Keywords
Global cerebral ischaemia, Neurodegenration, oxidative stress, punarnavine
Introduction
Ischemic hypoxic brain injury often causes irreversible brain damage. The cascade of events leading to neuronal injury and death in ischemia includes the release of cytokines and free radicals, and induction of inflammation, apoptosis, and excitotoxicity [1]. Reperfusion of ischemic areas could exacerbate ischemic brain damage through the generation of reactive oxygen species. Along with further studies on cerebral ischemia-reperfusion injury, discovering effective agents for the prevention and treatment of ischemic cerebrovascular disease has become urgent. The lack of effective and widely applicable pharmacological treatments for ischemic stroke patients may explain a growing interest in traditional medicines. Our emphasis on the plant Boerhaavia diffusa L. (family Nyctaginaceae) is due to its widespread distribution throughout India. It is widely distributed in the tropics and subtropics and low altitude mountains of India [2] (especially in the Dhar region of Punjab state of India where this study was conducted) as well as due to its highly claimed medicinal properties. Medicinal properties of the plant have been utilized since long in the indigenous system of medicine in India. Various types of extracts of different plant parts (especially roots and leaves) of B.diffusa have been documented to possess hepatoprotective [3], antiproliferative and antiestrogenic [4], antibacterial [5], spasmoytic [6], anti-fungal [7], anti-diabetic [8], immunomodulatory [9], anti-amoebic [10] and anti-convulsant [11] activity.
Most of the medicinal properties of the plant have been attributed to the alkaloidal constituent Punarnavine. It is also considered as the active principle in the plant extract [12] and recently many studies have documented the key role of Punarnavine in immunomodulatory [13] as well as anti-metastatic [14], activities of the plant. Although there has been some recent research [15] regarding the anti-oxidant potential of leaf extract of B.diffusa, yet to the best of our knowledge, no study has been documented till now exploring the neuroprotective potential of punarnavine, in global cerebral ischaemic injury. Therefore, the present study was designed to investigate the neuroprotective potential of punarnavine in bilateral common carotid artery occlusion induced global cerebral ischaemia in the mice. Edavarone (a potent free radical scavenger and known neuroprotective agent) served as positive control in the research protocol.
Materials and Methods
Animals
Swiss albino mice of either sex weighing 25±5 g were obtained from the departmental animal house. They were maintained on standard laboratory diet and had free access to tap water. Animals were exposed to normal cycle of light and dark before the initiation of study. The experimental protocol was duly approved by Institutional Animal Ethics Committee and care of the animals was carried out as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Reg. No.- 37/ 2007/ CPCSEA).
Drugs and Chemicals
Punarnavine (95% pure) and Edavarone were obtained as gift samples from Bio-gen Extracts Pvt. Ltd., Bangalore, India. Reduced Glutathione (GSH), 5, 5- dithiobis (2-nitro benzoic acid) (DTNB), and thiobarbituric acid were obtained from Loba Chem., Mumbai, India. 1, 1, 3, 3-tetramethoxy propane was procured from Sigma-Aldrich, USA. A terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick-end labeling (TUNEL) apoptosis detection kit was purchased from Tianjin Hao Yang Biotech Co., Ltd. All other chemicals and reagents such as formaldehyde, paraformaldehyde, Hematoxilin-eosin (HE) stain were purchased from Loba Chem., Mumbai, India and Sigma-Aldrich, USA. The chemicals and the reagents used were of Analytical grade. In the primary toxicological studies, when punarnavine was given as an intraperitoneal injection to mice, the maximal dosage (5000 mg/kg) showed no toxic effects, the median lethal dose (LD50) could not be determined, and the maximum tolerable dose (MTD) mice was 5 g/kg. Therefore, we selected the punarnavine dose of 110 mg/kg i.p, which was 1/45 of the MTD in mice. Mice were randomly divided into the sham-operated, control, punarnavine (110 mg/Kg i.p) treated and standard edavarone (8 mg/Kg i.p) treated groups with 10 mice in each group. The edaravone group received an intraperitoneal injection of edaravone (1 mL/kg) twice daily for 4 days. The sham-operated group and control group were administered intragastric 0.5% sodium carboxymethyl cellulose (1 mL/kg), and the punarnavine treated group was given punarnavine (110 mg/Kg i.p) at 08:00 once daily for 4 days. One hour after administration on day 4, the cerebral ischemia-reperfusion model was established following the method reported in the literature [16] by occluding the bilateral common carotid artery (CCA) with an artery clip for 20 min. Mice in the sham-operated group underwent surgical exposure of the bilateral CCA but it was not occluded. After suturing the skin, the mice continued to be medicated as above with rectal temperature maintained at 36.5–37.5°C on a heated laboratory table until awakening from anesthesia. At different times (1, 24, 48 h) after reperfusion, the mice were decapitated, the brain stem and cerebellar cortex were removed, and the brain tissues were examined.
Detection of Histomorphology in Hippocampal CA1
Twenty-four and 48 h after reperfusion, 1–4 mm of brain tissue (coronal slices, 5 μm) were removed from the posterior of the optic chiasm, to obtain hippocampal CA1 region. Slices were stained with hematoxylin and eosin (HE), fixed in formalin, and embedded in paraffin. In 5 visual fields of a highpower microscope (×400), the survival rate of nerve cells was counted (percentage of normal nerve cells among total nerve cells). In normal nerve cells, the nucleus was blue, the cytoplasm was red, the cell membrane was clear, and nucleoli could be seen
Detection of Nerve Cell Apoptosis in Hippocampal CA1
Twenty-four and 48 h after reperfusion, 1–4 mm of brain tissue was dissected from the posterior of the optic chiasm (the remainder was used to detect protein expression), fixed with 10% paraformaldehyde, embedded in paraffin, and 7- μm coronal sections were used to determine the apoptosis rate in the TUNEL assay.) [17] Three nonoverlapping views were taken of each section at high magnification (×400). According to morphologic criteria, TUNEL-positive nuclei with brown granules were considered as apoptotic cells, and normal nuclei were blue. The apoptotic rate was then calculated as the percentage of apoptotic cells among total nerve cells in hippocampal CA1.
Estimation of Serum thiobarbituric acid reactive substances (TBARS) Level
The quantitative estimation of thiobarbituric acid reactive substances (TBARS), an index of lipid peroxidation in isolated brain tissue was performed 48 hours after reperfusion according to the method of Fraga et al [18]. Lipid peroxides in the sample by repeat heating with acid would generate the second product which would react with thiobarbituric acid (TBA) to form TBARS with the maximal absorbance in 532 nm.
Estimation of Serum Glutathione (gSH) Level
The reduced glutathione (gSH) contents in the brain tissue of different groups were estimated 48 hours after reperfusion using method devised by Tietze [19].
Statistical Analysis
The statistical software package SPSS 15.0 (SPSS Inc., Chicago, IL, U.S.A.) was used for all analyses. Data are expressed as mean±S.D. One-way analysis of variance (ANOVA) was used for multigroup pairwise comparisons; the least significant difference test was used for analysis of homogeneity of variance, and Dunnett’s T3 test was used for analysis of heterogeneity of variance. p values of less than 0.05 were considered to represent a statistically significant difference.
Results
Comparison of Histomorphology in Hippocampal CA1
Twenty-four and 48 h after reperfusion, 2 or 3 layers of pyramidal cells were seen in hippocampal CA1 in the sham operated group (Figure 1) Those pyramidal cells were arranged closely and neatly, and the nuclei were large and round with 1 or 2 nucleoli. In the control group, pyramidal cells had lost their normal structure and were disorganized. Vacuolate degeneration and eosinophilic-like changes in nerve cells were seen in the cytoplasm, and many nuclei had become pyknotic (shrunken and dark). Compared with the sham-operated group, the survival rate of nerve cells decreased in the model group (p<0.01). At 24 h and 48 h after reperfusion, vacuolated degeneration, eosinophilic-like changes, and karyopyknosis of nerve cells were reduced in the punarnavine and edaravone groups compared with the control group and survival rates increased (all p<0.01); the increased survival rate in the edaravone group was more marked than that in the punarnavine group (both p<0.01).
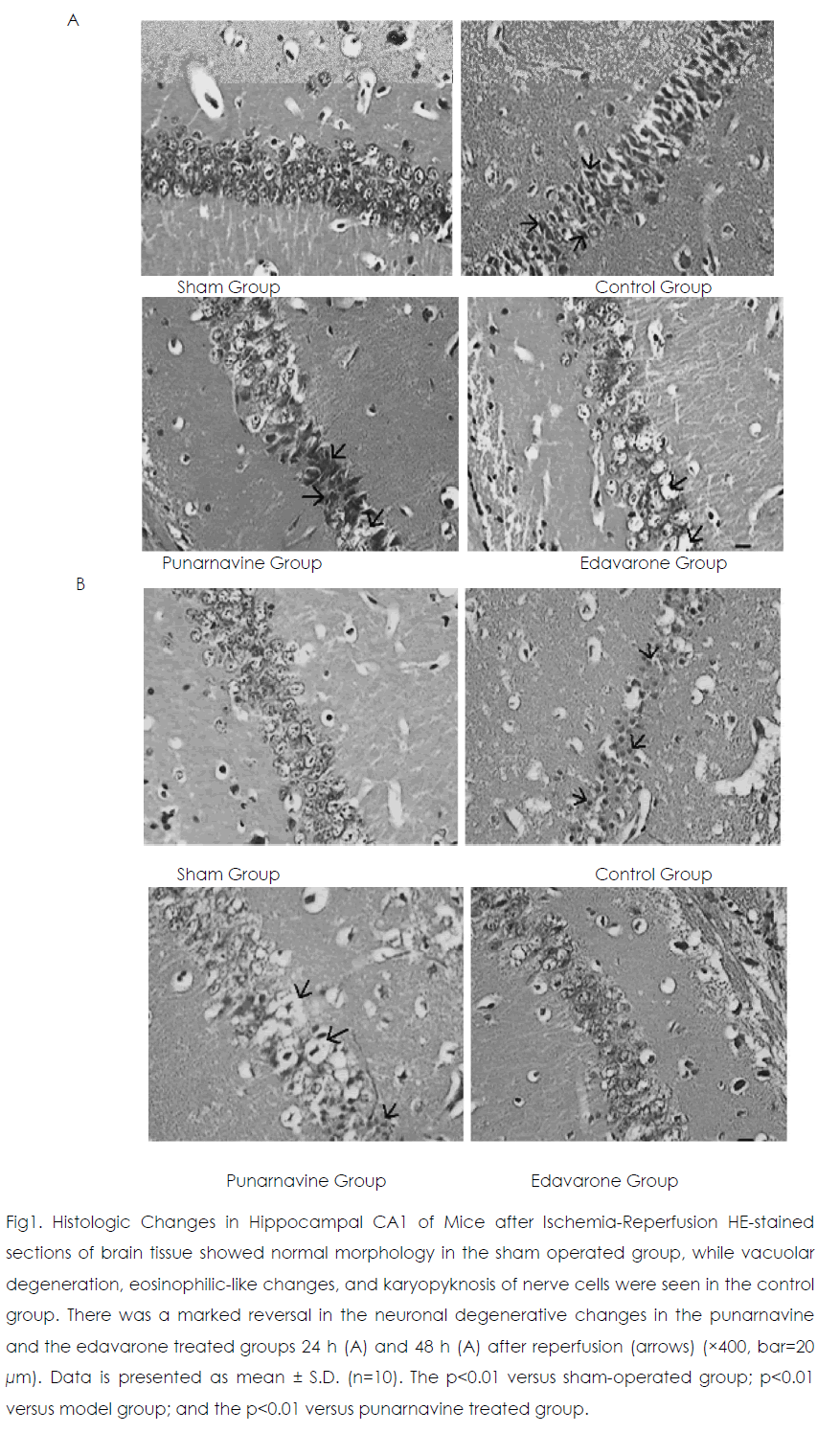
Figure 1: Histologic Changes in Hippocampal CA1 of Mice after Ischemia-Reperfusion HE-stained sections of brain tissue showed normal morphology in the sham operated group, while vacuolar degeneration, eosinophilic-like changes, and karyopyknosis of nerve cells were seen in the control group. There was a marked reversal in the neuronal degenerative changes in the punarnavine and the edavarone treated groups 24 h (A) and 48 h (A) after reperfusion (arrows) (×400, bar=20 μm). Data is presented as mean ± S.D. (n=10). The p<0.01 versus sham-operated group; p<0.01 versus model group; and the p<0.01 versus punarnavine treated group.
Comparison of Apoptosis in Hippocampal CA1
At 24 h and 48 h after ischemia-reperfusion, there were scattered individual apoptotic cells in hippocampal CA1 in the sham operated group. Numerous apoptotic cells were seen in the model group, and the apoptotic rate was significantly higher than that in the sham-operated group (both p<0.01) (Figure 2). Compared with the model group, the apoptotic rates were significantly decreased in the Punarnavine extract group and edaravone group 24 h and 48 h after reperfusion (all p<0.01), and the decreased apoptotic rate in the edaravone group was more obvious than that in the Punarnavine group (both p<0.01).
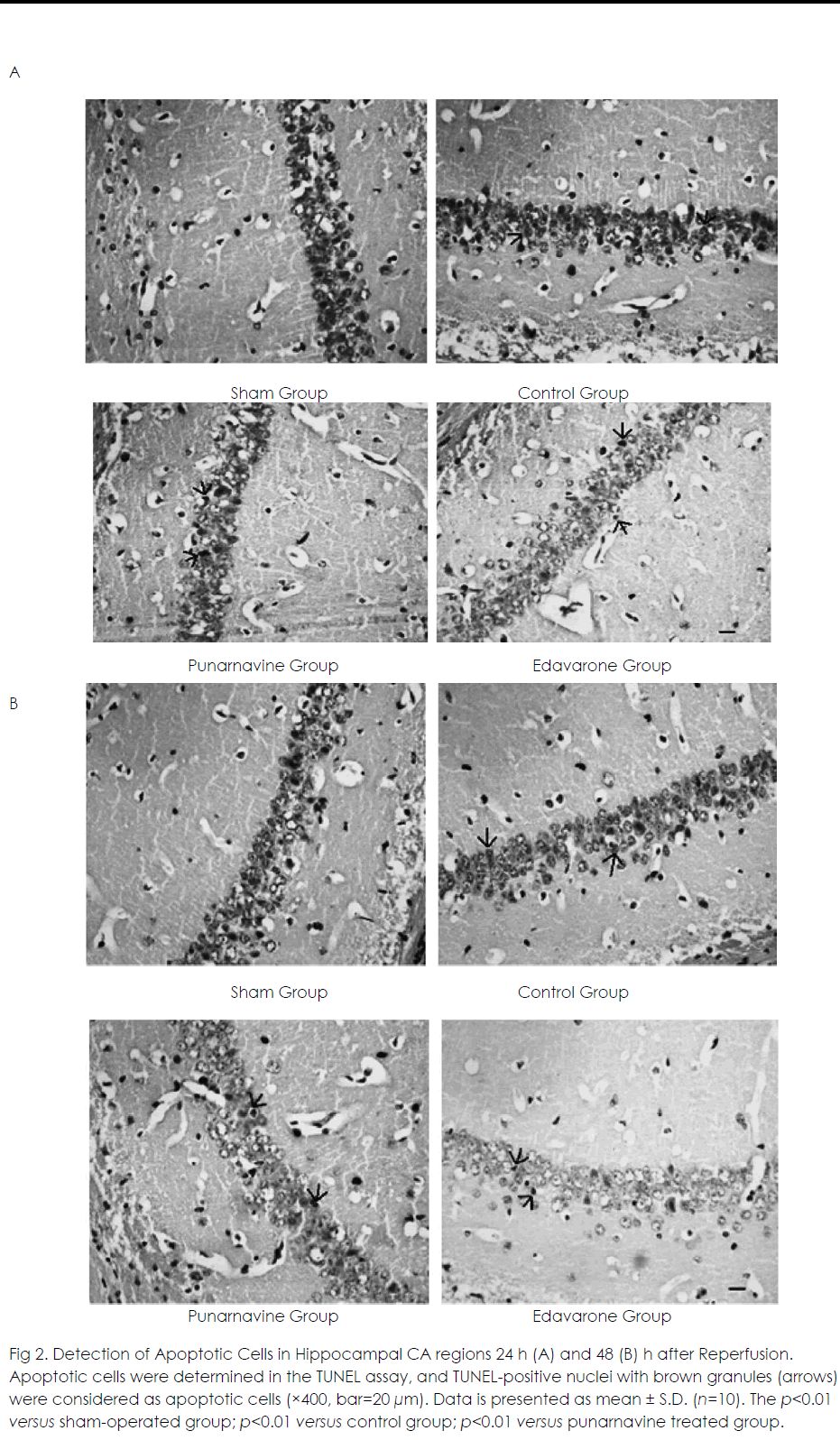
Figure 2: Detection of Apoptotic Cells in Hippocampal CA regions 24 h (A) and 48 (B) h after Reperfusion. Apoptotic cells were determined in the TUNEL assay, and TUNEL-positive nuclei with brown granules (arrows) were considered as apoptotic cells (×400, bar=20 μm). Data is presented as mean ± S.D. (n=10). The p<0.01 versus sham-operated group; p<0.01 versus control group; p<0.01 versus punarnavine treated group.
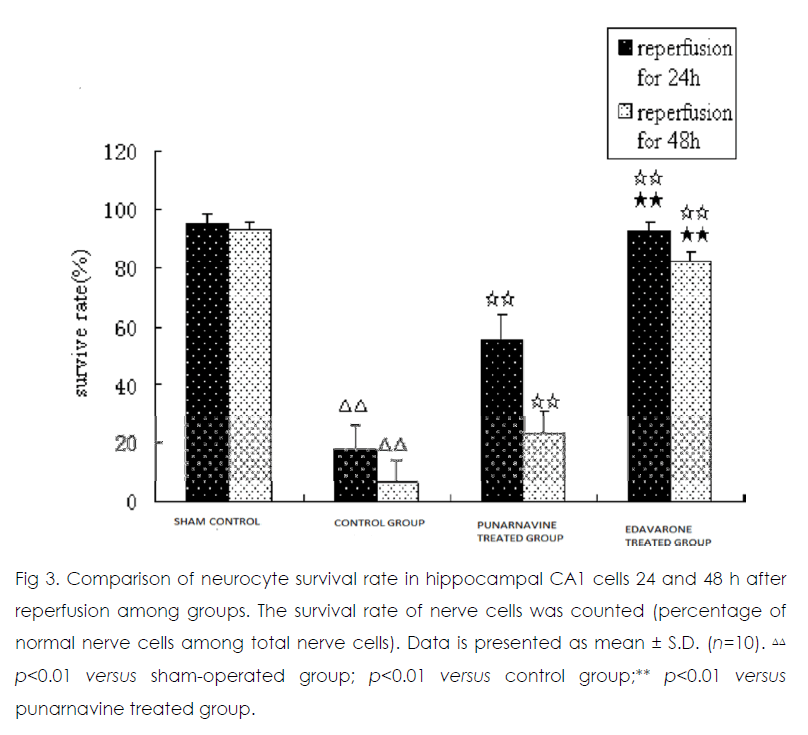
Figure 3: Comparison of neurocyte survival rate in hippocampal CA1 cells 24 and 48 h after reperfusion among groups. The survival rate of nerve cells was counted (percentage of normal nerve cells among total nerve cells). Data is presented as mean ± S.D. (n=10).ΔΔ p<0.01 versus sham-operated group; p<0.01 versus control group;** p<0.01 versus punarnavine treated group.
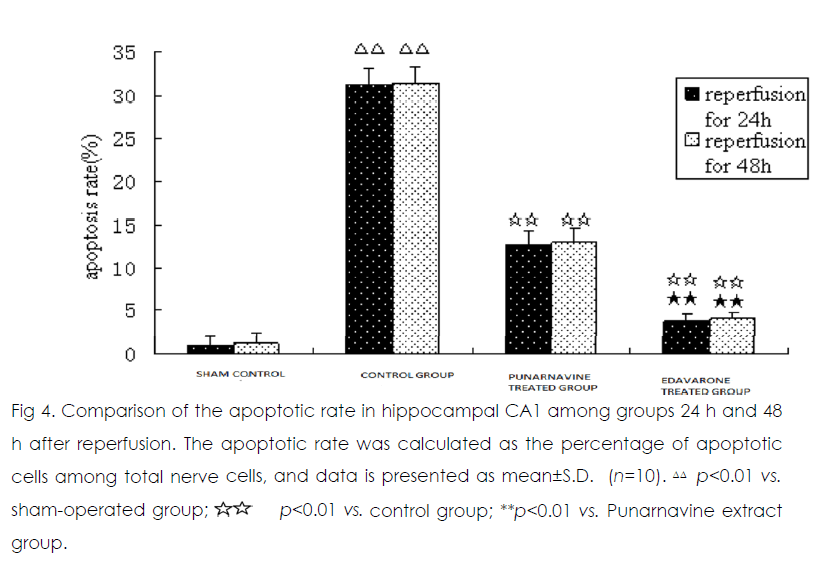
Figure 4: Comparison of the apoptotic rate in hippocampal CA1 among groups 24 h and 48 h after reperfusion. The apoptotic rate was calculated as the percentage of apoptotic cells among total nerve cells, and data is presented as mean±S.D. (n=10). ΔΔ p<0.01 vs. sham-operated group; p<0.01 vs. control group; **p<0.01 vs. Punarnavine extract group.
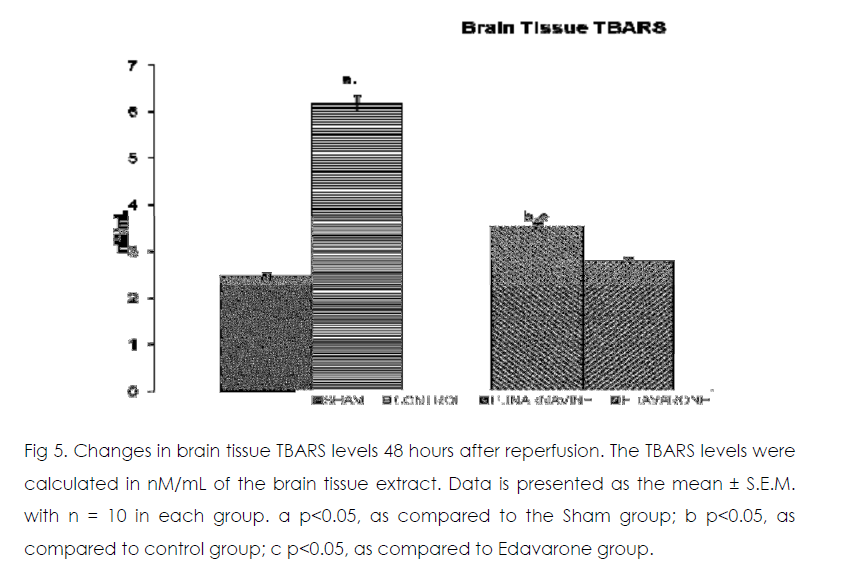
Figure 5: Changes in brain tissue TBARS levels 48 hours after reperfusion. The TBARS levels were calculated in nM/mL of the brain tissue extract. Data is presented as the mean ± S.E.M. with n = 10 in each group. a p<0.05, as compared to the Sham group; b p<0.05, as compared to control group; c p<0.05, as compared to Edavarone group.
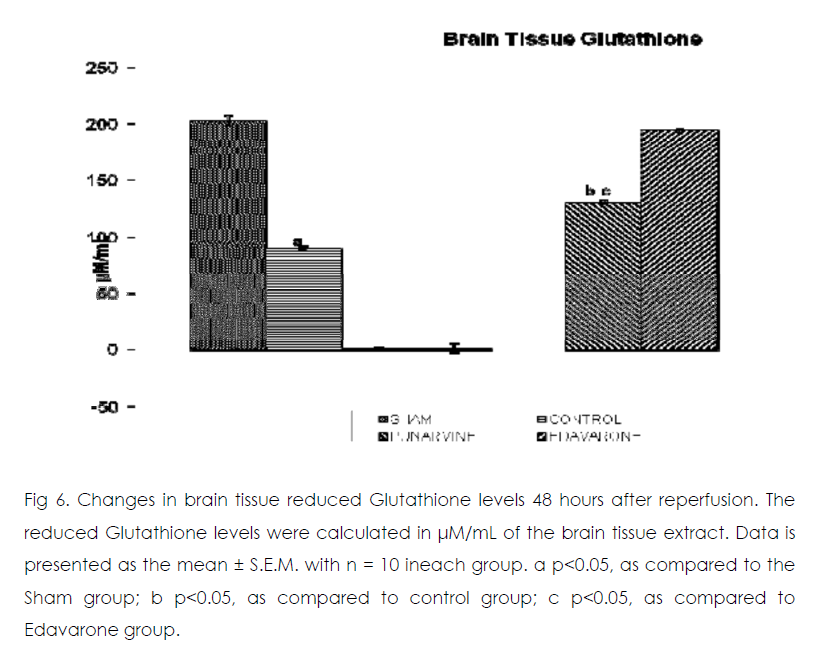
Figure 6: Changes in brain tissue reduced Glutathione levels 48 hours after reperfusion. The reduced Glutathione levels were calculated in μM/mL of the brain tissue extract. Data is presented as the mean ± S.E.M. with n = 10 ineach group. a p<0.05, as compared to the Sham group; b p<0.05, as compared to control group; c p<0.05, as compared to Edavarone group.
Effect of Punarnavine on cerebral ischaemiareperfusion induced changes in the brain oxidative stress markers enzymes
As compared to normal mice group occlusion of bilateral common carotid artery of control and drug treated mice for 20 min followed by 1-h reperfusion resulted in significant increase in brain tissue thiobarbituric acid reactive substances (TBARS) and decrease in reduced glutathione levels. Punarnavine treatment (110 mg/kg i.p) significantly attenuated ischaemia-reperfuison induced rise in TBARS and decrease in glutathione levels in the brain tissue.
Discussion and Conclusion
According to the patterns of manifestation after ischemia, brain damage is divided into two phases. The first is acute damage in a series of reactions following cerebral ischemia, which lead to acute neurocyte death. The main pathophysiologic mechanisms include energy metabolism disturbance; calcium overload; the production of oxygen free radicals, nitric oxide, and neurotoxic excitatory amino acids; and destruction of the BBB. The second phase is delayed neuronal damage (DND). After cerebral ischemia-reperfusion, ischemia triggers the mechanisms of secondary injury, further damaging nerve cells. Increasing evidence has indicated that ischemia/reperfusion occurs due to oxidative stress that may potentiate ischemic injury [20].The main pathophysiologic mechanisms of DND are inflammatory reaction and apoptosis. Oxidative stress and the antioxidant system have important roles in the pathophysiology of cerebral changes and brain disorders. The decreased concentration of GSH in the brain might be due to NADPH reduction or GSH utilization in the exclusion of peroxides [21]. GSHdependent enzymes offer a second line of defense because they detoxify noxious byproducts generated by ROS and also help to avert the dissemination of free radicals [22]. GSHBhatia Px detoxifies peroxides by reacting with GSH and converting it to GSSG, which is reduced to GSH by GSR [23]. GSHpx, glutathione reductase (GSR) and glutathione-S-transferase are basic antioxidant enzymes. Therefore, the significant decrease in the level of reduced glutathione in the brain is strongly linked with neurodegenerative diseases.
Here we showed that the administration of punarnavine in mice significantly improved the neurodegenerative outcomes after global cerebral ischemia and reperfusion in terms of protection against hippocampal damage and neuronal apoptosis rate and extent. At the same time, administration of punarnavine in mice significantly boosted the defense mechanism against cerebral ischemia by decreasing the levels brain tissue free radicals level (as evident by significant decrease in TBARS levels in brain) and by increasing antioxidant enzyme level activity related to lesion pathogenesis (as evident by significant increase in reduced glutathione levels in brain). Restoration of the antioxidant homeostasis in the brain after reperfusion may have helped the brain recover from ischemic injury. The positive control edaravone is a free radical scavenger against oxidative damage and apoptosis, [24, 25] which was also confirmed in the present study.
It was further observed that cell apoptosis and the apoptosis rate increased markedly 24 h and 48 h after cerebral ischemia-reperfusion. Punarnavine did attenuate nerve cell apoptosis, suggesting that it has an anti-apoptotic effect after cerebral ischemia-reperfusion.
It can be concluded from the present study that administration of punarnavine attenuated the pathological outcomes of global cerebral ischaemia reperfusion injury induced neuronal cell damage. This study also highlighted the antiapoptotic potential of punarnavine. However, further studies are still required to delineate the molecular mechanism on neuronal protection afforded by punarnavine in conditions of cerebral ischaemia-reperfusion induced neuronal cell injury.
7286
References
- Kuroda S, Siesjo BK. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci 1997; 4:199-212.
- CSIR. The Wealth of India: Raw Materials Vol. 7 B. CSIR, New Delhi, India. 1988. p-174.
- Olaleye MT, Akinmoladun AC, Ogunboye AA, Akindahunsi AA, Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen- induced liver damage in rats. Food Chem Toxicol; 2010, 48(8-9): 2200-2205.
- Sreeja S, Sreeja S. An in vitro study on antiproliferative and antiestrogenic effects of Boerhaavia diffusa L. extracts. J Ethnopharmacol 2009; 126(2): 221-225.
- Nair R, Kalariya T, Chanda S. Antibacterial activity of some plant extracts used in folk medicine. J Herb Pharmacother 2007; 7(3-4):191-201.
- Borrelli F, Ascione V, Capasso R, Izzo AA, Fattorusso E, Taglialatela-Scafati O. Spasmolytic effects of nonprenylated rotenoid constituents of Boerhaavia diffusa roots. J Nat Prod 2006; 69(6): 903-906.
- Agrawal A, Srivastava S, Srivastava MM, Antifungal activity of Boerhavia diffusa against some dermatophytic species of Microsporum. Hindustan Antibiot Bull 2004; 45-46(1-4): 1-4.
- Pari L, Amarnath SM. Antidiabetic effect of Boerhavia diffusa: effect on serum and tissue lipids in experimental diabetes. J Med Food 2004; 7(4): 472-476.
- Mehrotra S, Singh VK, Agarwal SS, Maurya R, Srimal RC. Anti-lymphoproliferative activity of ethanolic extract of Boerhaavia diffusa roots. Exp Mol Pathol 2002; 2(3): 236-242.
- Sohni YR, Kaimal P, Bhatt RM. The antiamoebic effect of a crude drug formulation of herbal extracts against Entamoeba histolytica in vitro and in vivo. J Ethnopharmacol 1995; 45(1): 43-52.
- Kaur, M., Goel, R.K., Anti-convulsant Activity of Boerhaavia diffusa: Plausible Role of Calcium Channel Antagonism. Evid Based Complement Alternat Med. 2009. [Epub ahead of print].
- Manu KA, Kuttan G. Immunomodulatory activities of Punarnavine, an alkaloid from Boerhaavia diffusa. Immunopharmacol Immunotoxicol 2009a; 31(3): 377-387.
- Manu, K.A., Kuttan, G., Anti-metastatic potential of Punarnavine, an alkaloid from Boerhaavia diffusa Linn. Immunobiol 2009b; 214(4): 245-255.
- Gulati V, Harding IH, Palombo EA. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement and Alter Med 2012; 12:77-85.
- Yang G, Kitagawa K, Matsushita K, Mabuchi T, Yagita Y, Yanagihara T, Matsumoto M. C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: selective neuronal death in the murine transient forebrain ischemia. Brain Res1997; 752: 209–218.
- Teshima Y, Akao M, Li RA, Chong TH, Baumgartner WA, Johnston MV, Marbán E. Mitochondrial ATP- sensitive potassium channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress. Stroke 2003; 34: 1796–1802.
- Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid- reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 1988; 4:155–161.
- Tietze, F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: application to mammalian blood and other tissues. Anal Biochem 1969; 27: 502-509.
- Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol 1991; 71:1185-1195.
- Yadav P, Sarkar S, Bhatnagar D. Action of Capparis deciduas against alloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res 1997; 36: 221–228.
- Gumieniczek A. Effects of repaglinide on oxidative stress in tissues of diabetic rabbits. Diab Res Clin Pract 2005; 68: 89–95.
- Maritim AC, Sanders RA, Watkins JB. Effects of α- lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem 2003; 14: 288–294.
- Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev 2006; 12: 9– 20.
- Rajesh KG, Sasaguri S, Suzuki R, Maeda H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am. J. Physiol. Heart Circ. Physiol 2003; 285: H2171– H2178.












