Keywords:
Pterygium; Conjunctiva; Auto graft
Introduction
Pterygium is the result of aberrant wound healing process characterised by centripetal growth of limbal epithelial cells followed by squamous metaplasia giving rise to pterygial epithelium with goblet cell hyperplasia. Underlying stroma consists of activated proliferating fibroblasts, neovascularisation, inflammatory cells with remodelled extra cellular matrix. Thus it can be considered as a degenerative condition of subconjunctival tissue triggered by some ocular surface disorder [1] leading to dysfunction of limbal stem cells. It has a proliferative and locally invasive apex where conjunctival epithelium abruptly transition into corneal epithelium at the advancing edge. Goblet cell hyperplasia is prominent in pterygium epithelium, compared with autologus normal conjunctiva [2]. Thus conjunctiva over the body of pterygium can be used for graft.
Treatment ranges from bare sclera to grafts [3]. But recurrence is the main complication whose rate is extremely variable and unpredictable. Any surgical treatment of pterygium is complicated by its recurrence. Bare sclera has highest recurrence rate and to reduce this high incidence of recurrence covering the exposed sclera with grafts were introduced. These includes Conjunctival auto grafts(CAG)with or without stem cells, amniotic membrane transplant (AMT).Different adjuvant therapy ranging from beta irradiation to antimitotic agents and more recently anti vasculoendothelial growth factors like bevacizumab [4] were also started. These were not devoid of complications due to cytotoxicity or offered no added advantage [4], hence lost their popularity. Pterygium itself has origins from cytotoxic effect of UV radiation. Moreover unstable tear film and cytotoxic effects of UV radiation can incite vasculoendothelial growth factors (VEGF) production which can give rise to pterygium formation [3]. Histopathologically conjunctiva is not at fault in pterygium. This is an elastotic degeneration of Subconjunctival tissues. So conjunctiva overlying pterygium can be used for covering bare sclera which can give stable tear film and protect from UV radiation thus preventing recurrence.
This severe form of OSD is mostly a tropical problem (Figure 1) [1].

Figure 1: All ages affected with equal gender distribution
Histopathological studies of pterygium has shown fibrovascular proliferation combined with subconjunctival elastotic degeneration (Figure 2). Its effect can be a cosmetic blemish due to altered ocular surface with recurrent inflammation. Visual defects can range from irregular astigmatism to total obstruction of visual axis
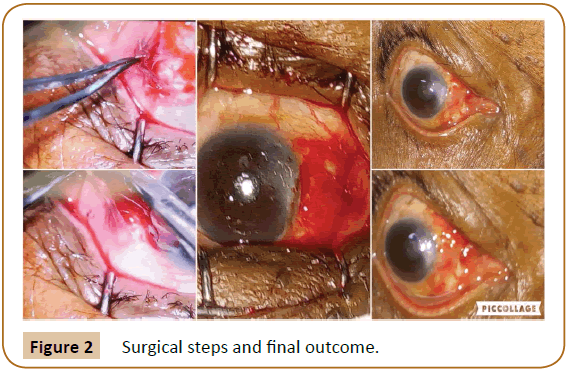
Figure 2: Surgical steps and final outcome.
Treatment options are excision, redirection, adjuvant medication in the form of thiotepa, beta radiation and cytotoxic drugs can give rise to necrosis of sclera tissue. Excision with graft has now become the treatment of choice. Graft could be amniotic membrane, limbal stem cells or conjunctiva itself and secured by sutures, glue or autologous fibrin. But altered ocular surface, the main trigger for pterygium formation still remains. As no procedure stood the test of time so no treatment of choice has evolved so far.
Aim of this study is to evaluate the result of a new surgical procedure of grafting of healthy conjunctiva overlying the body of pterygium with reversal of direction secured with autologous fibrin. This procedure is named as Conjunctival in situ auto graft (CISAG) .The theory behind this procedure is removal of triggering apical growth with alteration of direction of abnormal growth.
Outcome measure is recurrence which is defined as new fibro vascular growth across the limbus and measured as more than 1 mm regrowth. Minimum follow-up period is 1 year.
Methods
This is a single centre prospective interventional study. Informed consent from the patient and ethical committee approval from Institute was obtained for this study. Procedure was performed by same surgeon to ensure consistency. Operations were performed under peribulbar anesthesia using 2% Lignocaine injection. Surgical steps include standard aseptic preparation and draping. The conjunctiva overlying the pterygium is dissected 1 mm inside the limbus after subconjunctival injection of air (to delineate the extent of degeneration). Rest of the neck and apex is rrhexised with a McPherson forceps which leads to relatively smooth corneal surface. This will stabilize tear film post-operatively. Conjunctiva is dissected free of fibrotic Subconjunctival tissue. Curuncular end of the conjunctiva is placed along the limbus over the blood already clotted over bare sclera and CISAG is adhered by fibrin secreted from the clot below the graft. Eye was patched for 24 hours and the patient was advised not to rub the eye after removal of bandage .Topical Loteprednol and artificial tears were administered four times a day which was tapered over 6 weeks. Follow-up was done weekly for 4 weeks; this is the adhesion time and further monthly follow up for one year (Figure 3). Histopathological study of apical pterygial conjunctiva was done.
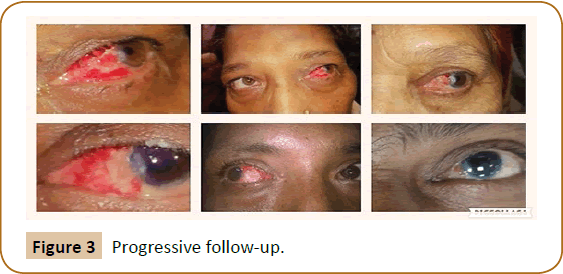
Figure 3: Progressive follow-up.
Results
10 cases of primary pterygium two cases of recurrent pterygium and 4 cases of combined cataract and CISAG were included in this study. Male female ratio was 1:1 with mean age of 49 years. No measurable recurrence was seen after one year of follow up. However, 1 cataract case had displaced graft in the immediate post of period which was re-placed under slit lamp and patching was done for 24 hours. Combining with cataract surgery procedure become little difficult technically. Conjunctival pigmentations and presence of Stocker’s line was found to be bad prognostic sign as these indicate reduced healing capacity of corneal epithelium covered by pterygium. Migration of epithelial cells is the primary process of corneal surface healing [5] which is a prerequisite for stable tear film. Unstable tear film has definite role in pathogenesis of pterygium [6].
Advantage of the procedure include no tissue wastage (as in excision), no normal area was traumatised (as in superior -temporal auto graft), no suture and no external glue required.
Discussions
Pterygium is a well-known external ocular condition of eye and is fairly common in tropical countries like India because of more cumulative yearly exposure to ultraviolet light, heat, dust and sun glare.
Globally prevalence of pterygium is 10.2% [1]. In India prevalence ranges from 9.5% to 13% [6], in our present study, males and female were equally affected with ratio of 1:1. Though, being male is considered a risk factor for pterygium [6], the equal incidence here can be explained by the fact that in this area where tribal population is more, more females are engaged as marginal workers.
Since the description of pterygium 1000 BC back by Sushruta, many surgical techniques evolved for management of pterygium. All are limited by most commonly recurrence and other complications. There is more aggressive growth of recurred lesion than the primary lesion.
Bare sclera technique was one of the earliest surgeries to be done for pterygium management. It involved surgical excision of pterygium with exposed sclera left bare. Though it was an easy and quick procedure done in office setting under topical or subconjunctival anesthesia, it has a high recurrence rate of 30%-80%. Therefore, it is seldom used today.
Then the technique of closing the post-pterygium excision defect by sliding conjunctival flaps harvested from inferior or superior bulbar conjunctiva was used. It has the recurrence rate of 1%- 5% but was limited by suboptimal cosmesis [7]. Conjunctival limbal autograft transplantation is a landmark in pterygium management, described by Kenyon in 1985 [8]. Associated recurrence rate ranged from 0% to 15% [9]. Rao et al reported the recurrence rate of 3.8% with this procedure done in 53 eyes with 36 primary and 17 recurrent pterygia on a mean follow-up of 18.9 ± 12.1 month [10].
P.E.R.F.E.C.T surgery (Pterygium Extended Removal Followed by Extended Conjunctival Transplant) introduced by Dr. Hirst has a recurrence rate of 0.5%. The main limitation being it is time consuming and technically challenging.
Many adjunctive are employed for prevention of recurrence. Radiation of bare sclera with Strontium 90 now abandoned due to development of sclera necrosis. Use of Mitomycin C during pterygium surgery decreases the recurrence rate. But it has serious sight threatening complications e.g. infectious scleritis, severe secondary glaucoma, corneal edema, perforation, iritis, incapacitating pain and photophobia [11].
Our technique of CISAG i.e. conjunctival in-situ auto graft involves reversal of direction of graft which is deterrent to recurrence compared to other techniques. Use of autologus fibrin over the subpterygial sclera as adhesive for graft also provides the advantage of no suture. More over limbal stem cells of affected area are also removed by meticulous dissection. This new technique has the benefit of not traumatising adjacent healthy ocular tissue. The one year follow-up of patients showed good cosmetic result as well as no recurrence. Patients are on follow up after mandatory one year period and have shown no signs of recurrence.
Our study has shown similar incidence in male and females which could be explained by more females are engaged as marginal workers in this part of world [1].
Many forms of graft have been tried in pterygium with good results. Recurrence rate is minimum with conjunctival auto graft with cyclosporine eye drops as shown in latest Meta- analysis [3]. This study also concluded that bare sclera technique to be abandoned due to high recurrence rate.
Our method has the lowest recurrence and is comparable with the best results where adjuvant was used (cyclosporine) with conjunctival graft [3]. CISAG is technically better as no suture was needed thus least irritation. Autologus fibrin secreted from the blood clot over the sub-pterygial sclera act as adhesive for the graft. Reversing the direction of graft was deterrent to recurrence.
Recently up regulation of micro RNA has been proved to be associated with pterygium along with involvement of specific oncogene. This may further open up research involving target therapy [12,13].
Conclusion
Conjunctival in situ auto graft or CISAG is a new technique of surgical treatment for pterygium.
This is more physiological nature as it uses conjunctiva from same site without traumatising healthy ocular tissues from some other part (Figures 4 and 5).
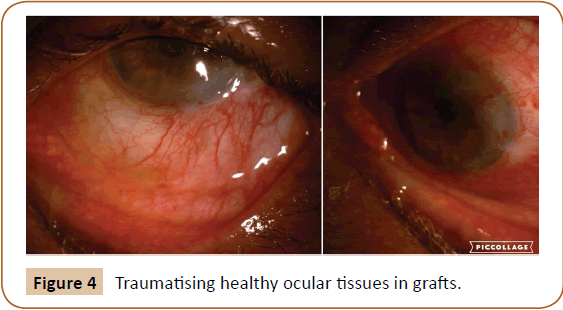
Figure 4: Traumatising healthy ocular tissues in grafts.
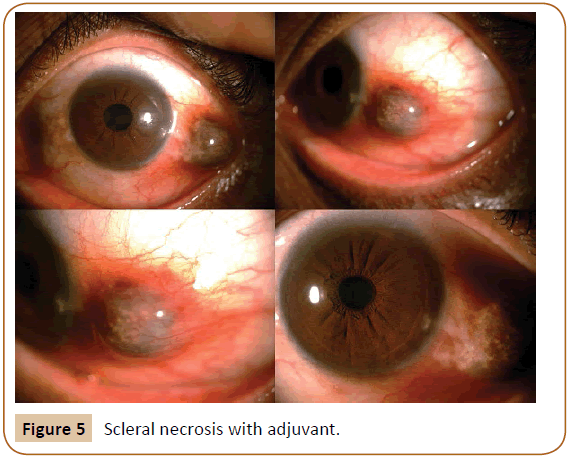
Figure 5: Scleral necrosis with adjuvant.
Limitations of our study are short number of cases and not a randomized control trial. However the theory behind this procedure has not violated any normal physiological principles.
We recommend this new procedure to be included in the treatment regimen and can be adopted by all ophthalmologists.
23515
References
- Luthra R, Nemesure BB, Wu SY, Xie SH, Leske MC (2001) Frequency and risk factors for pterygium in the Barbados Eye Study. Arch ophthalmo 119: 1827-1832.
- Chui J, Coroneo MT, Tat LT, Crouch R , Wakefield D, et al. (2011) Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathology 178: 817-827.
- Fonseca EC, Rocha EM, Arruda GV (2018) Comparison among adjuvant treatments for primary pterygium: a network meta-analysis. B J Ophthal 102: 748-756.
- Karalezli A, Kucukerdonmez C, Akova YA, Koktekir BE (2014) Does topical bevacizumab prevent postoperative recurrence after pterygium surgery with conjunctival autografting? Int J Ophthalmol 7: 512-516.
- Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, et al. (2013) Options and adjuvants in surgery for pterygium: A report by the American Academy of Ophthalmology. Ophthalmology 120: 201-208.
- Chui J, Di Girolamo N, Wakefield D, Coroneo MT (2008) The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf 6: 24-43.
- Davanger M, Evensen A (1971) Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229: 560-561.
- Nangia V, Jonas JB, Nair D, Saini N, Nangia P, et al. (2013) Prevalence and associated factors for pterygium in Rural Agrarian Central India. The Central India Eye and Medical Study. PLoS One 8.
- Tomas T (1992) Sliding flap of Conjunctival limbus to prevent recurrence of pterygium. Refract Corneal Surg; 8: 394-395.
- Kenyon KR, Wagoner MD, Hettinger ME (1985) Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 92:1461-1470.
- Al Fayez MF (2002) Limbal vs conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 109: 1752-1755.
- Rao SK, Lekha T, Mukesh BN, Sitalakshmi G, Padmanabhan P (1998) Conjunctival-limbal autografts for primary and recurrent pterygia: Technique and results. Ind J Ophthalmol 46: 203-209.
- Rubinfeld RS, Pfister RR, Stein RM, Foster CS, Martin NF, et al. (1992) Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology 99: 1647-1654.










