Introduction
|
| |
| Pharmaceutical tablets represent the most popular drug delivery systems. Tablets are manufactured by compressing a powder formulation in a die. Following this process the tablets are subjected to a bulk handling and other operations during which the bioavailability (disintegration, dissolution) behaviour and mechanical integrity must be maintained. The final properties of the tablets depend on the choice of ingredients used in the powder formulation, the details of the mixing process and selection of process parameters applied by tabletting equipmenti. The application of three or higher-level experimental design using the response surface methodology does not appear to have been reported in development and optimization of drug release methods until now. The relationship between one or more response variables and set a quantitative parameters can be examined well by using response surface models , such as central composite design or Box-Behnken design. In this present work a systemic analysis of the process parameters during compression process and explore their influence on the final properties of the tablets have been studied. Also illustrate how tablet properties are controlled by dependent variables of granulator and compression machine. |
| |
Material and Methods
|
| |
MATERIALS:
|
| |
| Atenolol was received by Ariane Org Chem. Ltd. (Hyderabad, India). Heavy magnesium carbonate and Gelatin was procured from Taurus chemicals (London, U.K.). Sodium Lauryl sulphate and maize starch were received from Cognis (Mumbai, India). Magnesium stearate was gift sample from Febro Ltd (London, U.K.). All chemicals used for this study were of analytical reagent grade. Freshly distilled water was used through out the work. |
| |
EXPERIMENTAL DESIGN
|
| |
|
Manufacturing of Tablets
|
| |
| Atenolol, heavy magnesium carbonate, maize starch and sodium lauryl sulphate (60-80 mesh, 250-177 µm) were mixed in Rapid Mixer Granulator (Sainath boilers and pneumatics, India) for 10 mins at different impeller and chopper speed. Purified water (50%) was heat and add gelatin in heated water with constant stirring until dissolve. 3.57 % w/w maize starch paste (granulating agent) was prepared with boiling purified water. Add the gelatin solution to starch paste and mixed properly. Add the granulating agent to the material over a period of 2 min at different impeller and chopper speed followed by kneading for about 2 min to get a good granular mass. Wet granular mass was dried in fluidized bed dryer (Bectochem, India, 20kg) at an internal temperature of 60 ± 5°C, outlet temperature 40±5°C till a loss on drying of 1.5–3.3 % was achieved on IR moisture balance in auto mode at 105°C. |
| |
| Dried granules were sifted through 18 mesh on vibratory sifter (Bectochem, India) and mill the retentions of granules through 1 mm screen of multimill (bectochem, India) with knives forward direction at slow speed. The dried granules were blended with 1% magnesium stearate in Octagonal Dried granules were sifted through 18 mesh on vibratory sifter (Bectochem, India) and mill the retentions of granules through 1 mm screen of multimill (bectochem, India) with knives forward direction at slow speed. The dried granules were blended with 1% magnesium stearate in Octagonal |
| |
| In the presented study, granulation process was optimized by taking three different lots, in which dependent variables were impeller speed, binder addition time, chopper speed, impeller mixing time and their effect on bulk density, true density, Carr’s index, and hausner ratio (Table 2). |
| |
| For compression process three levels Factorial Box- Behnken experimental design was used to evaluate effect of selected independent variables on the responses, to characterize physical properties of tablets and to optimize the procedure. This design is suitable for exploration of quadratic response surface and for construction of polynomial models, thus helping to optimize process by using a small number of experimental runs. For the three levels three factor Box and Behnken Experimental design, a total of 15 experimental runs, shown in Table 3, are needed. The generated models contain Quadratic term explaining the non linear nature of responses This design also resolves the three factor interaction effect of individual terms and allow a mid level setting (0), for the combination of the factors (montogomery,1991;Singh et al.,1995). |
| |
| The design consists of replicated center points and a set of points lying at the mid points of each edge of multidimensional cube that defines the region of interest .The model is of the following form: |
| |
| y = b0 + b1x1 +b2x2 +b3x3 +b4x1x2 +b5x2x3+b6x1x3 +b7x12+b8x22+b9x32 + E |
| |
| Where y is the selected response, b0-b9 are the regression coefficients, X1, X2 and X3 are the factors studied and E is an error term. The Box-Behnken experimental design is an orthogonal design. |
| |
| Therefore, the factor levels are evenly spaced and coded for low, medium and high settings; as -1, 0, +1 (Montgomery,1991; Singh et al.,1995;Karnachi and Khan ,1996). Factors studied in the Box and Behnken experimental design where: precompression force (X1), compression force (X2) and compression speed (X3). The factors levels are shown in Table 3. The selected responses were Hardness (Y1), friability (Y2) and Disintegration time (Y3). The responses studied and the constraints selected considering Atenolol Physical properties and regarding U.S.FDA guidelines, presented in Table 4. |
| |
|
Evaluation of Tablets
|
| |
|
Hardness
|
| |
| The hardness of the tablets was tested for 10 tablets by pharma hardness tester (Pharma Test, Germany) and average hardness (N) was being taken and compared with that of standard one. |
| |
|
Friability
|
| |
| Friability test was performed in accordance with USP (Electroleb friabilator, Mumbai) 5 tablets were selected randomly, their individual weight was taken and then kept in the friabilator and rotated for 4 min at a speed of 25 rpm the tablets were taken out and any loose dust from them was removed, the weight was registered and friability was calculated as a percentage weight loss. |
| |
|
Disintegration time
|
| |
| The disintegration of the tablets was tested in a disintegration tester (Pharma Test, Germany), six tablets were put in to a basket that was raised and lowered in a beaker containing preheated water at 37?? °C. The disintegration test was calculated as the mean value and as the range. |
| |
|
In-vitro dissolution studies
|
| |
| The release rate of atenolol from tablets (n=3) was determined according to British Pharmacopoeia (ref) using the Dissolution Testing Apparatus 2 (model TDT-60T, Electrolab, India) fitted with paddles. The dissolution test was performed using 900 mL of 0.1 N HCl, 37±0.5°C and 50 rpm. A 5 mL sample was withdrawn from the dissolution apparatus at predetermine time interval, and the samples were replaced with fresh dissolution medium. The samples were filtered through a 0.45µm membrane filter and diluted to a suitable concentration with 0.1 N HCl. Absorbance of these solution was measured at 275 nm using UV/VIS spectrophotometer (Jasco V530, Japan). Cumulative drug release was calculated using the equation generated from Beer Lamber’s calibration curve in the linearity range of µg/mL. |
| |
|
Statistical analysis
|
| |
| Statistical analysis of the Box-Behnken design batches was performed by multiple regression analysis using Microsoft Excel. To evaluate the contribution of each factor with different levels to the response, the two-way analysis of variance (ANOVA) was performed using the DESIGN EXPERT 6.0.11 (STAT-EASE) demo version software. To graphically demonstrate the influence of each factor on the response, the response surface plots were generated using DESIGN EXPERT 6.0.11 (STAT-EASE) demo version software. |
| |
Results and discussion
|
| |
| In the present investigation, combinations of three variables were studied using the Box-Behnken experimental design. The mathematical models developed for all the dependent variables using statistical analysis software are shown in equations (1)-(3): |
| |
 |
| |
 |
| |
 |
| |
| The hardness of all tablets was found to be below 61 N . |
| |
| In The Present investigation granulation and compression process were optimized. |
| |
|
Results and Discussion for Granulation Process :
|
| |
| In granulation process, impeller speed, binder addition time, chopper speed, impeller mixing time were studied by taking three different lots for dependent variables i.e. bulk density, true density, Carr’s index, and hausner ratio (Table 2). |
| |
|
Effect of impeller and chopper speed
|
| |
| Various granulation batches prepared to study the effect of impeller and chopper speed are listed in table 1 |
| |
|
Results and Discussion for Compression Process:
|
| |
| Combinations of three independent variables were studied using t he Box Behnken design. The mathematical models developed for all the dependent variables using design expert software are shown in equation (1)-(3) |
| |
|
Effect of Pre Compression Force , Compression force and Compression speed :
|
| |
| 15 batches had been prepared to study the effect of pre compression force is listed in table no 3. so, we took three different pre compression force -1 indicates there is no pre compression force,0 indicates pre compression force is 1tonne,+1 indicates precompression force is 2 tonnes,after preparing all the batches results showed that ,after applying pre compression force hardness becomes higher than limit in combination with lower compression force and lower compression force showed cracking tabletting defect (BB 4). So, result showed that Precompression force should not be given ,when we are applying higher compression force and ,and when Precompression force is not given and compression force is also lowered cause lower hardness and friability problems with high compression speed.(BB 7) |
| |
| All three independent variables also affects invitro dissolution studies ,if pre compression force is 2 tonnes and compression force 6 tonnes with lowe compression speed 25 rpm ,takes more time to dissolve and vice versa. |
| |
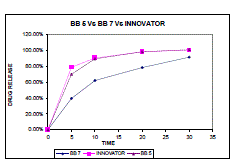
| Dissolution profiles for two Optimized batches BB 5 and BB 7 were analysed and compared with innovator product and calculated for similarity factor showed result given in table no. 7 |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
 |
 |
 |
 |
| Table 5 |
Table 6 |
Table 7 |
Table 8 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |











