgel preparation
in the treatment of acne, pimples, psoriasis, fungal infection,
osteoarthritis, and rheumatoid arthritis inflammation has been
demonstrated to be much more effective [83]. It can be used
for ophthalmic, vaginal, dental, and nose-to-brain medication
administration for the treatment of a variety of local and systemic
diseases such as alopecia, periodontitis, and Parkinson's disease, in
addition to transdermal use. In the cosmetics business, nanoemulgel
has been used as a UV absorber nanoemulgel to protect skin from
sunburn. The nanoemulgel technology has remarkable potential
to treat a wide range of local and systemic illnesses. Some
preparations are currently on the market, while others require more
clinical testing before being released [84, 85-105].
Conclusion
Topical Nanoemulgels have shown to be a more advantageous
choice for a reliable and practical drug delivery mechanism. In
comparison to previous formulations, the gel-like and nongreasy
qualities increase patient compliance and the absence
of oil as a basis improves drug release. With enhanced Spread
ability, problems with typical emulsions such as creaming and
phase separation are eliminated based on formulations of
nanoemulsion-gel may offer a better and more dependable
approach for the administration of hydrophobic medications.
Many of the drugs used to treat skin infections are hydrophobic in
nature. These drugs can be effectively delivered as Nanoemulgels
by first being integrated into the oil phase of the nanoemulsion
and then being combined with the gel basis. Despite a few
obstacles, nanoemulgel has a good chance of being the main
topical delivery system for lipophilic medicines in the future. It
offers a variety of delivery options for topical medications used to
treat a wide range of ailments, including the ability to adjust drug
release as well as high drug loading owing to improved solubilizing
efficiency. In addition to the transdermal application, it may be
utilized for the ocular, vaginal, dental, and nose-to-brain delivery
of medicine for the treatment of several local and systemic
disorders such as alopecia, periodontitis, and Parkinson's disease.
References
- Dandamudi M, McLoughlin P, Behl G, Rani S, Coffey L et al (2021) Chitosan-coated plea nanoparticles encapsulating triamcinolone acetonide as a potential candidate for sustained ocular drug delivery. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Jacob S, Nair AB, Shah J (2020) Emerging role of nanosuspensions in drug delivery systems. Biomaterials Research.
Indexed at, Google Scholar, Crossref
- Neetika B, Arsh D, Manish G (2012) an Overview on Various Approaches to Oral Controlled Drug Delivery System via Gastroretentive Drug Delivery System. Int Res J Pharm.
Indexed at, Google Scholar, Crossref
- Arora D, Kumar L, Joshi A, Chaudhary A, Devi P et al (2021) A Brief Review on Floating Drug Delivery System. J Drug Deliv Ther.
Indexed at, Google Scholar, Crossref
- Rashmitha V, Pavani S, Rajani T (2020) An Update on Floating Drug Delivery System: A Review. Int J Adv Pharm Biotechnol.
Indexed at, Google Scholar, Crossref
- Lodh H, Chourasia PK, Pardhe HA (2020) Floating Drug Delivery System: A Brief Review. Am J PharmTech Res.
Indexed at, Google Scholar, Crossref
- Farooq SM, Sunaina S, Rao MDS, Venkatesh P, Hepcykalarani D et al (2020) Floating Drug Delivery Systems: An Updated Review. Asian J Pharm Res.
Indexed at, Google Scholar, Crossref
- Sopyan I, Sriwidodo L, Wahyuningrum R, Norisca Aliza P (2020) A review: Floating drug delivery system as a tool to improve dissolution rate in gastric. International Journal of Applied Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Kumar AK, Srivastava R (2021) In vitro in vivo studies on Floating microspheres for Gastroretentive drug delivery system. Asian J Pharm Clin Res.
Indexed at, Google Scholar, Crossref
- Morais RP, Hochheim S, de Oliveira CC, Riegel-Vidotti IC, Marino CEB et al (2022) Skin interaction, permeation, and toxicity of silica nanoparticles: Challenges and recent therapeutic and cosmetic advances. Int J Pharmas.
Indexed at, Google Scholar, Crossref
- Pandey VN, Tiwari N, Pandey VS, Rao A, Das I et al (2019) Targeted drug delivery and gene therapy through natural biodegradable nanostructures in pharmaceuticals. Nanoarchitectonics in Biomedicine.
Indexed at, Google Scholar, Crossref
- Fereig SA, El-Zaafarany GM, Arafa MG, Abdel-Mottaleb MMA (2020) Tackling the various classes of nano-therapeutics employed in topical therapy of psoriasis. Drug Deliv.
Indexed at, Google Scholar, Crossref
- Mohd Nordin UU, Ahmad N, Salim N, Mohd Yusof NS (2021) Lipid-based nanoparticles for psoriasis treatment: a review on conventional treatments, recent works, and prospects. RSC Advances.
Indexed at, Google Scholar, Crossref
- Bhardwaj S, Tiwari A (2021) Nanoemulgel: a Promising Nanolipoidal-Emulsion Based Drug Delivery System in Managing Psoriasis. Dhaka Univ J Pharm Sci.
Indexed at, Google Scholar, Crossref
- Thakur S, Rajinikanth PS, Deepak P, Jaiswal S, Anand S et al (2021) Withdrawal Notice: Novel Treatment strategies for Management of Psoriasis: Current update and Future Perspective. Curr Drug Deliv.
Indexed at, Google Scholar, Crossref
- Tambe VS, Nautiyal A, Wairkar S (2021) Topical lipid nanocarriers for management of psoriasis-an overview. J Drug Del Sci &Techno.
Indexed at, Google Scholar, Crossref
- Shetty K, Sherje AP (2021) Nano intervention in topical delivery of corticosteroid for psoriasis and atopic dermatitis-a systematic review. J Mat Sci: Mat Med.
Indexed at, Google Scholar, Crossref
- Vildanova R, Lobov A, Spirikhin L, Kolesov S (2022) Hydrogels on the Base of Modified Chitosan and Hyaluronic Acid Mix as Polymer Matrices for Cytostatics Delivery. Gels.
Indexed at, Google Scholar, Crossref
- Soylu HM, Chevallier P, Copes F, Ponti F, Candiani G et al (2021) A Novel Strategy to Coat Dopamine-Functionalized Titanium Surfaces With Agarose-Based Hydrogels for the Controlled Release of Gentamicin. Front Cell Infect Microbiol.
Indexed at, Google Scholar, Crossref
- Singhal A, Schneible JD, Lilova RL, Hall CK, Menegatti S et al (2020) A multiscale coarse-grained model to predict the molecular architecture and drug transport properties of modified chitosan hydrogels. Soft Matter.
Google Scholar
- Jacob S, Nair AB, Shah J, Sreeharsha N, Gupta S et al (2021) Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- White JM, Calabrese MA (2022) Impact of small molecule and reverse poloxamer addition on the micellization and gelation mechanisms of poloxamer hydrogels. Colloids Surface A Physicochem Eng Asp.
Indexed at, Google Scholar, Crossref
- de Lima CSA, Balogh TS, Varca JPRO, Varca GHC, Lugão AB et al (2020) An updated review of macro, micro, and nanostructured hydrogels for biomedical and pharmaceutical applications. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Pyo SM, Maibach HI (2019) Skin Metabolism: Relevance of Skin Enzymes for Rational Drug Design. Skin Pharmacology and Physiology.
Indexed at, Google Scholar Crossref
- Kaur R, Ajitha M (2019) Transdermal delivery of fluvastatin loaded nanoemulsion gel: Preparation, characterization and in vivo anti-osteoporosis activity. Eur J Pharm Sci.
Indexed at, Google Scholar, Crossref
- Jain R, Sarode I, Singhvi G, Dubey SK (2020) Nanocarrier Based Topical Drug Delivery- A Promising Strategy for Treatment of Skin Cancer. Curr Pharm Des.
Indexed at, Google Scholar, Crossref
- Mani A, Mahalingam G (2020) Topical Delivery of Drugs for Skin Disease Treatment: Prospects and Promises. In: Nanotechnology in the Life Sciences. 2020.
Indexed at, Google Scholar, Crossref
- Dhaval M, Vaghela P, Patel K, Sojitra K, Patel M et al (2022) Lipid-based emulsion drug delivery systems-a comprehensive review. Drug Del & Translational Res.
Indexed at, Google Scholar, Crossref
- Shaker DS, Ishak RAH, Ghoneim A, Elhuoni MA (2019) Nanoemulsion: A review of mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Scientia Pharmaceutica.
Indexed at, Google Scholar, Crossref
- Chung SL, Yee MSL, Hii LW, Lim WM, Ho MY et al (2021) Advances in nanomaterials used in co-delivery of siRNA and small molecule drugs for cancer treatment. Nanomaterials.
Indexed at, Google Scholar, Crossref
- Rao MR, Sonawane A, Sapate S, Abhang K (2020) Exploring Recent Advances in Nanotherapeutics. J Drug Deliv Ther.
Indexed at, Google Scholar, Crossref
- Zhang C, Zhou X, Zhang H, Han X, Li B et al (2022) Recent Progress of Novel Nanotechnology Challenging the Multidrug Resistance of Cancer. Frontiers in Pharmacology.
Indexed at, Google Scholar, Crossref
- Purohit D, Manchanda D, Makhija M, Rathi J, Verma R et al (2020) An Overview of the Recent Developments and Patents in the Field of Pharmaceutical Nanotechnology. Recent Pat Nanotechnol.
Indexed at, Google Scholar, Crossref
- Elsewedy HS, Al-Dhubiab BE, Mahdy MA, Elnahas HM (2021) Basic concepts of nanoemulsion and its potential application in pharmaceutical, cosmeceutical, and nutraceutical fields. Res J Pharm Technol.
Indexed at, Google Scholar, Crossref
- Marzuki NHC, Wahab RA, Hamid MA (2019) an overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnology and Biotechnological Equipment.
Indexed at, Google Scholar, Crossref
- Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, et al (2019) Advances in transdermal insulin delivery. Advanced Drug Delivery Reviews.
Indexed at, Google Scholar, Crossref
- Bubic Pajic N, Nikolic I, Mitsou E, Papadimitriou V, Xenakis A et al (2018) Biocompatible microemulsions for improved dermal delivery of sertaconazole nitrate: Phase behavior study and microstructure influence on drug biopharmaceutical properties. J Mol Liq.
Indexed at, Google Scholar, Crossref
- Zhao W, Zhao Y, Wang Q, Liu T, Sun J et al (2019) Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small.
Indexed at, Google Scholar, Crossref
- Prajapati B (2018) ‘‘Nanoemulgel” Innovative Approach for Topical Gel Based Formulation. Res Rev Healthc Open Access J.
Indexed at, Google Scholar, Crossref
- Varghese J, Anderson KD, Widerström-Noga E, Mehan U (2020) A primary care provider’s guide to pain after spinal cord injury: Screening and management. Top Spinal Cord Inj Rehabil.
Indexed at, Google Scholar, Crossref
- Tran PHL, Duan W, Lee B-J, Tran TTD (2019) Nanogels for Skin Cancer Therapy via Transdermal Delivery: Current Designs. Curr Drug Metab.
Indexed at, Google Scholar, Crossref
- Choudhury H, Gorain B, Pandey M, Chatterjee LA, Sengupta P et al (2017) Recent Update on Nanoemulgel as Topical Drug Delivery System. Journal of Pharmaceutical Sciences.
Indexed at, Google Scholar, Crossref
- Anand K, Ray S, Rahman M, Shaharyar A, Bhowmik R et al (2019) Nano-emulgel: Emerging as a Smarter Topical Lipidic Emulsion-based Nanocarrier for Skin Healthcare Applications. Recent Pat Antiinfect Drug Discov.
Indexed at, Google Scholar, Crossref
- Morsy MA, Abdel-Latif RG, Nair AB, Venugopala KN, Ahmed AF et al (2019) Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Algahtani MS, Ahmad MZ, Shaikh IA, Abdel-Wahab BA, Nourein IH et al (2021) Thymoquinone loaded topical nanoemulgel for wound healing: Formulation design and in-vivo evaluation. Molecules.
Indexed at, Google Scholar, Crossref
- Harshitha V, Swamy MV, Kumar DP, Rani KS, Trinath A et al (2020) Nanoemulgel: A Process Promising in Drug Delivery System. Res J Pharm Dos Forms Technol.
Indexed at, Google Scholar, Crossref
- Bhardwaj S, Gaur PK, Tiwari A (2022) Development of Topical Nanoemulgel Using Combined Therapy for Treating Psoriasis. Assay Drug Dev Technol.
Indexed at, Google Scholar, Crossref
- Nagaraja S, Basavarajappa GM, Attimarad M, Pund S (2021) Topical nanoemulgel for the treatment of skin cancer: Proof-of-technology. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Padhy S, Sahoo BM, Kumar BVVR, Patra CN (2020) Development, Characterization, and Evaluation of Nanoemulgel Used for the Treatment of Skin Disorders. Curr Nanomater.
Indexed at, Google Scholar, Crossref
- Sharma P, Tailang M (2020) Design, optimization, and evaluation of hydrogel of primaquine loaded nanoemulsion for malaria therapy. Futur J Pharm Sci.
Indexed at, Google Scholar, Crossref
- Aithal GC, Narayan R, Nayak UY (2019) Nanoemulgel: A Promising Phase in Drug Delivery. Curr Pharm Des.
Indexed at, Google Scholar, Crossref
- Algahtani MS, Ahmad MZ, Ahmad J (2020) Nanoemulgel for improved topical delivery of retinyl palmitate: Formulation design and stability evaluation. Nanomaterials.
Indexed at, Google Scholar, Crossref
- Vidal-Casanella O, Nuñez N, Sentellas S, Núñez O, Saurina J et al (2020) Characterization of turmeric and curry samples by liquid chromatography with spectroscopic detection based on polyphenolic and curcuminoid contents. Separations.
Indexed at, Google Scholar, Crossref
- Pascual-Maté A, Osés SM, Fernández-Muiño MA, Sancho MT (2018) Analysis of Polyphenols in Honey: Extraction, Separation and Quantification Procedures. Separation and Purification Reviews.
Indexed at, Google Scholar, Crossref
- Whelan LC, Geary M, Healy J (2021) A Novel, Simple Rapid Reverse-Phase HPLC-DAD Analysis, for the Simultaneous Determination of Phenolic Compounds and Abscisic Acid Commonly Found in Foodstuff and Beverages. J Chromatogr Sci.
Indexed at, Google Scholar, Crossref
- Salem HF, Kharshoum RM, Abou-Taleb HA, Naguib DM (2019) Nanosized nasal emulgel of resveratrol: preparation, optimization, in vitro evaluation and in vivo pharmacokinetic study. Drug Dev Ind Pharm.
Indexed at, Google Scholar, Crossref
- Javed H, Shah SNH, Iqbal FM (2018) Formulation Development and Evaluation of Diphenhydramine Nasal Nano-Emulgel. AAPS Pharm Sci Tech.
Indexed at, Google Scholar, Crossref
- Hasan S, Bhandari S, Sharma A, Garg P (2021) Emulgel: A Review. Asian J Pharm Res.
Indexed at, Google Scholar, Crossref
- Jadach B, Świetlik W, Froelich A (2022) Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J Pharm Sci.
Indexed at, Google Scholar, Crossref
- Adnet T, Groo AC, Picard C, Davis A, Corvaisier S et al (2020) Pharmacotechnical development of a nasal drug delivery composite nanosystem intended for Alzheimer's disease treatment. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Uddin S, Islam MR, Chowdhury MR, Wakabayashi R, Kamiya N et al (2021) Lipid-Based Ionic-Liquid-Mediated Nanodispersions as Biocompatible Carriers for the Enhanced Transdermal Delivery of a Peptide Drug. ACS Appl Bio Mater.
Indexed at, Google Scholar, Crossref
- Silvestrini AVP, Caron AL, Viegas J, Praça FG, Bentley MVLB et al (2020) Advances in lyotropic liquid crystal systems for skin drug delivery. Expert Opin Drug Deliv.
Indexed at, Google Scholar, Crossref
- Ojha B, Jain VK, Gupta S, Talegaonkar S, Jain K et al (2022) Nanoemulgel: a promising novel formulation for the treatment of skin ailments. Polymer Bulletin.
Indexed at, Google Scholar, Crossref
- Sultan MH, Javed S, Madkhali OA, Alam MI, Almoshari Y et al (2022) Development and Optimization of Methylcellulose-Based Nanoemulgel Loaded with Nigella sativa Oil for Oral Health Management: Quadratic Model Approach. Molecules.
Indexed at, Google Scholar, Crossref
- Blichfeldt H, Faullant R (2021) Performance effects of digital technology adoption and product & service innovation–A process-industry perspective. Technovation.
Indexed at, Google Scholar, Crossref
- Hu D, Jiao J, Tang Y, Xu Y, Zha J et al (2022) how global value chain participation affects green technology innovation processes: A moderated mediation model. Technol Soc.
Indexed at, Google Scholar, Crossref
- Sungpud C, Panpipat W, Chaijan M, Yoon AS (2020) Techno-biofunctionality of mangosteen extracts loaded virgin coconut oil nanoemulsion and nanoemulgel. PLoS One.
Indexed at, Google Scholar, Crossref
- Chavda VP, Shah D (2017) Self-emulsifying delivery systems: One step ahead in improving the solubility of poorly soluble drugs. In: Nanostructures for Cancer Therapy.
Indexed at, Google Scholar, Crossref
- Choudhury H, Gorain B, Chatterjee B, Mandal UK, Sengupta P et al (2017) Pharmacokinetic and Pharmacodynamic Features of Nanoemulsion Following Oral, Intravenous, Topical, and Nasal Route. Curr Pharm Des.
Indexed at, Google Scholar, Crossref
- Güngör S, Kahraman E (2021) Nanocarriers Mediated Cutaneous Drug Delivery. Eur J Pharm Sci.
Indexed at, Google Scholar, Crossref
- Haider M, Abdin SM, Kamal L, Orive G (2020) Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Harwansh RK, Deshmukh R, Rahman MA (2019) Nanoemulsion: Promising nanocarrier system for delivery of herbal bioactive. J Drug Deliv Sci Technol.
Indexed at, Google Scholar, Crossref
- Yadav K, Soni A, Singh D, Singh MR (2021) Polymers in topical delivery of anti-psoriatic medications and other topical agents in overcoming the barriers of conventional treatment strategies. Prog Biomater.
Indexed at, Google Scholar, Crossref
- Hussain A, Singh S, Sharma D, Webster TJ, Shafaat K et al (2017) Elastic liposomes as novel carriers: Recent advances in drug delivery. Int J Nanomedicine.
Indexed at, Google Scholar, Crossref
- Lalu L, Tambe V, Pradhan D, Nayak K, Bagchi S et al (2017) Novel nanosystems for the treatment of ocular inflammation: Current paradigms and future research directions. J Control Release.
Indexed at, Google Scholar, Crossref
- Abu-Huwaij R, Al-Assaf SF, Hamed R (2021) Recent exploration of nanoemulsions for drugs and cosmeceuticals delivery. J Cosmet Dermatol.
Indexed at, Google Scholar, Crossref
- Rai VK, Mishra N, Yadav KS, Yadav NP (2018) Nanoemulsion as a pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations, and applications. J Control Release.
Indexed at, Google Scholar, Crossref
- Okur ME, Bülbül EÖ, Mutlu G, Eleftherıadou K, Karantas ID et al (2021) An Updated Review for the Diabetic Wound Healing Systems. Curr Drug Targets.
Indexed at, Google Scholar, Crossref
- Paul S, Roy T, Bose A, Chatterjee D, Chowdhury VR et al (2021) Liposome mediated pulmonary drug delivery system: An updated review. Res J Pharm Technol.
Indexed at, Google Scholar, Crossref
- Sharadha M, Gowda DV, Vishal Gupta N, Akhila AR (2020) an overview on topical drug delivery system-an updated review. Int J Pharm Sci Res.
Indexed at, Google Scholar, Crossref
- Mishra P, Handa M, Ujjwal RR, Singh V, Kesharwani P et al (2021) Potential of nanoparticulate based delivery systems for effective management of alopecia. Colloids and Surfaces B: Biointerfaces.
Indexed at, Google Scholar, Crossref
- Zheng Y, Deng F, Wang B, Wu Y, Luo Q et al (2021) Melt extrusion deposition (MEDTM) 3D printing technology-A paradigm shift in design and development of modified release drug products. Int J Pharm.
Indexed at, Google Scholar, Crossref
- Wöll S, Schiller S, Bachran C, Swee LK, Scherließ R et al (2018) Pentaglycine lipid derivates–rp-HPLC analytics for bioorthogonal anchor molecules in targeted, multiple-composite liposomal drug delivery systems. Int J Pharm.
Indexed at, Google Scholar, Crossref
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA et al (2021) . Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery.
Indexed at, Google Scholar, Crossref
- Bég OA (2019) Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med One.
Indexed at, Google Scholar, Crossref
- Tripathi J, Vasu B, Dubey A, Gorla RSR, Murthy PVSN et al (2020) A review on recent advancements in the hemodynamics of nano-drug delivery systems. Nanosci Technol.
Indexed at, Google Scholar, Crossref
- Chen M, Quan G, Sun Y, Yang D, Pan X et al (2020) Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J Control Release.
Indexed at, Google Scholar, Crossref
- Ramalheiro A, Paris JL, Silva BFB, Pires LR (2020) rapidly dissolving microneedles for the delivery of cubosome-like liquid crystalline nanoparticles with sustained release of rapamycin. Int J Pharm.
Indexed at, Google Scholar, Crossref
- Mokhtari H, Tavakoli S, Safarpour F, Kharaziha M, Bakhsheshi-Rad HR et al (2021) Recent advances in chemically-modified and hybrid carrageenan-based platforms for drug delivery, wound healing, and tissue engineering. Polymers.
Indexed at, Google Scholar, Crossref
- Mukhtar M, Bilal M, Rahdar A, Barani M, Arshad R et al (2020) Nanomaterials for diagnosis and treatment of brain cancer: Recent updates. Chemosensors.
Google Scholar
- Zottel A, Paska AV, Jovčevska I (2019) Nanotechnology meets oncology: Nanomaterials in brain cancer research, diagnosis, and therapy. Materials.
Indexed at, Google Scholar, Crossref
- Teleanu DM, Chircov C, Grumezescu AM, Teleanu RI (2019) Neuronanomedicine: An up-to-date overview. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Rajabi T (2020) Application of Nanomaterials in Brain Cancers Diagnosis and Treatment: A Mini-Review. Am J Biomed Sci Res.
Indexed at, Google Scholar, Crossref
- Sun Q, Barz M, De Geest BG, Diken M, Hennink WE et al (2019) Nanomedicine and macroscale materials in immuno-oncology. Chemical Society Reviews.
Indexed at, Google Scholar, Crossref
- Thakur K, Sharma G, Singh B, Chhibber S, Katare OP (2019) Nano-engineered lipid-polymer hybrid nanoparticles of fusidic acid: an investigative study on dermatokinetics profile and MRSA-infected burn wound model. Drug Deliv Transl Res.
Indexed at, Google Scholar, Crossref
- Wang W (2021) Nano Drug Delivery Strategies for the Treatment of Cancers. Nano Drug Delivery Strategies for the Treatment of Cancers.
Indexed at, Google Scholar, Crossref
- Tang L, Li J, Zhao Q, Pan T, Zhong H et al (2021) Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics.
Indexed at, Google Scholar, Crossref
- Raj S, Khurana S, Choudhari R, Kesari KK, Kamal MA et al (2021) Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Seminars in Cancer Biology.
Indexed at, Google Scholar, Crossref
- Perdomo SJ, Fonseca-Benítez A, Cardona-Mendoza A, Romero-Sánchez C, Párraga J et al (2021) Nano drug delivery strategies for the treatment and diagnosis of oral and throat cancers. In: Nano Drug Delivery Strategies for the Treatment of Cancers.
Indexed at, Google Scholar, Crossref
- Jain P, Kathuria H, Momin M (2021) Clinical therapies and nano drug delivery systems for urinary bladder cancer. Pharmacology and Therapeutics.
Indexed at, Google Scholar, Crossref
- Santos AM, Carvalho SG, Meneguin AB, Sábio RM, Gremião MPD et al (2021) Oral delivery of micro/nanoparticulate systems based on natural polysaccharides for intestinal diseases therapy: Challenges, advances, and future perspectives. Journal of Controlled Release.
Indexed at, Google Scholar, Crossref
- Xia W, Tao Z, Zhu B, Zhang W, Liu C et al (2021) Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int J Mol Sci.
Indexed at, Google Scholar, Crossref
- Sun W, Deng Y, Zhao M, Jiang Y, Gou J et al (2021) Targeting therapy for prostate cancer by pharmaceutical and clinical pharmaceutical strategies. Journal of Controlled Release.
Indexed at, Google Scholar, Crossref
- Paliwal R, Sulakhiya K, Paliwal SR, Singh V, Kenwat R et al (2022) Role of nanoparticles in neurotoxicity. In: Nanomedical Drug Delivery for Neurodegenerative Diseases.
Indexed at, Google Scholar, Crossref
- Zaid NAM, Sekar M, Bonam SR, Gan SH, Lum PT et al (2022) Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Design, Development, and Therapy.
Indexed at, Google Scholar, Crossref
Citation: Sharma RB, Kumar G,Thakur H,
Tomar S, (2023) Nanoemulgel: A Novel Approach
for Topical Delivery System: Updated Review. Int J Drug Dev Res J, Vol. 15 No. 1: 988.








