Keywords
Candida parapsilosis sensu stricto; Biofilm; Oral eubiosis; Oral dysbiosis
Introduction
Of the NAC species, Candida parapsilosis has arisen in recent years as a high-impact hospital pathogen [1], attracting the attention of medical staff. Candida parapsilosis belongs to a complex of three species (Candida parapsilosis sensu stricto, Candida orthopsilosis and Candida metapsilosis) which are phenotypically indistinguishable but genetically diverse, called Candida parapsilosis complex (psilosis complex) [2]. According to the literature, of the three species in the psilosis complex, C. parapsilosis sensu stricto predominates in different human ecological niches, both in sickness and in health, and is the species most frequently recovered from invasive infections, both in adults and in premature neonates [3-5].
For many years, Candida albicans was the Candida species most frequently isolated from the oral cavity, whether pathological or healthy. However, a recently published paper on a prospective and comparative study reported greater prevalence of Candida parapsilosis sensu stricto in the oral cavity, with 83.3% isolation frequency-higher than Candida albicans (56.7%)-in both pathological and healthy oral conditions, without significant differences between clinical groups [6]. Our research team conducted a pilot study in 2017, which showed that of the psilosis complex, the species C. parapsilosis sensu stricto was the most frequently isolated from oral cavity niches. Under inflammatory conditions, the probability of recovering it was almost four times higher, and it displayed increased biofilm forming capacity in vitro, with a significant difference over isolates of the same species obtained from conditions of eubiosis or health [7]. This outcome led us to hypothesize that an oral environment in dysbiosis overregulates virulence genes through epigenetic changes, promoting a more pathogenic phenotype. We therefore decided to evaluate the in vitro biofilm formation assay under two nutritional conditions, using two colorimetric methods for quantification. In addition, the results were validated with optical microscopy (OM).
Materials and Methods
We designed a basic, retrospective, cross-sectional comparative study using a collection of 50 C. parapsilosis sensu stricto oral isolates defined by conventional phenotypic methods (differential chromogenic medium “ CHROMagar- Candida” [Becton-Dickinson]; micromorphology, and Vitek2), and molecular methods (endpoint PCR with specific primers derived from the region ITS1-5.8S-ITS2; plus Sanger sequencing and bio informatic analysis with Nucleotide BLAST [blastn]). The isolates were obtained from a previous study on adult patients aged 18 to 65 years, immune competent, nonsmokers, with no history of use of antimicrobial agent use during the 6 months prior to sampling; with or without prosthetic devices; with or without diagnosed gingivalperiodontal disease, using the criteria of the American Association of Periodontology for diagnosis of periodontal diseases and conditions [8]. Samples were taken by a calibrated dentist following a procedure approved by the ethics committee of the School of Dentistry of Buenos Aires University, resolution number 012/2016CETICAFOUBA.
Isolates and media
To study the potential impact of a dysbiotic environment on the virulence of Candida parapsilosis sensu stricto, we used isolates of C. parapsilosis sensu stricto (defined by phenotypic and molecular studies) from patients diagnosed with gingivalperiodontal disease (GPD). GPD is a model par excellence for chronic inflammatory disease and is characterized by an association of alterations in oral pH, REDOX imbalance, oxidative stress, and changes in diversity and composition of the microbiota towards a polarization of the red and orange complex [9-11]. These attributes make GPD a good model for studying the impact of the oral microenvironment on the phenotype of a given microbial model.
For the experimental phase, we used an strain of Candida albicans ATCC 10231 as positive control for the run, because it has been declared a pathogen according to the criteria of the CLSI (Clinical and Laboratories Standard Institute) [12], in addition to the 50 isolates of C. parapsilosis sensu stricto taken from different sites in the oral mucosa (cheek, tongue, palate) and subgingival niches. The preserved isolates were reconstituted in BHI (brain-heart infusion) broth (Merck) at 37°C. The growths obtained in BHI broth were plated in Sabouraud Dextrose Agar (SDA) supplemented with chloramphenicol (Becton Dickinson), incubated at 28°C [13]. With cultures obtained in SDA, suspensions were prepared at 1×107 cells/ml in saline solution. The biofilm assay was conducted in two nutritional media: YPD broth supplemented with chloramphenicol (Becton Dickinson) 2X, and RPMI 1640 medium supplemented with L-glutamine (Life technologies) 1X.
Analysis of virulence in vitro
We measured in vitro virulence through biofilm-forming ability in 96-well polystyrene microtiter plates (catalog number 167008 from Nunc or from Techno Plastic Products AG) by total biofilm biomass quantification assay with crystal violet (CV), in two different nutritional conditions: 1) YPD broth 2X (supplemented with antibiotic), described by Peteers et al. [14], and 2) RPMI 1640 medium 1X (supplemented with Lglutamine), described by Treviño Rangel et al. [15]. Each sample was analyzed in 4 replicas and in two independent experiments. To increase the confidence level of the results, we used an alternative method for quantification of biofilm in vitro based on measuring metabolic activity in each well by enzymatic reduction of the tetrazolium salt XTT (2,3-bis[2- methoxy-4-nitro-5-sulfophenil]-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide) by mitochondrial dehydrogenases, which are only active in viable fungal cells. Only viable cells will be able to reduce XTT salt in a chromogenic product (formazan) whose optical density is measured at 490 nm [14].
The quantity of biofilm formed by an isolate was classified according to the guidelines by Sánchez et al., [16] and Treviño Rangel et al., [15] as: high producers (≥0.41), low producers (0.11-0.40), and null producers (≤0.10). Sánchez et al. break down high producers into strong and moderate biofilm producers (Table 1).
Table 1. Classification of biofilm-forming ability for optical density 595 and 490 nm
| Group |
Formation of biofilm |
OD 595/490nm |
CFU Log10 cells/ml |
| I |
Non-producer |
≤0.10 |
<0.1 x 108 |
| II |
Slight producer |
0.11-040 |
0.1-0.75 x 108 |
| III |
Moderate producer |
0.41-0.74 |
0.76-2 x 108 |
| IV |
Strong producer |
≥0.75 |
>2 x 108 |
Source: Sánchez et al. [37].
Evaluation of biofilm with optical microscopy (OM)
In order to observe Candida parapsilosis sensu stricto biofilm by MO, 4 isolates with different biofilm-forming capacity (high, low and null) according to the CV assay were selected at random. Suspensions of 1x107 cells per milliliter were prepared from each selected isolate to induce biofilm growth on 24-well polystyrene trays. A sterile Thermanox coverslip (Plastic Coverlips-Rochester, NY USA) 13 mm in diameter was placed at the bottom of each well of the polystyrene tray under laminar flow. In each well with its coverslip, 500 ul of the Candida parapsilosis inoculum was placed on 500 ul culture medium (YPD broth) to induce biofilm growth for 24 hours, with shaking, at 37°C. After the incubation period, the culture medium was removed by aspiration, and each well rinsed with 1 ml sterile saline solution. Each Thermanox coverslip was removed from the bottom of each well using sterile tweezers and left to dry on filter paper at 37°C for 20 minutes. Then the fixing and staining protocol was applied to each coverslip, and coverslip mounted on a slide for observation by MO, following the protocol described by Oggioni M et al. [17]. Biofilm morphology and topology were examined under optical microscope (Olympus).
Candida albicans ATCC 10231, confirmed as a pathogen by the CLSI (Clinical and Laboratory Standard Institute) and which has high biofilm-forming capacity was used as experimental control. For negative control, water was used instead of inoculum.
Images were obtained with a SAMSUNG Galaxy J7 and exported to TIFF format.
Statistical analysis
Data were processed and analyzed in Microsoft Excel 2010 and the InfoStat 2018 statistic package. Difference between means was evaluated by right-tailed Student’s t-test for two independent samples after verifying the assumptions of normality, independence and homogeneity of variances, with a 95% confidence interval, considering as significant a p value lower than error alpha (alpha=0.05). Homogeneity of variances was tested with the F test, establishing a significance level of 10%, with the aim of increasing the power and minimizing error type II when not rejecting the null hypothesis of homogeneity of variances. Data normality was tested with Q-Q plot and Shapiro Wilks’ test with 20% significance level. This was done in order to increase precision when not rejecting the null hypothesis of normality. To determine whether there is significant association between biofilm-forming phenotype and clinical source of isolates, ANOVA with two fixed factors was used and K=1 observation, and Bonferroni ’ s test for pairwise comparisons. ANOVA validness was tested with Shapiro Wilks ’ test, Q-Q plot test and Levene´s test on residuals. Presence or absence of outliers was determined by Grubb’s test for outliers (data not revealed). The correlation between variables was analyzed by Spearman test and scatter plot.
Sample size was calculated using statistical software from the InfoStat 2018 package.
Results
Measurement of biofilm biomass by crystal violet stain
In both nutritional conditions the high biofilm forming phenotype prevailed over the "low and null forming" phenotypes. Bonferroni ’ s test showed that high biofilmforming phenotypes differed significantly from low and null biofilm-forming phenotypes (p<0.0001), confirming that the observations are not random (Figures 1 and 2).
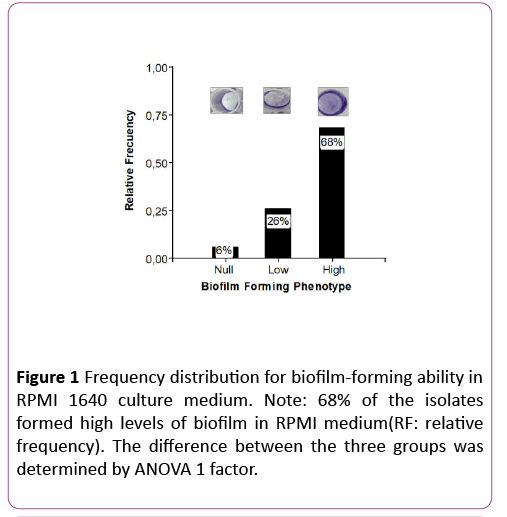
Figure 1: Frequency distribution for biofilm-forming ability in RPMI 1640 culture medium. Note: 68% of the isolates formed high levels of biofilm in RPMI medium(RF: relative frequency). The difference between the three groups was determined by ANOVA 1 factor.
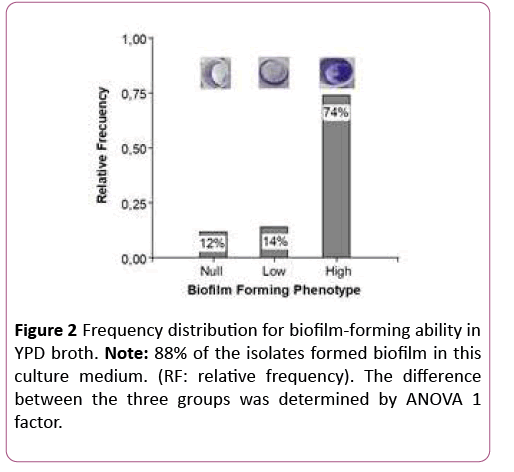
Figure 2: Frequency distribution for biofilm-forming ability in YPD broth. Note: 88% of the isolates formed biofilm in this culture medium. (RF: relative frequency). The difference between the three groups was determined by ANOVA 1 factor.
Spearman ’ s test showed positive correlation (0.49) and statistically significant association (p=0.0001) between CV-YPD absorbance values and CV-RPMI absorbance values. Figure 3 confirms the result of Spearman ’ s test, showing that the correlation between the two variables is not only positive, but also linear. Considering this agreement in results, the information obtained for biofilm-forming phenotype in the collection of study isolates is valid.
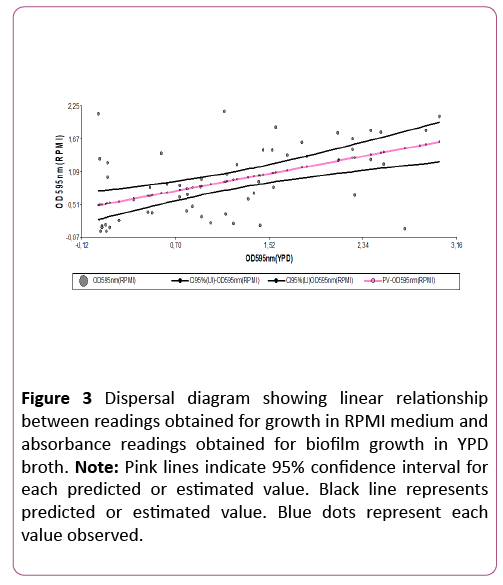
Figure 3: Dispersal diagram showing linear relationship between readings obtained for growth in RPMI medium and absorbance readings obtained for biofilm growth in YPD broth. Note: Pink lines indicate 95% confidence interval for each predicted or estimated value. Black line represents predicted or estimated value. Blue dots represent each value observed.
The optical density at 595 nm of the crystal violet solutions depending on the clinical origin (eubiosis=oral health, and dysbiosis=gingiva-periodontal disease), evidenced in both nutritional conditions (YPD broth and RPMI medium) a tendency of the Candida parapsilosis cells to form more biofilm biomass when they derive from an environment in ecological imbalance or dysbiosis. Nevertheless, comparison of means according to clinical origin using Student’s t-test found no significant difference between the two nutritional conditions tested (Table 2).
Table 2. Mean, standard deviation and p-value for absorbances recorded at 595nm and 490nm depending on the clinical origin of the isolates
| CV assay (OD595nm) |
Nutritional condition |
Eubiosis |
Dysbiosis |
p-value |
| Mean |
ED |
Mean |
ED |
| |
YPD |
1.08 |
0.95 |
1.33 |
0.83 |
0.047 |
| RPMI |
0.89 |
0.64 |
0.93 |
0.62 |
0.1 |
| XTT assay (OD490nm) |
RPMI only |
0.61 |
0.15 |
0.49 |
0.13 |
0.0025 |
Note: ED= standard deviation.
Therefore, we cannot accept the hypothesis that the virulence of Candida parapsilosis sensu stricto increases in dysbiotic oral environments.
Measurement of biofilm metabolic activity by reduction of XTT salt
The choice of this technique as an alternative method for measuring biofilm formation in vitro was based on the following criteria:
The high inter-assay variability observed in the results with CV;
The logarithmic relationship between the absorbance signal and the number of Candida albicans cells in two different strains in planktonic assays reported by Peteers et al. [14];
The correlation between absorbance values and microscopic image of biofilm reported by Pannanusorn et al., [18]; and
High repetitivity and minimal variation in absorbance among different experiments [18].
The XTT assay detected no null biofilm-forming ability. On the contrary, all isolates tested (50/50) displayed virulence. Figure 4 shows that only 16% of the isolates behave in vitro as low biofilm formers. The difference was statistically significant (p<0.0001), which also shows that within the species Candida parapsilosis sensu stricto there is intra-species variability in biofilm-forming ability, with a probability of error lower than 5% (Figure 4).
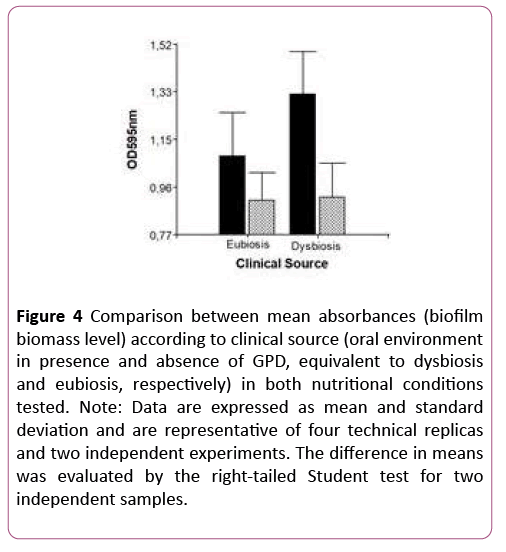
Figure 4: Comparison between mean absorbances (biofilm biomass level) according to clinical source (oral environment in presence and absence of GPD, equivalent to dysbiosis and eubiosis, respectively) in both nutritional conditions tested. Note: Data are expressed as mean and standard deviation and are representative of four technical replicas and two independent experiments. The difference in means was evaluated by the right-tailed Student test for two independent samples.
Upon contrasting mean values for the variable OD490 nm (metabolic activity in biofilm) according to clinical provenance, the t-test for two independent samples showed a statistically significant difference between clinical groups. Figure 5 clearly shows this difference in biofilm metabolic activity according to clinical provenance (Table 2). This result means that in vitro biofilm-forming ability in this Candida species apparently also depends on clinical provenance. This raises the question of whether the biofilm-forming phenotype of Candida parapsilosis sensu stricto is significantly associated to clinical provenance of isolates. The hypothesis was tested by ANOVA with two fixed factors, and Bonferroni’s test, which provided a p-value of 0.04, indicating significant association. This result indicates that the high biofilm-forming phenotype is associated to a dysbiotic oral environment (Figure 6).
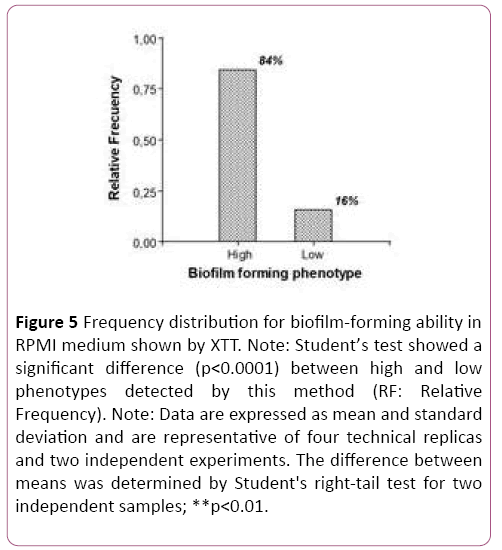
Figure 5: Frequency distribution for biofilm-forming ability in RPMI medium shown by XTT. Note: Student’s test showed a significant difference (p<0.0001) between high and low phenotypes detected by this method (RF: Relative Frequency). Note: Data are expressed as mean and standard deviation and are representative of four technical replicas and two independent experiments. The difference between means was determined by Student's right-tail test for two independent samples; **p<0.01.
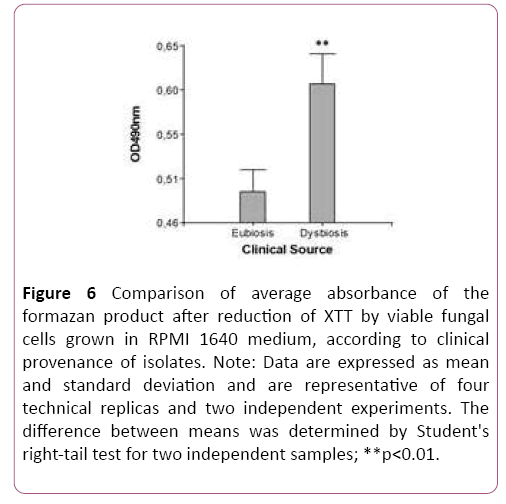
Figure 6: Comparison of average absorbance of the formazan product after reduction of XTT by viable fungal cells grown in RPMI 1640 medium, according to clinical provenance of isolates. Note: Data are expressed as mean and standard deviation and are representative of four technical replicas and two independent experiments. The difference between means was determined by Student's right-tail test for two independent samples; **p<0.01.
Due to the discrepancy between the results of the CV and XTT assays on biofilm-forming capacity and its association with clinical provenance, three isolates were randomly selected per phenotype and classified according to the CV assay results as high, low and null biofilm-forming, and used to evaluate the biofilm produced by OM. All C. parapsilosis sensu stricto isolates formed highly organized cell structures (purple) immersed in an extracellular substance (blue) with different topography and density of extracellular polymer substance (EPS), according to biofilm-forming capacity.
Discussion
The etiopathogenesis of GPD has evolved over the past 5 decades, from being considered an infectious disease in which the microbial challenge was sufficient to cause clinical lesion, to being considered-according to the latest model, a disease determined by polymicrobial synergy and dysbiosis. This model claims that GPD is a pathology associated to an imbalanced biofilm, with increased levels of keystone pathogens, which not only increase in quantity, but also fulfill specific functions such as: a) reducing the host’s defense and b) interacting with other, less abundant pathogens and modulating the virulence of the entire microbial community. The interaction between this dysbiotic biofilm and the susceptible host determines the onset and progression of the disease [19-21].
According to a review published this year by Rodríguez et al., the oral cavity under conditions of ecological equilibrium (eubiosis) will probably acquire Candida parapsilosis sensu stricto strains (from the environment or direct person-toperson contact) with different potential virulence; but in a favorable context, under ecological and immunological balance (Th1-Th2-Th17-Treg), strains with low or null virulence capacity or symbionts are selected in the different oral niches. On the contrary, if homeostasis conditions in the oral cavity are altered, this imbalance may favor the selection of more virulent strains of Candida species, or pathobionts [22]. A pathobiont is a microorganism which, although it expresses attributes of virulence, nevertheless continues living in symbiosis with the host and therefore does not cause a clinically verifiable lesion [23]. This is possible as long as the Th1-Th2-Th17-Treg balance remains intact, which will depend mainly on an efficient Th17 response, since recent evidence shows that this response is crucial to anti-fungal defense in the mucosa, particularly oral mucosa. When this balance breaks down, the clinical outcome may be oral candidiasis or invasive candidiasis [24,25].
We hypothesize that an oral environment in dysbiosis associated to overgrowth of pathobionts exacerbates the virulence of commensal C. parapsilosis sensu stricto strains through inheritable epigenetic changes, since the variability in DNA sequence between strains of this species has been shown to be minimal, even between isolates from different geographic regions [26]. In this regard, we found that isolates from oral niches under conditions of GPD or dysbiosis formed more biofilm biomass than isolates from conditions of eubiosis, although the difference was not significant according to the CV assay. However, the alternative method we used for measuring biofilm metabolic activity by reduction of tetrazolium salts showed statistically significant differences in the biofilm-forming ability between isolates of this Candida species according to clinical provenance (dysbiosis and eubiosis). Moreover, the XTT assay showed association between biofilm-forming phenotype and clinical provenance of isolates, since statistical analysis showed significant association between these two variables, suggesting that biofilm-forming ability in this fungal modal may also be modulated by the conditions in the ecological niche or the environment. These results were consistent with those published by Hasan et al., [27] and Jain et al., [28] who also suggested that, the provenance of the isolates may be an influencing factor in biofilm formation by Candida species. This is the first study to analyze the behavior of oral isolates of C. parapsilosis sensu stricto according to the clinical conditions in which they were isolated and the impact of the conditions of the oral environment on its pathogenicity.
The results for biofilm quantification using CV were corroborated with an alternative method based on measuring the metabolic activity present in the biofilm by using tetrazolium salts such as XTT salt. This is a colorimetric method for indirect quantification of biofilm, as it measures the activity of mitochondrial dehydrogenase. Viable cells will be capable of reducing the XTT to a water-soluble product (formazan) [14]. This solubility makes the product easily measurable in cellular supernatants. According to Pananusorn et al., this point is important in research into biofilm, as it enables biofilm to be studied intact, without alterations to its structure, and the method is being used increasingly in studies evaluating fungal growth and sensitivity to drugs [18]. We prioritized the results obtained with XTT over those obtained with CV, mainly based on the reports by Pananusorn et al., [18] and Sánchez et al., [16], who showed the existence of a direct association between colorimetric signal and number of cells. Nevertheless, some studies show that although this method is useful for comparisons involving a single strain, it is more problematic to use when different fungal strains and species are to be compared. With regard to this dilemma, evaluated the association between colorimetric signal (OD490 nm) and number of cells (different concentrations of a suspension of fungal cells), using two different strains and species of Candida (C. albicans and C. parapsilosis); at two different concentrations of XTT (1X-1 mg/mL and 5X-5 mg/mL). That study found significant differences in the absorbance signal between the two Candida species and between the two C. parapsilosis strains, suggesting that there are differences within the genus Candida regarding tetrazolium salt metabolizing capacity, both between strains and between species. The authors thus concluded that the method is 100% reliable for measuring biofilm within the same species, but when different Candida strains or species are to be compared, detailed standardization is required [29]. However, the sample size in the study is deficient, and results should be validated using a greater number of isolates. In our study, based on the research, we decided to use an inoculum of 1x107 cells per milliliter and a 1X concentration of substrate (XTT); because these were the two conditions showed the least variability among Candida parapsilosis strains analyzed [29].
The results obtained by colorimetric methods (CV and XTT) to define biofilm-producing capacity in each isolate in the study group were confirmed by morphology using optical microscopy (OM). This method confirmed the results of the XTT colorimetric study; and in agreement with reports by Pannanusorn et al., the biofilm-producing capacity as well as biofilm topography and morphology are highly dependent on the C. parapsilosis sensu stricto strain. Our study recognized different biofilm topographies according to biofilm-forming phenotype. With regard to topography, the isolates classified by CV as “high biofilm producers” formed denser and complex biofilm structures that looked like “cobwebs”, with plentiful cellular component and EPS. This is consistent with the observations of Pannanusorn et al., [30]. Among the isolates with low biofilm-forming phenotype, there was a single topography based on more- or less-dense aggregates of yeasts forming a network with more or less EPS deposit, in agreement with Pannanusorn et al., [30] and Serrano-Fujarte et al., [31]. The isolates categorized by CV test as "null producers" showed in the morphological study by OM ability to form intelligent cellular organizations, based on interconnected cell cords generating a reticular structure with low EPS (Figures 7-9). The imaging results confirmed the XTT colorimetric method results, which only recognized isolate with high and low biofilm-forming capacity.
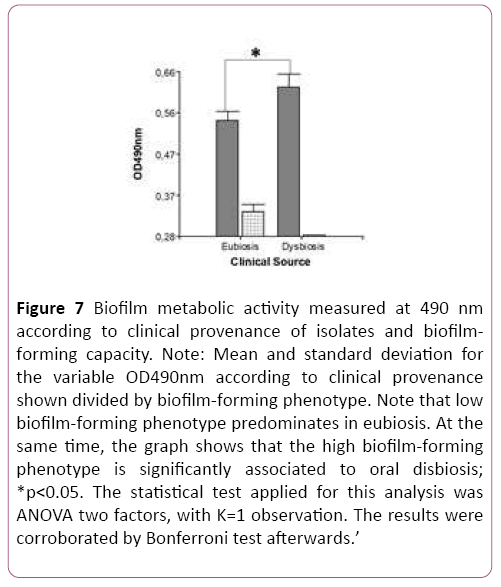
Figure 7: Biofilm metabolic activity measured at 490 nm according to clinical provenance of isolates and biofilmforming capacity. Note: Mean and standard deviation for the variable OD490nm according to clinical provenance shown divided by biofilm-forming phenotype. Note that low biofilm-forming phenotype predominates in eubiosis. At the same time, the graph shows that the high biofilm-forming phenotype is significantly associated to oral disbiosis; *p<0.05. The statistical test applied for this analysis was ANOVA two factors, with K=1 observation. The results were corroborated by Bonferroni test afterwards.’
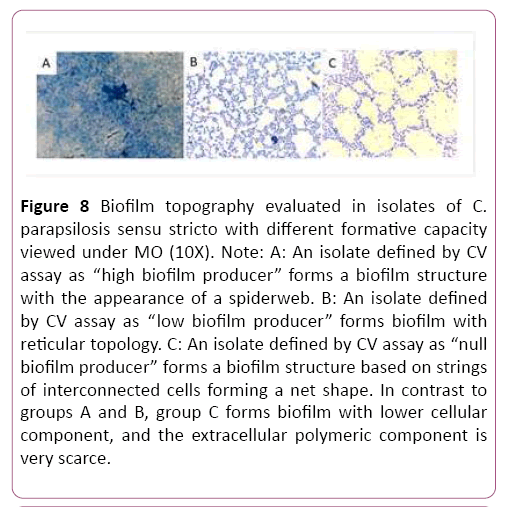
Figure 8: Biofilm topography evaluated in isolates of C. parapsilosis sensu stricto with different formative capacity viewed under MO (10X). Note: A: An isolate defined by CV assay as “high biofilm producer” forms a biofilm structure with the appearance of a spiderweb. B: An isolate defined by CV assay as “low biofilm producer” forms biofilm with reticular topology. C: An isolate defined by CV assay as “null biofilm producer” forms a biofilm structure based on strings of interconnected cells forming a net shape. In contrast to groups A and B, group C forms biofilm with lower cellular component, and the extracellular polymeric component is very scarce.
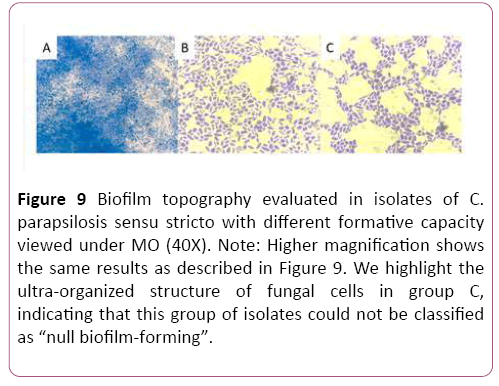
Figure 9: Biofilm topography evaluated in isolates of C. parapsilosis sensu stricto with different formative capacity viewed under MO (40X). Note: Higher magnification shows the same results as described in Figure 9. We highlight the ultra-organized structure of fungal cells in group C, indicating that this group of isolates could not be classified as “null biofilm-forming”.
The findings in this study suggest a new hypothesis on the role of the epigenome in the regulation of genes in this fungal mode. To date, the impact of the epigenome on virulence has only been studied in C. albicans, and it has been suggested that the phenotypical variation described for C. albicans may be regulated by epigenetic changes [32]. At the same time, the results of this study provide an important basis for designing and conducting further longitudinal studies which could reliably demonstrate that the oral cavity under pathological conditions may be a source of invasive infections by Candida species in groups of high risk. With respect to this, a longitudinal and prospective study published in 2014 and conducted in Brazil reported the existence of concordance at the species and karyotype level between oral and blood isolates of Candida albicans and Candida parapsilosis in 50% of infants who complicated with candidemia. The authors indicated the relevance of monitoring the oral microbiota, as a possible source of fungal infection, and assisting to develop appropriate therapeutic strategy [33].
Further, our results suggest that Candida parapsilosis sensu stricto could work as an accelerator of periodontal disease, especially in the clinical form of periimplantitis. This is due to the fact that, previous studies have shown a greater frecuency of isolation of this yeast in subjects with periodontitis, depending on the severity of the disease and in the presence of bio-devices such as implants [34-36].
Conclusions
Biofilm-forming capacity in C. parapsilosis sensu stricto species is intrinsically heterogenous. Nevertheless, the conditions of the ecological environment or niche may impact or condition its potential virulence. Our results suggest that the oral cavity under dysbiosis can transform commensal strains of Candida parapsilosis sensu stricto into pathobionts, probably via epigenetic regulation. We suggest that these observations should be corroborated at transcriptional level, and through approaches evaluating the epigenome in this fungal model.
Thankfulness
To the Licensed Eliana Alcaraz for her valuable collaboration in the realization of this study.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
The study was supported by UBACYT subsidy from the University of Buenos Aires with number 2002017100050BA-2018.
24812
References
- Trofa D, Gácser A, Nosanchuk JD (2008) Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 21: 606-625.
- Gago S, García-Rodas R, Cuesta I (2014) Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis virulence in the non-conventional host Galleria mellonella. Virulence 5: 278-285.
- Canton E, Pemán J, Quindos G (2011) Prospective multicenter study of the epidemiology, molecular identification, and antifungal susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis isolated from patients with candidemia. antimicrob agents chemother 55: 5590-5596.
- Constante C, Monteiro A, Alves S (2014) Different risk factors for candidemia occurs for Candida species belonging to the C. parapsilosis complex. Medical Mycology 52: 403-406.
- Caggiano G, Lovero G, De Giglio O (2017) Candidemia in the neonatal intensive care unit: A retrospective, observational survey and analysis of literature data. BioMed Research International 1-13.
- Peters B, Wu J, Hayes R (2017) The oral fungal mycobiome: characteristicsand relation to periodontitis in a pilot study. BMC Microbiology 17: 157.
- Rodríguez L, Rosa A, Rodríguez J (2017) The oral cavity: a reservoir that favors colonization and selection of Candida parapsilosis sensu stricto strains with high pathogen potential under conditions of gingival-periodontal disease. J Dent Sci Ther 2(1).
- Armitage G (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4: 1-6.
- Bullon P, Morillo J, Ramirez-Tortosa M (2009) Metabolic syndrome and periodontitis: is oxidative stress a common link? Journal of Dental Research.
- D’Aiuto F, Nibali L, Parkar M (2010) Oxidative stress, systemic inflammation, and severe periodontitis. Journal of Dental Research.
- Monzón J, Acuña M, and Cuzziol F (2015) Salivary as an indicator of changes in periodontal tissues. Revista de la Facultad de Odontología FOUNNE 8: 7-20.
- Susewind S, Lang R, Hahnel S (2015) Biofilm formation and Candida albicans morphology on the surface of denture base materials. Mycoses 58: 719-727.
- Durán E, Mujica T, Jewtuchowicz V (2007) Examination of the genetic variability among biofilm-forming Candida albicans clinical isolates. Revista Iberoamericana de Micología 24: 268-271.
- Peeters E, Nelis H, Coenye T (2008) Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. Journal of Microbiological Methods 72: 157-165.
- Treviño R, Rodríguez I, Rosas A (2015) Biopelícula formation and genetic variability of BCR1 gene in the Candida parapsilosis complex. Rev Iberoam Micol.
- Sanchez L, Estrada D, Pozos A (2013) Biopelícula formation by oral clinical isolates of Candida species. Archives of Oral Biology 58: 1318-1326.
- Oggioni M, Trappetti C, Kadioglu A (2006) Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Molecular Microbiology. 61: 1196-1210.
- Pannanusorn S, Fernandez V, Romling U (2013) Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses 56: 264-272.
- Kinane D, Stathopoulou P, Papapanou P (2017) Periodontal disease. Nat Rev Dis Primers 3(17038).
- Michaud D, Fu Z, Shi J (2017) Periodontal disease, Tooth Loss, and Cancer Risk. Epidemiol Rev 39: 49-58.
- Tomás I, Camelo A, Balsa C (2016) Nuevo modelo de patogenia de la periodontitis crónica: de la enfermedad infecciosa a la disbiosis polimicrobiana. RCOE 21: 131-145.
- Rodríguez ML, Jewtuchowicz VM, Rosa AC (2018) La mucosa oral como fuente potencial de candidemia por Candida parapsilosis sensu stricto, bajo condiciones patológicas SOJ Microbiol Infect Dis. 6: 1-10.
- Hornef M (2015) Pathogens, Commensal Symbionts, and Pathobionts: Discovery and Functional Effects on the Host. ILAR Journal 56: 159-162.
- Richardson J, Moyes D (2015) Adaptive immune responses to Candida albicans infection. Virulence 6: 327-337.
- Gow N, van de Veerdonk F, Brown A (2012) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nature review microbiology 10: 112-122.
- Tavanti A, Hensgens L, Mogavero S (2010) Genotypic and phenotypic properties of Candida parapsilosis sensu strictu strains isolated from different geographic regions and body sites. BMC Microbiology 10: 1-11.
- Hasan F, Xess I, Wang X (2009) Biofilm formation in clinical Candida isolates and its association with virulence. Microbes and Infection 11: 753-761.
- Jain N, Kohli R, Cook E (2007) Biofilm formation by and antifungal susceptibility of Candida isolates from urine. Appl Environ Microbiol 73: 1697-1703.
- Kuhn D, Balkis M, Chandra J (2003) Uses and limitations of the XTT assay in studies of Candida growth and metabolism. Journal of Clinical Microbiology 41: 506-508.
- Pannanusorn S, Ramírez B, Lünsdorfet H (2014) Characterization of Biofilm Formation and the Role of BCR1 in Clinical Isolates of Candida parapsilosis. Eukaryotic Cell 13:438-451.
- Serrano I, López E, Reyna G (2015) Influence of culture media on biofilm formation by Candida species and response of sessile cells to antifungals and oxidative stress. Biomed Res Int 783639.
- Lohse M, Johnson A (2010) Temporal anatomy of an epigenetic switch in cell programming: the white-opaque transition of C. albicans. Molecular Microbiology 78: 331-343.
- Batista G, Krebs V, Ruiz L (2014) Oral colonization: A possible source for candidemia in low-weight neonates. Journal of Medical Mycology 24: 81-86.
- Jewtuchowicz V, Mujica M, Brusca M (2008) Phenotypic and genotypic identification of Candida dubliniensis from subgingival sites in immunocompetent subjects in Argentina. Oral Microbiology Immunology 23: 505-509.
- Canabarro A, Valle C, Farias MR (2013) Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J Periodontal Res 48: 428-432.
- Alrabiah M, Alshagroud R, Alsahhaf A (2019) Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin Implant Dent Relat Res 1-5.














