Yang Zhang1, Katharina Singh Kang1, Andrea Clarke2, Samantha Donaldson3, Manjit Cartlidge3, Pallavi Bedi3, Catherine J Doherty1, Thamarai Schneiders1, John RW Govan1 and Adam T Hill1-3*
1School of Medicine and Veterinary Medicine, The Chancellor’s Building, University of Edinburgh, Edinburgh EH16 4SB, UK
2Department of Respiratory Medicine, Royal Infirmary of Edinburgh, 51 Little France Crescent, Old Dalkeith Road, Edinburgh EH16 4SA, UK
3MRC Centre for Inflammation Research, Queen's Medical Research Institute, University of Edinburgh, 47 Little France Crescent, Edinburgh, EH16 4TJ, UK
- *Corresponding Author:
- Adam T Hill
School of Medicine and Veterinary Medicine, The Chancellor’s Building
University of Edinburgh, Edinburgh, UK
Tel: +4401316502252
E-mail: adam.hill318@nhs.net
Received date: Feb 26, 2016; Accepted date: Jun 02, 2016; Published date: Jun 13, 2016
Citation: Hill AT, Zhang Y, Kang KS, et al. Optimum Condition for Storage of Sputum from Patients with Bronchiectasis Infected with Haemophilus influenzae. Arch Clin Microbiol. 2016, 7:4. doi:10.4172/1989-8436.100052
Copyright: © 2016 Hill AT et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Bronchiectasis; Sputum processing; Haemophilus influenza; Temperature; Medium
Introduction
Bronchiectasis is a common chronic debilitating respiratory condition with permanent airway damage that leads to patients having recurrent cough, sputum production and recurrent chest infections. Almost two thirds of bronchiectasis patients are chronically infected with bacterial pathogens [1]. Assessing sputum microbiology is important in the management of patients, and in practice, the sputum sample is processed as soon as possible. However, we know from experience that there can be a long time between sample collection and processing. Long-term storage of specimens can also offer the opportunity to do future research on them [2], which makes the storage of samples important, as it may have a direct influence on the recovery of viable organisms. Nearly 55% of bronchiectasis patients are chronically infected with H. influenzae, which is the most common isolated species from sputum [1-3]. H. influenzae was first described in 1892 by Richard Pfeiffer. It usually colonizes the upper airways, but it is also frequently isolated from the lower airways of patients with chronic obstructive pulmonary disease or bronchiectasis [4]. To the author’s best knowledge, there have been no studies addressing the optimum storage conditions for the isolation of H. influenzae in sputum samples from patients with bronchiectasis. This study aims to identify the optimum time, temperature and medium for the storage of sputum samples from patients with bronchiectasis chronically infected with H. influenzae. This study shows that for short term storage of sputum from bronchiectasis patients chronically infected with H. influenzae, the ideal sputum storage condition is room temperature up to 48 h. For long term storage, the optimal condition is storage in glycerol at -70°C.
Methods
Patients
20 clinically stable patients with bronchiectasis and a history of chronic H. influenzae infection were recruited from the Bronchiectasis Clinic at the Royal Infirmary of Edinburgh in Scotland, UK. The study received ethical approval from the Lothian research ethics committee and NHS Lothian R+D approval.
In this study we only included patients with bronchiectasis confirmed by chest CT according to the features described by Naidich et al. [5] and clinical symptoms including chronic cough and sputum production when clinically stable. Only patients that cultured H. influenzae alone were included in the study. We excluded current or ex-smokers who stopped smoking less than one year ago or ex-smokers with a history of more than 20 pack-years of smoking. We also excluded patients who were on long-term oral or inhaled antibiotics longer than 28 days before the study.
Out of the 20 patients, three were recruited to assess reproducibility for quantitative microbiology. For the experiments three samples were plated in triplicate. 17 patients were recruited for the sputum storage experiments.
Sputum collection
All patients were taught chest clearance techniques using the active cycle breathing technique. Patients were asked to provide a spontaneous sputum sample into a sterile container at the Royal Infirmary of Edinburgh to allow immediate processing.
Sputum processing and storage
Sputum was collected and each sputum sample was immediately aliquot into separate sterile containers. 11 samples were stored short term (2, 4, 6, 24 and 48 hours) at room temperature, 4°C, -20°C and -70°C (Figures 1A and 1B). Samples 1-5 were each aliquot into 10 specimens, which were divided as follows: one specimen was processed immediately after collection, five specimens were stored at room temperature (23°C) for 2, 4, 6, 24 and 48 hours, two specimens were stored at 4°C for 24 and 48 h and two specimens were stored at -20°C for 24 and 48 h. Sample 6 was aliquot into 12 specimens, which were divided as follows: one specimen was processed immediately after collection, five specimens were stored at room temperature (23°C) for 2, 4, 6, 24 and 48 hours, two specimens were stored at 4°C for 24 and 48 h, two specimens were stored at -20°C for 24 and 48 h and two specimens were stored at -70°C for 24 and 48 hours. Samples 7-11 were each aliquot into 10 specimens, which were divided as follows: one specimen was processed immediately after collection, five specimens were stored at room temperature (23°C) for 2, 4, 6, 24 and 48 hours, two specimens were stored at 4°C for 24 and 48 h and two specimens were stored at -70°C for 24 and 48 hours.
Figure 1: Method of sputum sample short term storage and processing (A) sputum samples 1-6 , B) sputum samples 6-11, C) one specimen from each sample was processed immediately after collection, 3 specimens from each sample were stored with 25% glycerol at 4°C, -20°C and -70°C
6 sputum samples were each aliquot into 10 specimens and stored long term (7 days). 1 specimen from each sample was processed immediately after collection, 3 specimens from each sample were stored with 25% glycerol at 4°C, -20°C and -70°C. 3 specimens from each sample were stored with 50% skimmed milk at 4°C, -20°C and -70°C. 3 specimens from each sample were stored neat at 4°C, -20°C and -70°C (Figure 1C).
Sputum processing
At the designated time points for processing, samples were prepared for qualitative and quantitative bacteriology as described by Pye et al. [6]. Briefly, sputum was homogenized and liquified using an equal volume of Sputolysin, and serially diluted using sterile 0.9% saline to achieve dilution factors of 10-1 to 10-4. Chocolate blood agar plates were inoculated with 100 μl 10-1 and 10-4 sample, and then incubated in an aerobic atmosphere enriched with 5-10% carbon dioxide at 37°C for 48 hours. Colonies were then counted to determine the sputum H. influenzae density expressed as log10 c.f.u. ml-1. In addition the viable rate was calculated by comparing the colony numbers from the samples processed immediately after collection to all other time points, temperatures and storage media (observed bacterial load/ original bacterial load × 100).
Identification of H. influenzae
The identification of H. influenzae was based on the requirement of both X and V factor on blood agar.
Statistics
Statistical analysis was performed using Graph Pad Prism version 5.0 (Graph Pad Software). The data are presented as means with their standard deviations for normally distributed data and medians with their interquartile ranges for data that were not normally distributed. A paired t-test or Wilcoxon analysis for two groups was performed. An ANOVA was used to compare multiple groups. We use the coefficient of variation to assess internal consistency. A two-tailed P value of <0.05 was considered statistically significant.
Results
Out of the 20 patients, 3 were recruited to assess reproducibility for quantitative microbiology (2 females and 1 male, aged 67, 79 and 35 years). 17 patients (9 females and 8 males) with an average age of 65 years old (range 22 to 84 years) were recruited for the sputum storage experiments. On computed tomography, there were a median of 4 (2.75 to 5) bronchiectatic lobes involved.
In the three experiments to assess reproducibility for quantitative microbiology, the coefficients of variation were 2%, 4% and 9%.
All samples stored at any conditions grew H. influenzae alone. There was no significant reduction in H. influenzae density between specimens processed immediately after collection and the specimens stored at room temperature from 2 to 48 hours (Figure 2A). The average viable rate for the sample processed at 2 hours was 71%, at 4 hours was 124% and at 6 hours was 134%. After storage at room temperature for 24 and 48 hours, the viable rates were 110% and 81% respectively.
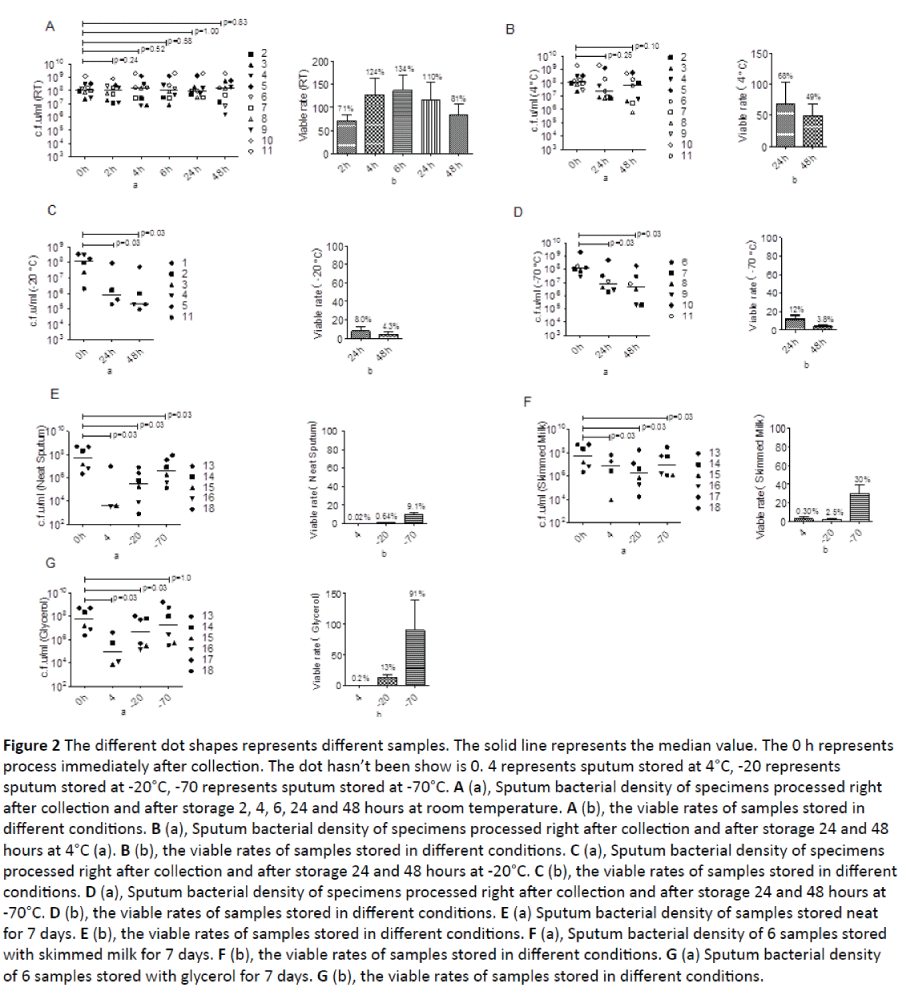
Figure 2: The different dot shapes represents different samples. The solid line represents the median value. The 0 h represents process immediately after collection. The dot hasn’t been show is 0. 4 represents sputum stored at 4°C, -20 represents sputum stored at -20°C, -70 represents sputum stored at -70°C. A (a), Sputum bacterial density of specimens processed right after collection and after storage 2, 4, 6, 24 and 48 hours at room temperature. A (b), the viable rates of samples stored in different conditions. B (a), Sputum bacterial density of specimens processed right after collection and after storage 24 and 48 hours at 4°C (a). B (b), the viable rates of samples stored in different conditions. C (a), Sputum bacterial density of specimens processed right after collection and after storage 24 and 48 hours at -20°C. C (b), the viable rates of samples stored in different conditions. D (a), Sputum bacterial density of specimens processed right after collection and after storage 24 and 48 hours at -70°C. D (b), the viable rates of samples stored in different conditions. E (a) Sputum bacterial density of samples stored neat for 7 days. E (b), the viable rates of samples stored in different conditions. F (a), Sputum bacterial density of 6 samples stored with skimmed milk for 7 days. F (b), the viable rates of samples stored in different conditions. G (a) Sputum bacterial density of 6 samples stored with glycerol for 7 days. G (b), the viable rates of samples stored in different conditions.
There was no significant decrease in H. influenzae density between the specimen processed right after collection and specimens stored at 4°C for 24 and 48 hours (Figure 2B). The viable rates for specimens processed after 24 and 48 hours was 68% and 49% respectively.
There was a significant reduction in H. influenzae density between the specimen processed right after collection and specimens stored at -20°C for 24 and 48 hours (Figure 2C). The viable rates for specimens processed after 24 and 48 hours was 8.0% and 4.3% respectively.
There was a significant reduction in H. influenzae density between the specimen processed right after collection and specimens stored at -70°C for 24 and 48 hours (Figure 2D). The viable rates for specimens processed after 24 and 48 hours was 12% and 3.8% respectively.
There was a significant reduction in H. influenzae density between the specimen processed right after collection and specimens stored neat at 4°C, -20°C and -70°C for 7 days (Figure 2E). The viable rates for specimens stored neat at 4°C was 0.02%, at -20°C was 0.64%, and at -70°C was 9.1%.
There was a significant reduction in H. influenzae density between the specimen processed right after collection and specimens stored in skimmed milk at 4°C, -20°C and -70°C for 7 days (Figure 2F). The viable rates for specimens stored in skimmed milk at 4°C, -20°C and -70°C was 0.3%, 2.5% and 30% respectively.
There was a significant reduction in H. influenzae density between the specimen processed right after collection and specimens stored in glycerol at 4°C and -20°C for 7 days (Figure 2G). There was, however, preserved bacterial density at -70°C for 7 days. The viable rates for specimens stored in glycerol at 4°C, -20°C and -70°C was 0.2%, 13.9% and 91%.
Discussion
In practice, due to the time spent on sputum collection and transportation, sample processing time is often delayed in the hospital and laboratory setting. Further studies incorporating a patient empowerment model considering the patient as the key member of the health team and care managers as key health care collaborators may enhance and support services to patients provided by physicians in the primary health care system and facilitate sputum processing [7].
The results showed that there was no significant difference in the density of H. influenzae cultured from the samples stored for any time points at room temperature and 4°C following collection. There was however improved viability with samples stored at room temperature. These results indicate that the preferred storage temperature for sputum samples with H. influenzae is room temperature for up to 48 hours. For long term storage of up to 7 days, the sputum sample stored in glycerol at -70°C led to no significant difference in the density and viability of H. influenzae, indicating glycerol as the preferred storage medium at -70°C. At room temperature, data on the average viable rates at 24 h and 48 h (110% and 81% respectively) were higher than at 4°C (63% and 46% respectively), which suggests that room temperature is a better temperature for the storage of sputum sample than 4°C.
The study of investigated storage time of sputum samples in bronchiectasis patients chronically infected with Pseudomonas aeruginosa. In their study, however, storage at room temperature for 24 and 48 h was associated with a significant increase in bacterial load [8] and they found the optimum storage temperature to be 4°C. Comparing the results of this study it is clear that the characteristic of various pathogens differ. It is thus important to find the optimum storage conditions to preserve multiple bacterial species from sputum samples.
In the short term storage at -20°C and -70°C, there was a significant decrease in the density of H. influenzae processed after 24 and 48 hours when compared to samples processed right after collection. Studies states that the effect of freezing on several kinds of bacteria. They found that 82% of Pseudomonas aeruginosa and 60% of Achromobacter were killed by a single freezing and thawing cycle [9]. Our results showed, that 92-96% of H. influenzae was killed after storage at -20°C for 24 or 48 hours and nearly 88-96% were killed at -70°C, which shows that the major harm to H. influenzae is caused by freezing and thawing. The storage medium, however, can influence H. influenzae viability during freezing and thawing. In our long term storage experiments, two mediums were compared to neat freezing: glycerol and skimmed milk. The results show, that after 7 days of storage before processing, only the sample stored at -70°C in glycerol showed no significant difference in the density of H. influenzae and a viable rate of 91%. All the other storage conditions (sputum neat at 4°C, -20°C, -70°C; sputum in skimmed milk at 4°C, -20°C, -70°C; sputum in glycerol at 4°C, -20°C) showed a significant reduction in the density of H. influenzae and viable rate. Glycerol shows a protective role at increasing the consistency of the sputum and its freezing point, which prevents the formation of ice crystals. In addition independent of the storage medium used, the viable rate was higher at -70°C, which suggests that the lower temperature is better for long term sputum storage. Very low temperatures, like liquid nitrogen (-196°C) may be the best choice for sputum storage, but further studies are needed [10].
A limitation of the study was the small sample size. The patients however were well characterized and excluded confounders, in particular those on long term antibiotics, as this could have skewed the results. We have also not explored long term storage more than seven days and further studies are needed.
In conclusion, this study shows that for short term storage of sputum from bronchiectasis patients chronically infected with H. influenzae, the ideal sputum storage condition is room temperature up to 48 h. For long term storage, the optimal condition is storage in glycerol at -70°C.
9643
References
- Angrill J, Agusti C, Celis RD, Rano A, Gonzalez J, et al. (2002) Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 57: 15-19.
- Acha SJ, Kuhn I, Mbazima G, Navarro PC,Mollby R (2005) Changes of viability and composition of the Escherichia coli flora in faecal samples during long time storage. J Microbiol Methods 63: 229-238.
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, et al. (2012) Short- and Long-Term Antibiotic Treatment Reduces Airway and Systemic Inflammation in Non-Cystic Fibrosis Bronchiectasis. Am J RespirCrit Care Med 186: 657-665.
- Hare KM, Binks MJ, Grimwood K, Chang AB, Leach AJ, et al. (2012) Culture and PCR detection of Haemophilusinfluenzae and Haemophilushaemolyticus in Australian Indigenous children with bronchiectasis. J ClinMicrobiol 50: 2444-2445.
- Naidich DP, Mccauley DI, Khouri NF, Stitik FP, Siegelman SS (1982) Computed-Tomography of Bronchiectasis. J Comput Assist Tomography 6:437-444.
- Pye A, Stockley RA, Hill SL (1995) Simple Method for Quantifying Viable Bacterial Numbers in Sputum. J Clin Path 48: 719-724.
- Ciccone MM, Aquilino A, Cortese F, Scicchitano P, Sassara M, et al. (2010) Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes. Vasc Health Risk Manag 6:297-305.
- Murray MP, Doherty CJ, Govan JR, Hill AT (2010) Do processing time and storage of sputum influence quantitative bacteriology in bronchiectasis. J Med Microbiol 59: 829-833.
- Haines RB (1938) The effect of freezing on bacteria. Proc R SocLond 124: 451.
- Bonavia A, Thompson M, Schryver B, Ehrhardt R (2012) Bacteria Cryopreservation Protocol. Protocol Exchange.







