Ahmed Samir1*, Ahmed Fahmy2, Essam Hatem M1 and Ahmed Orabi1
1Department of Microbiology, Faculty of Veterinary Medicine, Cairo University, Egypt
2Veterinary Officer, K9 Police Academy, Ministry of Interior, Egypt
*Corresponding Author:
Dr. Ahmed Samir
Department of Microbiology, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt
Tel: +201006700376
Fax: +20235725240
E-mail:ahmed.samir@cu.edu.eg ahmedsamir121@hotmail.com
Received Date: May 06, 2015; Accepted Date: June 15, 2015; Published Date: June 19, 2015
Citation: Samir A, Fahmy A, Hatem ME, et al. Occurrence of Canine Borreliosis in Egyptian Dogs: A Public Health Threat. Transl Biomed. 2015, 6:1. DOI: 10.21767/2172-0479.100009
Keywords
Lyme borreliosis; Dogs; Egypt
Introduction
Lyme disease, an Ixodes spp. tick-associated zoonosis caused by Borrelia burgdorferi, causes significant morbidity in humans and dogs [1]. Infected dogs may develop fever, anorexia, fatigue, renal failure and most commonly limb and joint disorders [2]. Several animal species can be infected by B. burgdorferi spirochete, including rodents, deer, dogs, cats, cows, horses, reptiles and birds, and since tick species could infest such animals [3], however, dogs are considered the most important reservoir for ticks in the home environment [4]. In Egypt, available data about the incidence and prevalence of Lyme disease are scarce [5]. Few old and recent studies have pointed out the evidence of occurrence of B. burgdorferi in animals, ticks and humans [6-8]. The aim of the current study is to detect B. burgdorferi in dogs, tick reservoirs and human contacts by isolation of the pathogen in a specific medium, BSK-H, detection of its DNA by PCR and investigate the specific IgM-ELISA in sera of dogs and companion humans.
Methods
Sampling
A total of 100 samples of (70 blood samples from stray dogs hosted in animal shelter, 15 blood samples from human contacts working in the shelter who have accepted to let us draw blood samples from them relying on ethically approved consent forms, 15 hard ticks infesting some dogs from which blood samples were collected) during the period from 2012 till 2013. Dog and human blood were subjected for culture and PCR. Sera from the same samples were separated and preserved at -20° C for serology. Ticks from canine were identified as Rhipicephalus sanguineus, and subjected to culture and PCR. All samples were taken prior any antibiotic administration
Culture
One drop of whole blood or gut contents of the collected ticks was inoculated into 2-3 tubes containing BSK-H medium (Sigma Co., St. Louis, MO). All the inoculated tubes were incubated at 33°C for up to 8 weeks under a microaerophilic condition. Cultures were being examined weekly under the dark-field microscope to observe the characteristic morphology and motility of B. burgdorferi indicating the viability of the pathogen [9]. DNA extraction from whole blood: Total genomic DNA was extracted from whole blood using QIAamp® DNA blood Mini kit (Qiagen, Valencia, CA) as per manufacturer's instructions.
DNA extraction from ticks
Briefly, tick specimens were cleaned by sonication for 3-5 minutes in ethanol and then washed twice in sterile distilled water. Afterwards, individual tick specimens were divided into pieces, placed in a microcentrifuge tube filled with 180 μL lysing buffer solution supplied in the Kit and then homogenized with a sterile tissue grinder. The homogenate was centrifuged at room temperature and the supernatant was further processed and DNA was extracted by using QIAamp® DNA Mini kit (Qiagen, Valencia, CA) in accordance with manufacturer’s instructions.
DNA amplification by polymerase chain reaction
DNA extracted from blood and tick specimens as well as the positive control, B. burg
dorferi B31 (ATCC# 35210) and negative control, Leptospira interrogans serovar Icterohaemorrhagiae (NVSL# 17) were used as template for PCR amplification. A specific primer sequence targeted OspA gene of B. burgdorferi sensu lato was used which was 5′-AATGTTAGCAGCCTTGACGAGAA-3′ and 5′-GATCGTACTTGCCGTCTTTGTTT-3′ [10]. All PCR reagents and Taq polymerase were obtained and used as recommended by the supplier (Sigma Co., St. Louis, MO). Briefly, a total of 0.2 μmol of the appropriate primers and various amounts of template DNA were used in each 50 μL reaction mixture. The PCR amplification was performed with a programmable thermocycler for 35 cycles with denaturation at 94°C for 1 minute, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute. Amplified DNA products were electrophoresed in 2% agarose gels in Tris-Borate-EDTA (TBE) buffer and visualized under ultraviolet (UV) light after staining with ethidium bromide. DNA marker (100-1000 bp) was obtained from Stratagen Co., USA, Cat. No. 201115. It was used for detection of the expected size of the target genes at 309 bp (Figure 1).
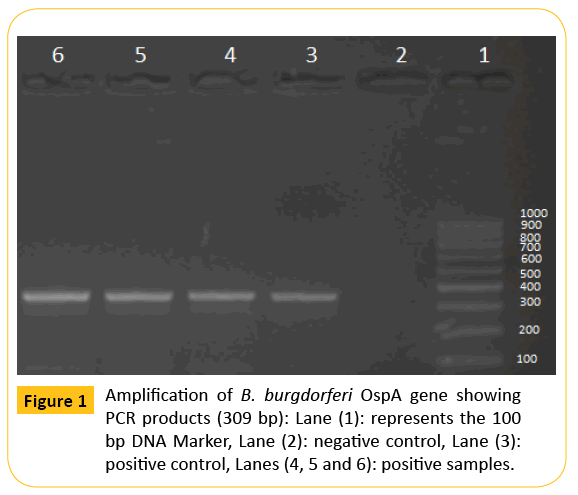
Figure 1: Amplification of B. burgdorferi OspA gene showing PCR products (309 bp): Lane (1): represents the 100 bp DNA Marker, Lane (2): negative control, Lane (3): positive control, Lanes (4, 5 and 6): positive samples.
ELISA
Sera from dogs were tested for the presence of IgM class antibodies against B. burgdorferi using (DBGM96-Dog EIA Borrelia IgM, TestLine, Czech Republic), while human sera were tested using (ab108711-Anti-Borrelia burgdorferi IgM Human ELISA Kit,abcam, UK).
Results
Culture result
None of the dogs, ticks or human samples showed recovery for B. burgdorferi.
PCR result
Out of 15 collected ticks from dog origin, 6 were positive for amplification of ospA gene (4%), while 13 (including those infested with positive ticks) out of 70 dogs were positive (18.5%). On the other hand, none of the blood samples collected from persons in close contact to such dogs was positive. 50 out of 70 canine sera (71.4%) were positive by ELISA, including those 13 which are positive by PCR, while only 3 out of 15 human serum samples (20%) were reactive.
Discussion
Lyme borreliosis is a worldwide zoonotic disease caused by the spirochete Borrelia burgdorferi, which is transmitted by a tick bite, primarily from Ixodes scapularis and Ixodes pacificus. It is characterized by multi-systemic disorders
In Egypt, Lyme disease has been recently reported in animals and transmitted by tick species out of family Ixodidae, Rhipicephalus sanguineus [8]. This finding was confirmed in the current study as we could detect the specific ospA gene of B. burgdorferi in 4% of the collected ticks.
Herein the current study, B. burgdorferi could not be recovered from the given samples when cultured in BSK-H medium. Although culture is the gold standard diagnostic test, isolation of B. burgdorferi is labor-intensive, more expensive, and much slower, requiring up to 12 weeks of incubation before being considered negative [7,11] and this makes culturing as a lower feasible method.
Our results showed that 50 out of 70 serum samples (71.4%) of the collected canine sera were sero-reactive and contained antiborrelia IgM. Anti-borrelia IgM are produced one week postinfection, and remain elevated for two months [12,13]. From those 50 sero-reactive dogs, B. burgdorferi DNA was detected in 13 animals and also in 6 Rhipicephalus sanguineus ticks that have been infesting such dogs. Moreover, 20% of human contacts were sero-reactive to B. burgdorferi.
The current study gives an evident to the existence of B burgdorferi in stray dogs in which Rhipicephalus sanguineus is the only tick species that has been identified so far. Although this kind of ticks has not been considered the main vector for B. burgdorferi, the present investigation and a previous one [8] suggest that Rhipicephalus sanguineus is incriminated in the epidemiology of B. burgdorferi infection.
Conclusions
This information is useful and may be of epidemiological value in monitoring the circulation of such pathogen, determining potential zoonotic risk and implementing prevention and intervention measures like vaccination and control programs which are not applied in Egypt.
It is also important to increase awareness about Lyme borreliosis among veterinarians and physicians in Egypt and to strengthen laboratory capacity for its diagnosis in veterinary and infectious hospitals.
Competing Interests
:Nothing to declare
6604
References
- Levy SA, Magnarelli LA (1992) Relationship between development of antibodies to Borreliaburgdorferi in dogs and the subsequent development of limb/joint borreliosis. J Am Vet Med Assoc 200: 3 44-347.
- Summers BA, Straubinger AF, Jacobson RH, Chang YF, Appel MJ et al. (2005) Histopathological studies of experimental lyme disease in the dog. J CompPathol 133: 1-13.
- Straubinger RK, Straubinger AF, Summers BA, Jacobson RH (2000) Status of Borreliaburgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J Infect Dis 181: 1069-1081.
- De Lacerda AA, Cuhna MR, Antunes DR (2004) Frequency of antibodies against Borreliaburgdorferi in dogs from the metropolitan region of Rio de Janeiro. Pesq Vet Bras 24: 203-206.
- Haberberger RL, Constantine NT, Schwan TG, Woody JN (1989) Lyme disease agent in Egypt? Trans R Soc Trop Med Hyg 83: 556.
- Hammoud NA, Hegazy IH, El-Sawy EH (1995) ELISA screening for Lyme disease in children with chronic arthritis. J. Egypt Soc. Parasitol 25: 525-533.
- Adham FK, Abd El-Samie EM, Gabre RM, El Hussein H (2010) Detection of tick-blood parasites in Egypt using PCR assay. II-Borreliaburgdorferisensulato. J Egypt SocParasitol 40: 553-564.
- Elhelw RA, El-Enbaawy MI, Samir A (2014) Lyme borreliosis: A neglected zoonosis in Egypt. ActaTropica 140: 188-192.
- Pollack RJ, Telford SR, Spielman A (1993) Standardization of medium for culturing Lyme disease spirochetes. J. Clin. Microbiol 31: 1251-1255.
- Sparagano OA, Allsopp MT, Mank RA, Rijpkema SG, Figueroa JV et al. (1999) Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): a review. ExpApplAcarol 23: 929-960.
- Schmidt BL (1997) PCR in laboratory diagnosis of Borreliaburgdorferi infections.ClinMicrobiol Rev 10: 185.
- Hovius KE, Rijpkema SG, Westers P, van der Zeijst BA, van Asten FJ, et al. (1999) A serological study of cohorts of young dogs, naturally exposed to Ixodesricinus ticks, indicates seasonal reinfection by Borreliaburgdorferisensulato. Vet Q 21: 16-20.
- Greene CE, Appel MJ, Straubinger RK (2006) Borreliosis. In Greene CE, editor. Infectious Diseases of the Dog and Cat. Elsevier; St. Louis 417-435.






