Meltem Ugras1*, Ozgur Kavak2, Faruk Alpay2, Selim Karabekir H3 and Suat Bicer4
1Yeditepe University, Medical Faculty, Department of Pediatrics, Pediatric Gastroenterology, Hepatology and Nutrition, Istanbul, Turkey
2Afyon Kocatepe University, Medical Faculty, Department of Pediatrics, Afyonkarahisar, Turkey
3Dokuz Eylül University, Medical Faculty, Department of Neurosurgery, Izmir, Turkey
4Yeditepe University, Medical Faculty, Department of Pediatrics, Istanbul, Turkey
*Corresponding Author:
Dr. Meltem Ugras
Associate Professor, Address. Yeditepe University, Medical Faculty, Department of Pediatrics, Division of Pediatric astroenterology, Hepatology and Nutrition, Devlet Yolu, Ankara Cad No 102-104, Kozyatagi 34752 Istanbul, Turkey.
Tel: +90 216 5784000; +90 5327834398
Fax: +90 216 4693796
E-mail: meltemkorkut@yahoo.com, meltem.ugras@yeditepe.edu.tr
Received Date: Oct 01, 2015; Accepted Date: Feb 15, 2016; Published Date: Feb 20, 2016
Keywords
Encephaloceles; Lissencephaly; Hydrocephalus; Cerebellar malformation; Meckel-Gruber syndrome
Introduction
An encephalocele results from failure of the surface ectoderm of to separate from the neuroectoderm. This leads to a bony defect in the skull table, which allows herniation of the meninges or of brain tissue [1]. The prevalance ranges from 0.8 to 4 per 10.000 live births [2-10]. The occiput is the most common site of this type of neural tube defect in the United States and Western Europe. Approximately 90% involve the midline. Currently, most cases are diagnosed prenatally. Other malformations and/or chromosomal defects are related in 60% of patients with encephalocele [11,12].
Here we describe 5 cases with occipital encephalocele, their clinical findings, surgical intervention and follow-up.
Material and Methods
Between January 2008 and October 2011 newborn babies who were diagnosed with occipital encephalocele were searched through the *hospital records retrospectively. Gestational week, gestation history, folic acid administration during early pregnancy, physical findings, medical and surgical treatment history as well as postoperative problems and follow-up consequences were evaluated. All patients and families were called for a thorough evaluation of physical findings and neurological examination. Consent was taken from all the families.
Case Reports
In a 4-years’ period, 5 female patients were diagnosed with occipital encephalocele (Table 1). The diagnosis was made by prenatal ultrasonography. Among five mothers one (Case 2) was over-aged (45-years old). Medical history revealed that only 2 mothers used folic acid (FA), -tablets containing 5 mg folic acid, once daily, beginning after being aware of the pregnancy- neither initiated preconceptionally, nor consumed regularly. The remaining 3 mothers did not use any supplements. No mothers used any kind of drugs during pregnancy, and none were diabetic. All the families were natives of the city, and no close consanguinity was revealed. None of the families had another child with any kind of congenital anomalies, including cranial defects. Mother of Case 2 was exposed to radiation as she was not aware of her pregnancy at 5 months of gestation. None of the babies were products of IVF. All babies were born with cesarean sectio. The size of occipital encephalocele varied between 7 cm × 6 cm and 28 cm × 20 cm (Figure 1). Case 1 had dismorphic face and contractures at elbows. Case 4 additionally had thoracal meningomyelocele (Figure 2). Pre- and postoperative weight, height and head circumference and relevant demographic data are given in Table 1. All patients had normal thyroid, liver and renal function tests. Abdominal ultrasonography revealed no abnormality in any child. Case 5 had anencephaly and occipital encephalocele (Figure 3), and was lost in the 48th hour of her life, due to respiratory failure. Cases 2, 3, 4, and 5 had afebrile convulsions, all those baby were administered phenobarbital orally. There were no terminations of pregnancy for encephalocele at our hospital during the 4 year period-due to religious reasons.
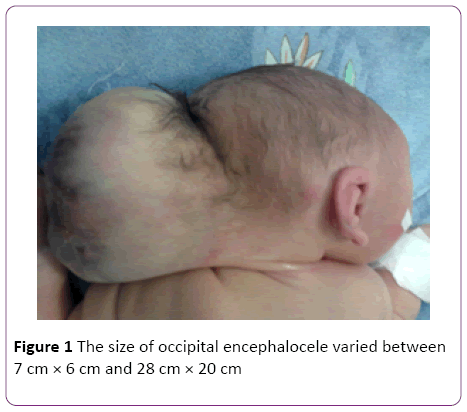
Figure 1: The size of occipital encephalocele varied between 7 cm × 6 cm and 28 cm × 20 cm
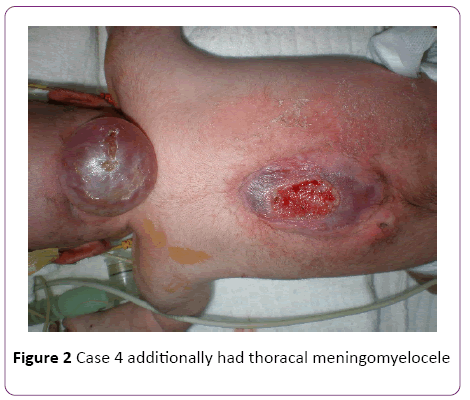
Figure 2: Case 4 additionally had thoracal meningomyelocele
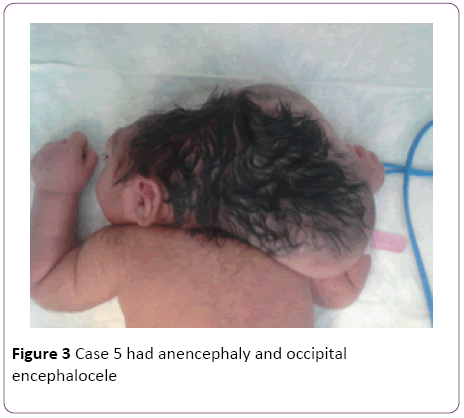
Figure 3: Case 5 had anencephaly and occipital encephalocele
| |
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
| Gestational week |
34 |
36 |
37 |
36 |
38 |
| Prenatal diagnosis |
Yes |
Yes |
Yes |
Yes |
Yes |
| Maternal age |
24 |
45 |
24 |
24 |
24 |
| Paternal age |
28 |
54 |
29 |
33 |
28 |
| Consanguinity |
No |
No |
Yes (30) |
No |
No |
| No of pregnancy |
3 |
8 |
2 |
2 |
1 |
| FA consumption |
Irregular |
None. |
No |
Irregular |
No |
| Apgar score |
NA |
7-9 |
NA |
2-5 |
0-6 |
| Sex |
Girl |
Girl |
Girl |
Girl |
Girl |
| Birth weight(p) |
1860 gr (25-50) |
2630 gr (50-75) |
2960 gr (50-75) |
2790 gr (25-50) |
2360 gr (10) |
| Birth height(p) |
41 cm (3-10) |
45 cm (10-25) |
50 cm (75-90) |
45 cm (10-25) |
49 cm (50-75) |
| Birth HC (p) |
30 cm (10-25) |
28 cm (>3p) |
33 cm (25-50 ) |
31 cm (10-25) |
30 cm (under 10p ) |
| Encephalocele size |
7× 6cm |
28× 20cm |
14× 10cm |
8× 9cm |
15× 18cm |
| Other anomaly |
Dismorphic face, narrow chest |
none |
None |
none |
none |
| Convulsion |
No |
Yes |
Yes |
Yes |
No |
| Chromosomeanalysis |
Performed |
N/D |
N/D |
N/D |
N/D |
| Current age |
10 months |
14 months |
26 months |
5 months |
exitus |
| Currentweight (p) |
4660 gr(<3p) |
4360 gr(<3p) |
12.5 kg(50p) |
6060 gr (3-10) |
exitus |
| Current height(p) |
58 cm(<3p) |
63 cm(<3p) |
83 cm(<3p) |
62cm (3-10) |
exitus |
| Current head circum.(p) |
38(<3p) |
37(<3p) |
46(3-10p) |
37cm (<3p) |
exitus |
Table 1: The demographic data, preopretive and postoperative physical examination findings of the patients with occipital encephalocele
In all cases, encephaloceles were resected totally with primary closure by neurosurgery department. Ventriculoperitoneal (V-P) shunts were placed to all of the cases because of hydrocephaly. Case 5 was operated on the second day of her life and was lost due to respiratory failure after operation.
All the 4 cases were alive at the time of review, with the longest lifetime being 30 months. The karyotype analysis of Case 1 was normal and the dismorphic feature did not match with a known syndrome. The babies have feeding difficulties and Case 2 is feeding via nasogastric tube. All babies were frequently hospitalized due to lower respiratory and urinary tract infections. All are under physical rehabilitation programmes. Case 2 is hospitalized due also to feeding problems and once for formula aspiration, she has weak gag reflex; yet, the family is not in favour of any kind of gastrostomy. Case 3 had bacterial meningitis when she was 18 months old and was recovered by antibiotic therapy and shunt revision. The babies are under the 3rd centile for height and most are at the low centiles for their ages. In the presented study the oldest patient is 2,5 years old. Case 2, 3 and 4 are receiving anticonvulsant drugs as they had convulsions during their follow-up period and did not have any convulsions later on.
Discussion
Encephalocele represents one end of the spectrum of open neural tube diagnoses [1]. The prenatal ultrasonographic diagnosis of an encephalocele is based on the demonstration of a cranial defect with varying degrees of brain herniation. The size of the bony defect can vary from a few millimeters to centimeters, and the sac can be larger than the fetal skull itself as in Case 2 and Case 5 of our series. Rarely prenatal ultrasonographic findings may mimic scapl cysts [13]. Pathological evaluation of the encephalocele specimens in our study revealed brain tissue in all cases. Because skull ossification begins at 10th gestational week, diagnosis is usually not possible before this time. In the present study the diagnosis of all cases were made prenatally.
Although all encephaloceles are thought to be sporadic, once an encephalocele is diagnosed, a search should be made for associated anomalies, such as Meckel-Gruber syndrome (which is characterized by an occipital encephalocele), microcephaly, cystic dysplastic kidneys, and polydactyly [11,14]. Meckel-Gruber syndrome may be more easily diagnosed in the first trimester, when the amniotic fluid volume is usually normal. In the second trimester, oligohydramnios may hamper visualization of polydactyly and the encephalocele. Walker-Warburg syndrome, which also is associated with encephaloceles, is a lethal complex of the central nervous system (CNS) and eyes [15]. The diagnosis is established by the detection of lissencephaly, hydrocephalus, and a cerebellar malformation. Recognizing these syndromes is important because they are autosomal recessive conditions and such syndromes are more frequent in countries where consanguinity marriages take place as in Turkey. Among the cases presented here, only one had dismorhic features but also had normal karyotype, and her findings did not match the above syndromes.
In the presence of an encephalocele, there is a 60-80% risk of associated structural abnormalities like optical, choroidal and retinal dysplasia, [16], severe ocular alterations [17], central nervous system anomalies [18], dermoid cyst [19], tectocerebellar dysraphia [20], and necrosis [21]. More than 60% of these patients may also develop hydrocephalus requiring a V-P shunt [22,23]. Survival rates and morbidity of encephalocoeles vary most strongly with anatomical sites being 100 and 50% respectively in the case of anterior defects and 55 and 83% respectively in the case of posterior defects [24].
FA deficiency or low consumption during early gestation is suggested to be a predisposing factor for neural tube defect. Recently it is reported that food fortification with folic acid has significantly reduced neural tube defects including encephaloceles [1]. There are similar reports from different countries showing that fortifying foods and FA supplementation reduces the incidence of encephaloceles [25]. Although among our cases only two mothers irregularly used FA and three did not use any FA, which hampers the fact of FA defficiency as a possible cause of the defects.
Although advances in surgical technique have improved outcomes in some types of encephalocele, overall morbidity and mortality remain high. Postoperative prognosis of posterior and occipital encephalocele is reported to be worse than parietal counterparts [13]. In a study of 167 encephalocele cases 70% were livebirths, 76% were isolated, the abnormality was more common among females infants and those weighing less than 2.5 kg. Infants weighting more than 3.5 kg had lower prevalence rates, while prevalence did not vary significantly with maternal age or plurality [26]. In our series, all the newborns were females, and only one mother was aged over 35 who had multiplurality.
This report has some drawbacks; we cannot estimate the prevalence of encephalocele because our hospital is a university hospital of the city, while there are four more hospitals (private and government) in the same city. As our hospital is a university hospital the complex cases are internalised so our population cannot reflect the whole population. One other factor is the exact birth numbers (livebirth and stillbirths) could not be taken from all those hospitals.
Conclusion
In conclusion, occipital encephalocele is a life-threatening cranial anomaly. The overall outcome of the patient depends on the site and dimension of the lesion, as well as the presence of accompanying congenital anomalies. Close multidisciplinary follow-up helps improvement of quality of life after surgery. Folic acid supplementation should be accepted and performed as national health policy particularly in countries where consanguious marriages take place.
Signed Statement
The author(s) transfer(s) all copyright ownership of the manuscript entitled (title of article) to The Turkish Journal of Pediatrics in the event the work is published. The author(s) warrant(s) that the article is original, is not for consideration by another journal, has not been previously published (except in a congress report), and has been prepared according to the manuscript rules".
8542
References
- Ghatan S (2011) Encephalocele : Cranial development Abnormalities:Pediatrics. In:Winn RH(ed),Youmans Neurological Surgery. Philadelphia,PA: Elsevier London1898-1905.
- Bell WO, Nelson LH, Block SM (1996) Prenatal diagnosis and pediatric neurosurgery. Pediatr Neurosurg24:134-137.
- Boyd PA, Wellesley DG, De Walle HE (2000) Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. J Med Screen. 7:169-174.
- Budorick NE, Pretorius DH, McGahan JP (1995) Cephalocele detection in utero: sonographic and clinical features. Ultrasound Obstet Gynecol5:77-85.
- Goldstein RB, LaPidus AS, Filly RA (1991) Fetal cephaloceles: diagnosis with US. Radiology180:803-808.
- Jeanty P, Shah D, Zaleski W (1991) Prenatal diagnosis of fetal cephalocele: a sonographic spectrum. Am J Perinatol8:144-149.
- Mernagh JR, Mohide PT, Lappalainen RE (1999) US assessment of the fetal head and neck: a state-of-the-art pictorial review. Radiographics19:229-2241.
- van Zalen-Sprock RM, van Vugt JM, van Geijn HP (1996) First-trimester sonographic detection of neurodevelopmental abnormalities in some single-gene disorders. Prenat Diagn16:199-202.
- Levine D, Barnes PD (1999) Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology210:751-758.
- Köhrmann M, Schellinger PD, Wetter A (2007) Nasal meningoencephalocele, an unusual cause for recurrent meningitis. Case report and review of the literature. J Neurol254:259-260.
- Stoll C, Alembik Y, Dott B (2007) Associated malformations in cases with neural tube defects. Genet Couns 18:209-215.
- Chen CP, Chern SR, Wang W (2000) Rapid determination of zygosity and common aneuploidies from amniotic fluid cells using quantitative fluorescent polymerase chain reaction following genetic amniocentesis in multiple pregnancies. Hum Reprod 15:929-934.
- Shahabi S, Busine A (1998) Prenatal diagnosis of an epidermal scalp cyst simulating an encephalocoele. Prenat Diagn18:373-377
- Wininger SJ, Donnenfeld AE (1994) Syndromes identified in fetuses with prenatally diagnosed cephaloceles. Prenat Diagn14:839-843.
- Martinez-Lage JF, Garcia Santos JM, Poza M (1995) Neurosurgical management of Walker-Warburg syndrome. Childs Nerv Syst 11:145-153.
- Mayer U, Klinger M, Rott HD (1982) A rare form of optical, choroidal and retinal dysplasia combined with an occipital encephalocele. Graefes Arch Clin Exp Ophthalmol219:72-75.
- Sertie AL, Quimby M, Moreira ES, Murray J, Zatz M, et al. (1996) A gene which causes severe ocular alterations and occipital encephalocele (Knobloch syndrome) is mapped to 21q22.3. Hum Mol Genet5:843-847.
- Lin HJ, Cornford ME, Hu B, Rutgers JK, Beall MH, et al. (1996) Occipital encephalocele and MURCS association: Case report and review of central nervous system anomalies in MURCS patients. Am J Med Genet61:59-62.
- Martinez-Lage JF, Poza M, Ramos J, Almagro MJ, Sempere M, et al. (1992) Occipital encephalocele associated with a dermoid cyst. J Child Neurol7:427-430.
- Chowdhary UM, Ibrahim AW, Ammar AS, Dawodu AH (1989) Tectocerebellar dysraphia with occipital encephalocele. Surg Neurol31:310-314.
- Sinha A, Ojha S, Mahajan JK (2005) Gangrene of an occipital encephalocele. Indian J Pediatr72:451
- Walia B, Bhargava P, Sandhu K (2005) Giant occipital encephalocele. MJAFI61:293-294.
- Hoving E, Blaser S, Kelly E, Rutka JT (1999) Anatomical and embryological considerations in the repair of a large vertex cephalocele. J Neurosurg90:537-541.
- Lorber J, Schofield JK (1979) The prognosis of occipital encephalocele. Z Kinderchir Grenzgeb28:347-351.
- Amarin ZO, Obeidat AZ (2010) Effect of folic acid fortification on the incidence of neural tube defects. Paediatr Perinat Epidemiol24:349-351.
- Rowland CA, Correa A, Cragan JD, Alverson CJ (2006) Are encephaloceles neural tube defects? Pediatrics118:916-923.








