Keywords
Peppermint; Human diseases; Peppermint oil; Quantum chemistry; Molecular docking
Abbreviations
PO: Peppermint oil; WHO: World Health Organization; HOMO: The highest occupied molecule orbital LUMO: The lowest un-occupied molecular orbital; MEP: Molecular electrostatic potentials; NAT: Arylamine N- acetyltransferase; SD: Standard deviation; IBS: Irritable bowel syndrome; HSV=Herpes simplex virus (DNA virus); VACV=Vaccinia virus
Introduction
Medicinal plants have received more attention because of their health benefits, such as anti-infectious properties, since ancient times [1-6]. The term of medical plants is referred to the natural remedies that have used for treatment of human diseases [4,7-10]. These medicinal plants can be considered as a valuable source of ingredients which can be used in drug development [5,11-13]. On the other hand, medical plants significantly affected the human life across the entire world [5,7,14,15]. The use of herbal medicine is leading modality, followed in Middle East, Europe and certain other advance countries, in order to treat of catastrophic human diseases [16]. Based on the WHO reports, the advanced countries have used medicinal plant for both clinical therapy and food industries significantly [16,17].
Medicinal plants have significant potentials for human societies and consumed by people across the entire world. Although most of their health benefits have not investigated yet, their medical activities can be considered in the treatment of present or future diseases [7]. Currently, more than 80% of the world population use the traditional medicine and medicinal plants (especially plant extracts and essential oils) for their primary health needs [18]. Peppermint or mint (Mentha piperita L.), a perennial aromatic herb belonging to the Lamiaceae (Labiatae) family, is a natural hybrid between spearmint (Mentha spicata L.) and water mint (Mentha aquatic L.) [19,20]. Although it is a native genus of the Mediterranean regions, it cultivated all over the world for its use in flavor, fragrance, medicinal, and pharmaceutical applications [21]. Members of the mint genus are characterized by their volatile oils which are of great economic importance, being used by the flavor, fragrance, and pharmaceutical industries [22].
This plant is widely used in folk remedies and traditional medicine for treatment of digestive disorders and nervous system actions because of its antitumor and antimicrobial properties, chemopreventive potential, its renal actions, antiallergenic effects, and also for lessening cramping, digestive complaints, anorexia, nausea and diarrhea [23,24]. Preparations of peppermint include leafs, leaf extracts and water, however, the plant is cultivated mainly for its essential oil, which is obtained by distillation from freshly grounded leaves [25-28]. PO is composed of menthol and menthone together with several other minor constituents, including pulegone, menthofuran and limonene, and its chemical composition may vary with plant maturity, geographical region and processing conditions [28-30].
Menthol occurs naturally as a colorless crystal or powder [31]. It is greatly responsible for the spasmolytic nature of peppermint [32]. Menthol has reported to stimulate bile flow [33], reducing the tone in the esophageal sphincter [34], facilitating belching [35], as well as having antibacterial properties [36]. In addition, peppermint is also a rich source of polyphenolic compounds and hence the strong antioxidant properties [8,22,26,28,37]. Among all countries in the world, India is the largest producer, exporter [38] and consumer of mint oil [39]. Currently China is a major importer of peppermint [39].
HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) orbitals are very important parameters used in quantum chemistry [40-42]. Based on their characteristics, it can be specified how a molecule would interact with other molecules [40]. The HOMO orbitals can be considered as an electron donor group, while the LUMO orbitals as free sites able to accept them [40,43-46]. Energy of the HOMO orbitals can be directly linked to the ionization potential, whereas the LUMO orbital energy can be associated with the electron affinity [40,44]. The difference between the orbital energies of HOMO and LUMO is referred to as energy gap (ΔE) which is an important parameter that can determine the reactivity or stability of molecules [40,44-46]. Since quantum chemistry and molecular docking studies have not been reported, the present study aims at determining the optimized molecular geometry, HOMO-LUMO energies of peppermint main compounds, using Hartree-Fock, 3-21G basic set and also indicates the binding mode of these compounds into a selected receptor. Also, the most abundant medicinal benefits of peppermint have reviewed.
Methods
Herein the therapeutic application of volatile oil of peppermint is discussed and also chemical descriptors are calculated to determine the electron parameters of peppermint active constituents to search for biological activities of these compounds.
Molecular quantum studies
All computational calculations were performed at the Hartree- Fock model on a Pentium IV/2.8 GHz personal computer using Spartan 10 software Wavefunction, Inc. [47]. The geometry of the peppermint active constituents in the ground state is fully optimized.
Molecular docking
The 3D structure of NAT enzyme (PDB ID: 2IJA) was obtained from PDB database (https://www.rcsb.org/pdb/home/home.do) and selected as receptor against peppermint chemical compounds. The molecular docking (blind docking) was done by Molegro virtual Docker 4.2.0 version. Visualization of docking results was performed by MOE software (https://www.chemcomp.com/MOE-Molecular_Operating_Environment.htm).
Nomenclature, botany and cultivation
Peppermint has more than 101 local names in different countries (Table 1) [48-51]. The principle of naming of mint is considered based on local culture and customs.
| Country |
Local name |
| Iran |
Nanafelfeli |
| Brazil |
Nortela pimento |
| USA |
Lab Mint, mint |
| Norway |
Peppermynte |
| Poland |
Pepparmunta |
| Spain |
Mentainglesa |
| Portugal |
Hortelanapimentosa |
| Swedish |
Pepparmynt |
| China |
Po Ho |
| India |
Urdu, mint, Pudina, Pudyana, Puthina |
| Turkey |
Nana |
| Russia |
Myataperechnaya |
| Uruguay |
Menta |
| French |
Menthe |
| Iraq |
Nana |
| Bogota |
Yerba Beuna |
| Denmark |
Pebermynte |
| Germany |
Peppermint |
| England |
Brandy Mint |
| Mexico |
Mentapiperita |
Table 1: The most abundant local names of mint around the world.
In botany, Mentha piperita L. is the common name for genus of peppermint [19]. The genus Mentha includes 25 to 30 species [52] which is a perennial herb and native to Europe, naturalized in the northern USA and Canada, and cultivated in many part of the world [53,54].
The mint is a sterile hybrid of spearmint (Mentha spicata) and water mint (Mentha aquatica) from the Lamiaceae family (Figure 1) [20,27].
Figure 1: A schematic illustration of peppermint hybrid.
The most relevant of mint species with commercial or medicinal usage are listed in (Table 2).
| Species |
Usage |
References |
| MenthaspicataL. |
Medicine |
[55] |
| Menthasuaveolens |
Ornamental Consumption |
[56] |
| MentharequieniiBenth. |
Ornamental Consumption |
[57] |
| MenthapulegiumL. |
Medicine |
[58] |
| MenthapiperitaL. |
Medicine, Ornamental consumption, commercial |
[59-61] |
| MenthacitrataEhrh |
Medicine |
[62] |
| MenthalongifoliaL |
Medicine, Commercial |
[63,64] |
| Menthacardiaca |
Medicine |
[65] |
| Menthaarvensis |
Medicine |
[66] |
| Menthacanadensis |
Weed |
[67] |
| Menthaflavouring |
Ornamental consumption, Medicine |
[68,69] |
Table 2 The list of the most abundant mint species and their functions.
Peppermint grows particularly well in lands with high waterholding capacity soil [55-70]. All commercial mint varieties are seed sterile and are propagated using the underground stolons (runners or rootstock) produced by existing plants [71]. The stolons can’t be stored for more than a few days since they deteriorate rapidly due to heat or dehydration [71]. In general, mints tolerate a wide range of conditions, and can also be grown in full sun [72].
Chemical properties
Many studies showed that peppermint essential oil is composed of various secondary metabolites [27,28,31,33,34,38,53,54,73,74]. The mint main chemical compounds consist of limonene, cineole, menthone, menthofuran, isomenthone, menthyl acetate, isopulegol, menthol, pulegone and carvone (Figure 2 and Table 3) [38,74].
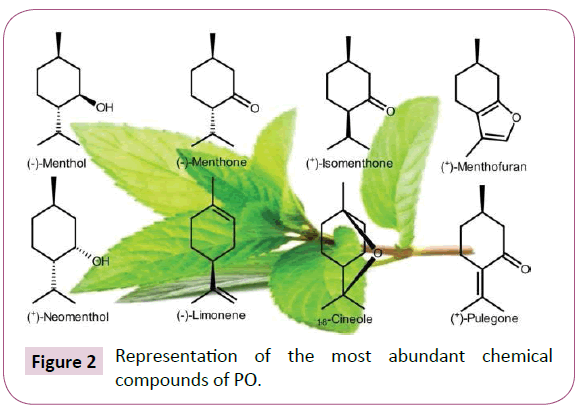
Figure 2: Representation of the most abundant chemical compounds of PO.
| Compounds |
IUPAC name |
Percentage (%) |
References |
| Limonene |
1-Methyl-4-(1-methylethenyl)-cyclohexene |
1 to 5 |
[38] |
| Cineole |
1,3,3-Trimethyl-2-oxabicyclo[2,2,2]octane |
3.5 to 14 |
[23,76] |
| Menthone |
(2S,5R)-2-Isopropyl-5-methylcyclohexanone |
14 to 32 |
[31] |
| Menthofuran |
3,6-Dimethyl-4,5,6,7-tetrahydro-1-benzofuran |
1 to 9 |
[23,28] |
| Isomenthone |
(2R,5R)-5-methyl-2-propan-2-ylcyclohexan-1-one |
1.5 to 10 |
[27] |
| Menthyl acetate |
Acetic acid [(1R,2S,5R)-2-isopropyl-5-methylcyclohexyl] ester |
2.8 to 10 |
[77] |
| Isopulegol |
5-methyl-2-prop-1-en-2-ylcyclohexan-1-ol |
0.2 |
[73] |
| Menthol |
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexanol |
30 to 55 |
[23,31,33,38,49,53,77] |
| Pulegone |
p-Menth-4(8)-en-3-one |
4 |
[78] |
| Carvone |
2-Methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one |
1 |
[79] |
Table 3 The most abundant active compounds of Mentha spp.
Other constituents include flavonoid glycoside (eg. Narirutin, Luteolin-7-o-rutinoside, Isorhoifolin and Hesperidin etc) [75] polyphenols (e.g Rosmaric acid, Eriocitrin, Cinamic acid, Caffeic acid and Narigenin-7-oglucoside); luteolin-diglucoronide and eriodictyol glucopyranosyl-rhamnopyranoside were also purified from aerial parts of mint [75-79].
The amount of peppermint compounds is different in various species [80]. Various factors including physiological variations, environmental conditions, geographic differences and genetic factors cause differences in chemical composition of these plants [80].
The most abundant chemical compounds that isolated form peppermint are largely classified into monoterpenes [81]. Currently, peppermint is the best model system for the study of monoterpene metabolism [82]. The pathway of monoterpene biosynthesis in peppermint has been well characterized by in vivo and systems biology studies (Figure 3) [83-85]. and all of the enzymes involved have been described [81,84]. According to the traditional view [86,87] monoterpenes are amongst the major constituents of essential oils and common secondary metabolites of plant metabolism, and as such they generally have been regarded as metabolic deadlock [83,84,87]. As shown in figure 3, the peppermint monoterpene-derived compounds separate from primary metabolism by conversion of isopentenyl diphosphate and dimethylallyl diphosphate, via the action of the prenyltransferase geranyl diphosphate synthase (EC 2.5.1.29), to geranyl diphosphate, which undergoes subsequent cyclization by limonene synthase (EC 4.2.3.16) to (4S)-(-)-limonene [84,88]. In peppermint a microsomal cytochrome (Cyt) P450 limonene- 3-hydroxylase (EC 1.14.13.47) adds an oxygen molecule in an allylic location to produce (-)-trans-isopiperitenol and thereby establishes the oxygenation pattern of all subsequent derivatives [81,88,89].
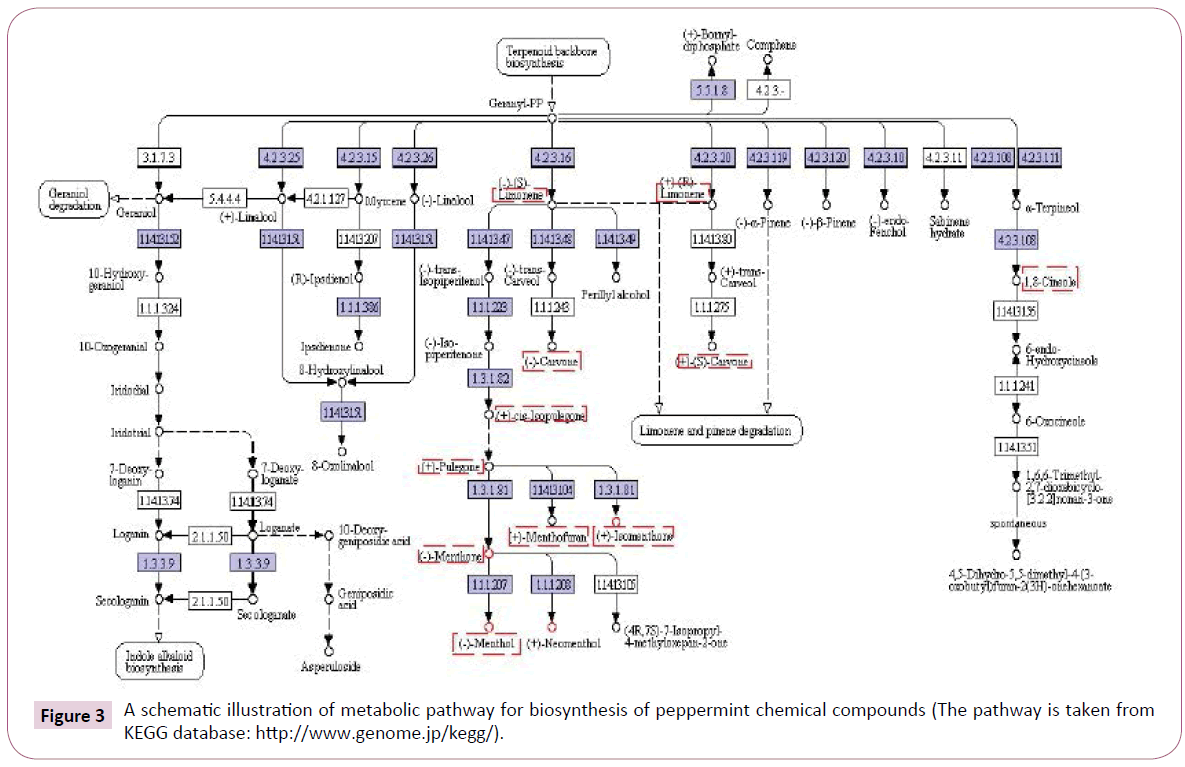
Figure 3: A schematic illustration of metabolic pathway for biosynthesis of peppermint chemical compounds (The pathway is taken from KEGG database: https://www.genome.jp/kegg/).
A soluble NADP-dependent dehydrogenase (EC 1.3.1.82) oxidizes the alcohol to a ketone, (-)-isopiperitenone, thereby activating the adjacent double bond for reduction by a soluble, NADPHdependent, regiospecific reductase to afford (+)-cis-isopulegone. An isomerase next moves the remaining double bond into conjugation with the carbonyl group, yielding (+)-pulegone. A NADPH-dependent reductase then converts (+)-pulegone to (+)-isomenthone and (-)- menthone, which predominates [89].
Finally, two stereo-selective NADPH-dependent reductases convert (-)-menthone and (+)-isomenthone to (-)-menthol and (+)-neoisomenthol, respectively, and (-)-menthone and (+)-isomenthone to (+)-neomenthol and (+)-isomenthol, respectively [81,88,89]. In these pathways, (-)-limonene is the first committed intermediate for biosynthesis of other compounds in the peppermint species. However, production of monoterpenes in peppermint id restricted to developing oil glands of young leaves [88,90,91], and the correlation between in vitro activity for the several enzymatic steps of menthol biosynthesis and the rate of biosynthesis measured in vivo suggests that monoterpene production is controlled by the coordinately regulated activity of relevant biosynthetic enzymes [82,90,92]. As mentioned above, (-)-Menthol greatly important among the menthol isomers (often exceeding 50% of the essential oil) and is primarily responsible for the characteristic flavor and cooling sensation of peppermint [31,89,93,94].
HOMO and LUMO orbitals analysis
The HOMO and LUMO orbitals are very important in quantum chemistry calculations [95,96]. The HOMO energy determines the electron donating ability while the LUMO designates the electron accepting ability and the HOMO–LUMO energy gap (ΔEgap) (ELUMO-EHOMO) [97,98] is an important value for stability index [96,99]. A large ΔEgap implies a good thermodynamic stability of the compound, in the sense of its lower reactivity in chemical reactions [100,101]. However, the magnitude of the HOMO-LUMO gap has very important chemical implications, even if qualitatively evaluated [102]. To determine stability and reactivity of peppermint main chemical compounds according to Hartree-Fock model 3-21G basis set calculation for water solution, the gap energies were measured (Table 4).
| Compounds |
HOMO (eV) |
LUMO (eV) |
(eV) |
| Limonene |
-9.1 |
5.0 |
14.1 |
| Cineole |
-10.1 |
6.3 |
16.4 |
| Menthone |
-10.6 |
4.2 |
14.8 |
| Menthofuran |
-8.4 |
4.8 |
13.2 |
| Isomenthone |
-10.3 |
4.4 |
14.7 |
| Menthyl acetate |
-11 |
5.0 |
16.0 |
| Isopulegol |
-9.7 |
5.0 |
14.7 |
| Menthol |
-10.9 |
6.0 |
16.9 |
| Pulegone |
-9.3 |
3.3 |
12.6 |
| Carvone |
-9.6 |
3.0 |
12.6 |
Table 4 HOMO and LUMO orbitals energy values for peppermint main chemical compounds in water, calculated with Spartan 10 V1.1.0, software, Hartree-Fock, 3-21G basic set.
Based on table 4 data, menthol, cineole and isopulegol have higher stability than other compounds. The increase of stability that showed by ΔEgap promotes the low reactivity of these compounds in a chemical reaction. The relationship between ΔEgap energy, stability and reactivity is well known described in many studies [103-105]. According to Hartree-Fock, 3-21G basic set calculation, the highest and lowest gap energies is related to menthol (16.9 eV), pulegone (12.6 eV) and carvone (12.6 eV) respectively. Our result about stability of menthol is similar to result that reported by Harlod and coworkers [106]. Froehlich et al. reported that in the aqueous ethanolic solutions, pulegone was unstable and it can be degraded to other products [107]. This case confirmed our molecular orbitals analysis for pulegone. Also, surfaces for the frontier orbitals were drawn to understand the bonding scheme of present compounds. The features of these molecular orbitals can be seen in (Figure 4).
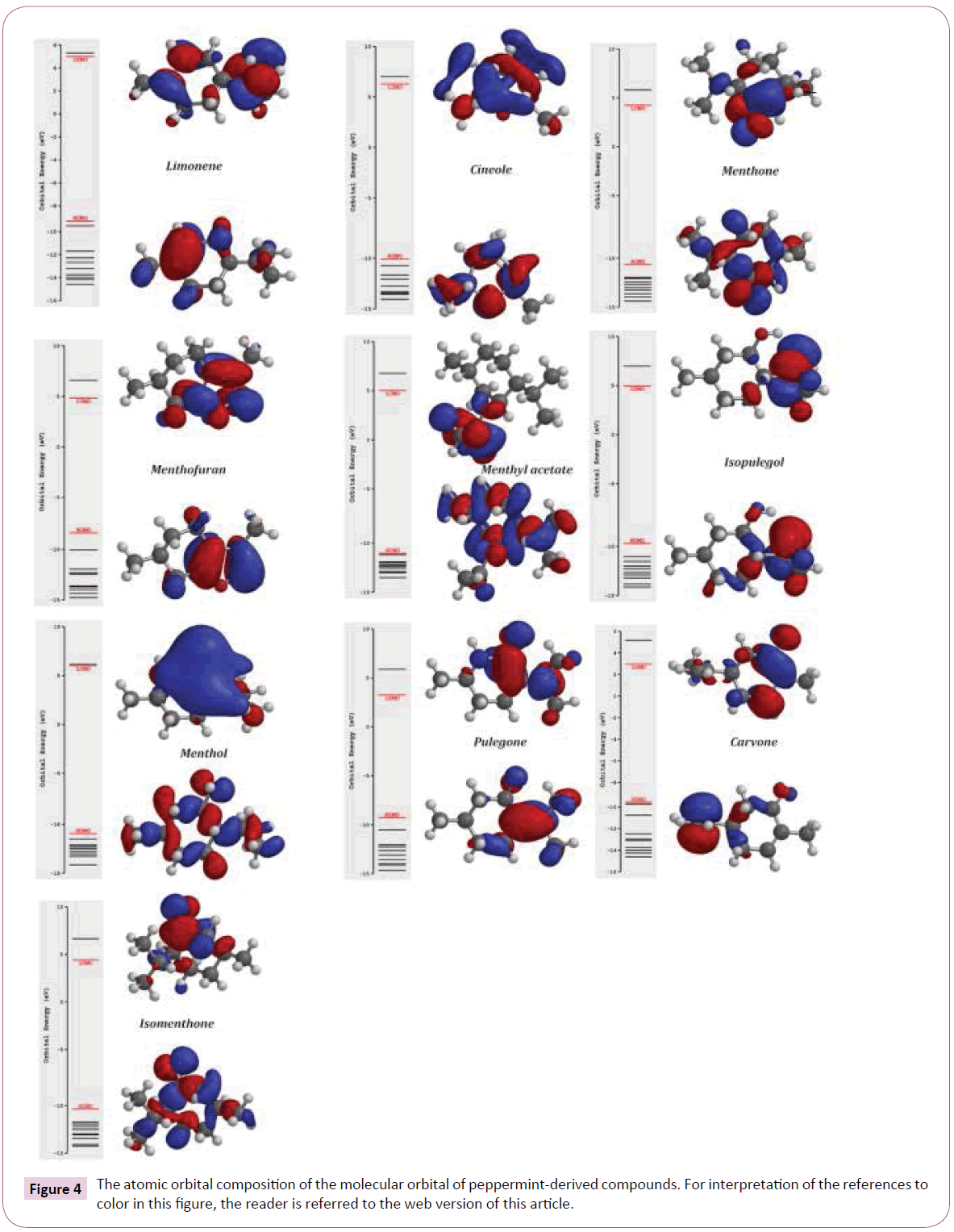
Figure 4: The atomic orbital composition of the molecular orbital of peppermint-derived compounds. For interpretation of the references to color in this ? gure, the reader is referred to the web version of this article.
MEP analysis
The electrostatic potential of a molecule is an established tool in medicinal chemistry, modeling, and computational chemistry [108,109]. The MEP employed abundantly for predicting potentials have been and interpreting the reactive behavior of a wide range of chemical system in both electrophilic and nucleophilic reactions, the study of biological recognition processes and hydrogen bonding interactions [109-111]. To predict reactive sites for electrophilic and nucleophilic attack for the peppermint chemical compounds, MEP was calculated at Hartree-Fock, 3-21G basic set optimized geometries. In the most of the MEP, while the maximum negative site which preferred region for electrophilic attack indications as red color, the maximum positive region which preferred site for nucleophilic attack symptoms as blue color [112,113]. In the present study, 3D plot of molecular electrostatic potential of studied compounds has been drawn in (Figure 5). In this plot the different values of electrostatic potential at surface are represented by different colors. Potential increase in order red113].
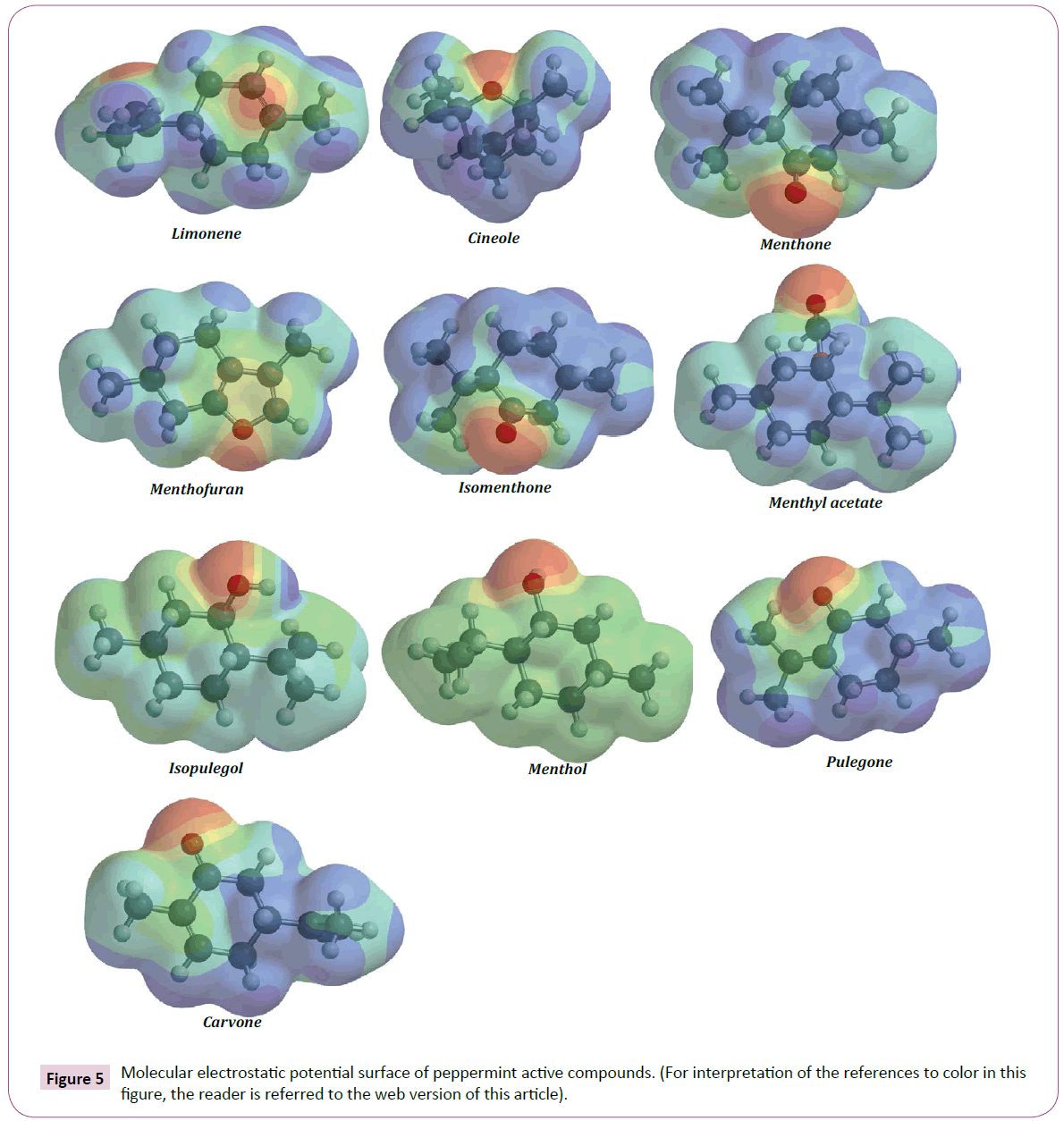
Figure 5: Molecular electrostatic potential surface of peppermint active compounds. (For interpretation of the references to color in this ?gure, the reader is referred to the web version of this article).
As shown in (Figure 5), the regions having the negative potential are over the electronegative atom oxygen, respectively. Thus, it would be predicted that an electrophile would preferentially attack peppermint compounds at the oxygen positions. In addition, we found the positive regions over hydrogen atoms of methyl group of peppermint compounds and indicating that these sites can be the most probably involved in nucleophilic processes. Red and blue colors in peppermint compounds map refer to the regions of negative and positive potentials and correspond to electron rich and electron-poor regions, respectively, whereas the green regions signify the neutral electrostatic potential. The MEP surface map of peppermint compounds provides necessary information about reactive sites. These results can be used for design and development of the stable peppermint-derived drugs. The importance and application of MEP map in drug development is discussed in many studies [114-117].
Antiviral Activity
Nowadays, the development of phytotherapies aiming at the inhibition of viral diseases [118], in combination with classical anti-viral therapies, is among the most intensively studied approaches for the treatment of pathogenic viruses [119]. Infectious viral diseases remain an important worldwide problem, since many viruses have resisted prophylaxis or therapy longer than other microorganisms [120]. At the moment, only few effective antiviral drugs are available for the treatment of viral diseases [121]. There is need to find new compounds with not only intracellular but also extracellular antiviral properties [122]. There are several reports showed that various peppermint extracts has signi?cant antiviral activities [123-126]. It seems, peppermint helps to immune system and protect the body from viruses [127-137]. Table 5 presents a comprehensive list of antivirus effect of peppermint extracts.
| Extracts |
Virus |
References |
| Aqueous |
HSV-1/2 |
[128,129] |
| HIV-1 |
[130] |
| Influenza A virus |
[131] |
| Newcastle disease virus |
[132] |
| VACV in egg |
[132] |
| Semliki Forest |
[133] |
| West Nile viruses |
[133] |
| Alcohol |
Influenza A virus |
[134] |
| HSV |
[135] |
| Essential oil |
HSV-1 |
[122,136,137] |
| HSV-2 |
[122,137] |
Table 5 Antiviral activity of different peppermint extracts.
Antibacterial Properties
Medicinal plants have been broadly used in common medicine and therefore, plant secondary metabolites are increasingly of interest as antimicrobial agents today [138,139]. Currently, biologically active compounds from peppermint sources have always been a great interest for scientists working on infectious diseases [140]. PO and extracts showed a good antimicrobial activity against: 1) Escherichia coli, 2) Salmonella pullorum, 3) Comamonas terrigena, 4) Streptococcus faecalis, 5) Acinatobacter sp, 6) Streptococcus thermophiles, 7) Lactobacillus bulgaricus, 8) Staphylococcus pyogenes, 9) Staphylococcus aureus, 10) Streptococcus pyogenes, 11) Serratia marcescens, 12) Mycobacterium avium, Salmonella typhi, 13) Salmonella paratyphi A/B, 14) Proteus vulgaris, 15) Enterobacter aerogenes, 16) Yersinia enterocolitica and 17) Shigella dysenteriae [131,141-143]. Studies showed that the antibacterial activity of peppermint leaves extract against Gram negative bacilli was higher than of its stem extract [131]. A number of studies demonstrated that essential oil from leaves of peppermint exhibited the highest antibacterial activity with 11.58 to 17.24 mm ± 0.87 SD, zone of inhibition [1,62,125,133], while the effect of extract obtained from the stem of peppermint is an average zone of inhibition 15.82 mm ± 3.56 SD, respectively [131]. On the other hand, PO has strongly effects against Enterococcus faecium ATCC10541, Salmonella choleraesuis, Staphylococcus aureus and Bacillus subtilis [140- 144]. There are differences in the chemical composition of peppermint essential oil from different parts of its structure [131]. As mentioned above, this differences can be effect on antibacterial activity of peppermint species [133]. Generally, mint oil and menthol have moderate antibacterial effects against both Gram-positive/negative bacteria [131]. It seems peppermint can become a novel target for synthesis of plant-derived drugs against a large spectrum of multidrug resistance bacteria.
Antifungal Activity
In-vitro data suggested that PO and extracts are good fungicidal against Candida albicans, Aspergillus albus and dermatophytic fungi [145]. The leave oils of Mentha spicata exhibited moderate activity against Aspergillus fumigatus (with 16 mm ± 0.5 SD, zone of inhibition) and A. niger (with 14 mm ± 0.5 SD) [146].
Allelopathic Effects
Allelopathy is one kind of stress that plays a signi?cant role in agro-ecosystems, and affects the growth, quality and quantity of the crops [147,148]. It was reported that water extract of peppermint (at concentration 10% v/v) is able to inhibits the growth of the tomato seedlings [149]. Skrzypek and Coworkers [150], demonstrated that aqueous extracts of peppermint (at concentration 15% v/v) decreases non-photochemical and photochemical quenching and vitality index of photosystem II in sunflower.
Medicinal Uses
Currently, PO has become most considered agent as treatment for a large body of human diseases [38]. The major health benefits of PO are shown in (Figure 6). In addition to medicinal uses, its extract is broadly used as flavoring in food industries [151]. As mentioned in pervious sections, among all chemical compounds that purified from PO [31], menthol is common ingredient and widely is used for respiratory congestion [152,153], headache [154], and skeletal muscle pain [155]. The best dosage of PO for consumption in adult was reported 0.2 to 0.4 mL of oil three times daily in enteric-coated capsules [156].
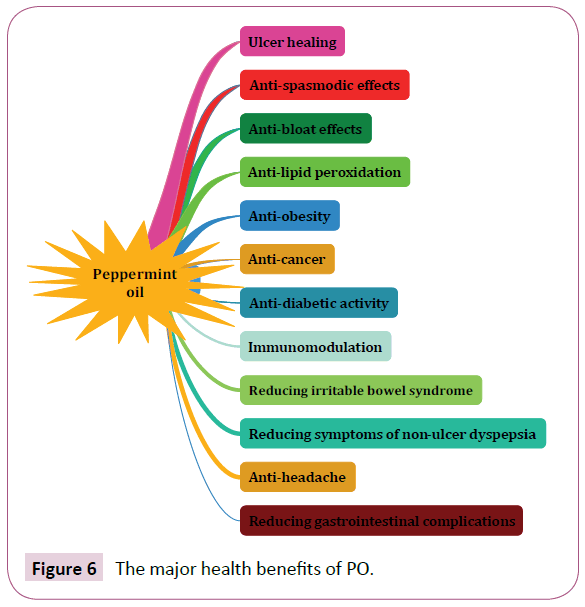
Figure 6: The major health benefits of PO.
Anti-angiogenic/Inflammatory effects
Angiogenesis, the formation of new arterioles from preexisting vessels, is a multistep event involving degradation and remodeling of the underlying basement membrane and the surrounding extracellular matrix with subsequent proliferation and migration of vascular endothelial cells into the tissue to be vascularized [157-159]. Inflammation is regarded as an important baseline reaction responsible for manifestations of various chronic diseases such as cancer, septic shock, diabetes, atherosclerosis and obesity [18,160]. Recent data have expanded the concept that inflammation is a critical component of tumor progression [143]. There are several reports that peppermint compounds have crucial roles in prevention of inflammation and angiogenesis [161-163]. Methanol extract of peppermint has cytotoxic effect on L1210 cancer cells [164]. Lin and colleagues [165] showed that apparently menthol, in higher doses, effects on NAT activity in the human liver tumor cell line J5 [166]. The NAT is responsible for the biotransformation of numerous arylamine drugs and carcinogens [141]. This enzyme has three critical residues consist of Cys68, His107 and Asp122 [167]. These residues corresponding to active site of NAT enzyme [142]. Herein, we performed a molecular docking to find the binding mode of peppermint compounds into NAT enzyme as receptor (Figure 7).
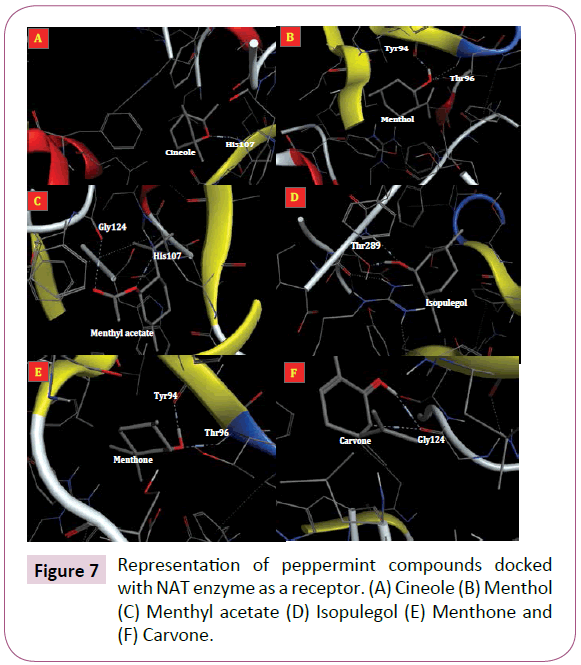
Figure 7: Representation of peppermint compounds docked with NAT enzyme as a receptor. (A) Cineole (B) Menthol (C) Menthyl acetate (D) Isopulegol (E) Menthone and (F) Carvone.
Docking results showed that cineole and menthyl acetate interact with His107 residue and therefore, they are able to inhibit NAT enzyme activity (Figure 7A and 7C). The docking energies for cineole, menthol, menthyl acetate, isopulegol, menthone and carvone were -11.2, -13.4, -11.91, -9.82, -7.83 and -10.11 kcal/mol, respectively. The His107 is one of critical residues in the active site of NAT enzyme and it is important for its activity [168]. Lin and Co-workers [165] reported that menthol a possible uncompetitive inhibitor to NAT activity in cytosols. Our docking result showed that menthol is able to interact with two residues (Tyr94 and Thr96) from NAT enzyme with a great probability (Figure 7B). In other hand, menthon was also able to interact with these two residues from NAT enzyme (Figure 7E). Other docked compounds (i.e. isopulegol and carvone) interact with different residues of receptor (Figure 7D and 7F).
Antispasmodic effects
PO relaxes gastrointestinal smooth muscle [169] by reducing calcium influx in both large intestine and jejunum [170]. PO and menthol are inhibitor for calcium channel activity in rats and guinea pig atrial and papillary muscle, rat brain synaptosomes, and chick retinal neurons [171,172].
Treating Irritable Bowel Syndrome
IBS is defined as a chronic disorder of altered bowel function characterized by symptoms of diarrhea, constipation, or alternating bowel habits accompanied by pain or discomfort and may include a constellation of other symptoms, e.g., bloating, urgency, and incomplete evacuation [156,171,173-175]. This syndrome affects 9 to 23% of the population across the world [176]. It was reported that PO is a safe and effective short-term treatment for IBS [177,178]. Also, PO acts as inhibitor for calcium channel activity in the intestine and therefore it can able to reduce symptoms of IBS [31]. Other postulated mechanisms for PO in treatment of IBS include inhibition of potassium depolarizationinduced and electrically stimulated responses in the ileum [179]. Also, it was reported that PO has crucial effects on histamine, serotonin, and cholinergic receptors in the gastrointestinal tract may also mediate some of its antiemetic effects [180]. Cappello et al. showed that a four weeks treatment with PO improved abdominal symptoms in patients with IBS [181]. The similar results also were reported in other studies [176,182-184]. Taken together, peppermint is the most encouraged plant for treatment of gastrointestinal disorders.
Anti-headache activity
Since ancient times, herbal therapy has been used as treatment for headache disorders [185]. Consumption of peppermint and derivatives is the best target for headache therapy [186]. Gobel et al. showed some bene?t from peppermint and eucalyptus oil in combination in relieving patients’ headache pain [186]. Also, similar result was reported by Levin [187].
Effect on hepatic enzymes
Maliakal and Wanwimolruk reported that aqueous extract of peppermint (at concentration 2% v/v) can modulate of phase I and phase II drug metabolizing enzymes [188]. In phase I, a variety of enzymes act to introduce reactive and polar groups into their substrates [189]. Phase II biotransformation reactions generally serve as a detoxifying step in drug metabolism [190]. Khodadust et al. showed that peppermint alcoholic extract ameliorated the adverse effects of CCl4 on growth performance and liver function, therefore they indicated that it might be useful for the prevention of oxidative stress-induced hepatotoxicity in broilers [191].
Radioprotective Effects
The radioprotective activity of peppermint oil and aqueous extract has well been documented [192,193]. Kaushik et al. demonstrated the effectiveness of peppermint alcoholic extract against radiation induced morbidity and mortality using the optimum dose of 100 mg/kg for 3 consecutive days [192]. Samarth and Coworkers suggested the antioxidant and free radical scavenging activities of leaf extract of peppermint are directly related to its mechanism of radiation protection [193]. Several mechanisms such as antioxidant activity, immune response, and enhanced recovery of bone marrow have been suggested for chemoprevention and radioprotection of peppermint extracts [194].
Side Effects and Toxicity
Although peppermint is a considered medicinal plant for treatment of human diseases, it was reported that in rats, PO caused cyst-like changes in the white matter of the cerebellum and nephropathy at doses of 40-100 mg/kg per day for 28-90 days [195].
Adverse reactions to enteric coated PO capsules are rare [174], but may include hypersensitivity reaction, contact dermatitis, abdominal pain, heartburn, perianal burning, bradycardia and muscle tremor [175,196].
In patients with chronic cough, pre-inhalation of menthol reduces cough sensitivity to inhaled capsaicin and in?uences inspiratory ?ows [197]. In rats, doses of 80 and 160 mg of pulgeone for 28 days caused atonia, weight loss, decreased blood creatinine content, and histopathological changes in the liver and the white matter of the cerebellum [198]. Menthol causes hepatocellular changes in rats [195].
Marketing
The market for PO in the entire world is divided into local and international buyers. The local buyers included small buyers and companies from chemical and pharmaceutical, as well as food and flavoring industries. The international buyers are divided into flavor and fragrance houses, cosmetics and personal health care, aromatherapy and food manufacturers who buy in large quantities [199]. The peppermint industry is the largest commercial herb industry in the United States (more than 4000 tons per year). Keeping in view multiple benefits of peppermint, various dosage forms are available in market for treatment of various human lifestyle diseases (Figure 8).

Figure 8: Different dosage forms of PO alone or in combination with other chemical ingredients are available in market.
Conclusion Remarks
Regarding to health benefits of peppermint, it can be concluded that this plant has great potentials for treatment of human diseases and also it has strong future in the world marketing. Further studies are need to exploration of cellular and molecular mechanisms of peppermint and its compounds on human body. Although peppermint plant has great beneficial and economical role in human society, researches must be considered its minor side effects and toxicity. The future in vivo human studies are needed to determine the molecular mechanism of PO in human health. Currently PO is most frequently traded essential oil in the entire world and in many developed and developing countries it considered as a valuable target for both food and pharmaceutical studies.
Acknowledgment
We gratefully thank Yoshihiro Kawaoka (editor in chief of Journal of Archives of Clinical Microbiology) for his kind invitation to write the current manuscript.
Conflicts of Interest
Authors certify that no actual or potential conflict of interest in relation to this article exists.
19955
References
- Nabavi SM, Marchese A, Izadi M, Curti V, Daglia M, et al. (2015) Plants belonging to the genus thymus as antibacterial agents: From farm to pharmacy. Food Chem173: 339-347.
- Akbari M, Rasouli H, Bahdor T (2012) Physiological and pharmaceutical effect of fenugreek: A review. IOSRPHR2: 49-53.
- Wang J, Yang D, Wang Z, Chen B, Yao S (2009) Simultaneous of illegal additives in dietary supplements and traditional medicines by high performance liquid chromatography–electrospray ionization mass spectrometry. Food Chem113: 227-232
- Xiao J (2016) Report of the international symposium on phytochemicals in medicine and food. Food Chem204: 497-498
- Jaberian H, Piri K, Nazari J (2013) Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem136: 237-244
- Rezaie-Tavirani M, Fayazfar S, Heydari-Keshel S, Rezaee MB, Zamanian-Azodi M, et al. (2001) Effect of essential oil of rosa damascena on human colon cancer cell line sw 742. Gastroenterol Hepatol Bed Bench 6.
- Hassan BAR (2012) Medicinal plants(importance and uses). Pharm Anal Acta3: 1000-1139.
- Wong CC, Li HB, Cheng KW, Chen F (2006) A systematic survey of antioxidant activity of 30 chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem97: 705-711.
- Rasouli H, Farzaei MH, Mansouri K, Mohammadzadeh S, Khodarahmi (2016) R Plant cell cancer: May natural phenolic compounds prevent onset and development of plant cell malignancy? A literature review. Molecules21: 1104.
- Farzaei MH, Bahramsoltani R, Rahimi R, Abbasabadi F, Abdollahi (2016) M A systematic review of plant-derived natural compounds for anxiety disorders. Curr. Top. Med. Chem16: 1924-1942
- Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some nigerian medicinal plants. Afr J Biotechnol4: 685-688.
- da Silva DX, de Souza MW, Corrêa CdS, Moya HD (2013) A critical study of use of the fe(ii)/3-hydroxy-4-nitroso-2,7-naphthalenedisulfonic acid complexes in the quantification of polyphenols in medicinal plants. Food Chem138: 1325-1332.
- Li Y, Zhou YC, Yang MH, Ou Yang Z (2012) Natural occurrence of citrinin in widely consumed traditional chinese food red yeast rice, medicinal plants and their related products. Food Chem132: 1040-1045
- Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z (1985) Medicinal plants in therapy. Bull World Health Organ63: 965.
- Zamaninan Azodi M, Ardeshirylajimi A, Ahmadi N, Rezaee MB, Jalilian FA, et al. (2013) Antibacterial effects of scrophularia striata seed aqueous extract on staphilococcus aureus. J Pharm Sci4: 1-11.
- Ahmad R, Ahmad N, Naqvi A.A, Shehzad A, Al-Ghamdi (2016) M.S. Role of traditional islamic and arabic plants in cancer therapy.J Tradit Complement Med 1-10.
- Hong Kong S (2013) Traditional medicine strategy. World Health Organization: China 20: 113.
- McKay DL, Blumberg JB (2006) A review of the bioactivity and potential health benefits of peppermint tea (mentha piperita l.). Phytother Res20: 619-633.
- Khalil AF, Elkatry HO, El Mehairy, HF (2015) Protective effect of peppermint and parsley leaves oils against hepatotoxicity on experimental rats. Ann. Agric. Sci60: 353-359.
- Spirling LI, Daniels IR (2001) Botanical perspectives on health peppermint: More than just an after-dinner mint. J R Soc Promot Health121: 62-63
- Iscan G, KIrimer N, Kürkcüoglu Mn, Baser HC, DEMIrci F (2002) Antimicrobial screening of mentha piperita essential oils. J Agri Food Chem50: 3943-3946.
- Dorman HD, Kosar M, Kahlos K, Holm Y, Hiltunen R (2003) Antioxidant properties and composition of aqueous extracts from mentha species, hybrids, varieties, and cultivars. J Agri Food Chem51: 4563-4569.
- Keifer D, Ulbricht C, Abrams TR, Basch E, Giese N, et al. (2008) Peppermint (mentha xpiperita) an evidence-based systematic review by the natural standard research collaboration. J Herb Med7: 91-143.
- Saeidnia S, Gohari AR, Yassa N, Shafiee A (2005) Composition of the volatile oil of achillea conferta dc. From iran. DARU13: 34-36.
- Cosentino M, Bombelli R, Conti A, Colombo ML, Azzetti A, et al. (2009) Antioxidant properties and in vitro immunomodulatory effects of peppermint (mentha x piperita l.) essential oils in human leukocytes. J Pharm Sci Res1: 33-43.
- Mairapetyan S, Mamikonyan V, Alexanyan J, Tovmasyan A, Daryadar M(2016) Productivity, biochemical indices and antioxidant activity of peppermint (mentha piperita l.) and basil (ocimum basilicum l.) in conditions of hydroponics. J Aquac Res Development7: 2.
- Uribe E, Marín D, Veg Gálvez A, Quispe-Fuentes I, Rodríguez A (2016) Assessment of vacuum-dried peppermint (mentha piperita l.) as a source of natural antioxidants. Food Chem190: 559-565.
- Riachi LG, De Maria (2015) C.A.B. Peppermint antioxidants revisited. Food Chem176: 72-81.
- Ansari M, Vasudevan P, Tandon M, Razda R (2000) Larvicidal and mosquito repellent action of peppermint (mentha piperita) oil. Biosci Rep71: 267-271.
- Chen H, Zhong Q (2015) Thermal and uv stability of β-carotene dissolved in peppermint oil microemulsified by sunflower lecithin and tween 20 blend. Food Chem174: 630-636.
- Hawthorn M, Ferrante J, Luchowski E, Rutledge A, Wei X, et al. (1988) The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment Pharmacol Ther2: 101-118
- Peat J, Frazee C, Kearns G, Garg U (2016) Determination of menthol in plasma and urine by gas chromatography/mass spectrometry (gc/ms) 205-211.
- Arab Ameri S, Samadi F, Dastar B, Zerehdaran S (2016) Effect of peppermint (mentha piperita) powder on immune response of broiler chickens in heat stress. Iranian J App Anim Sci6: 435-445.
- Oktemer T, Ipçi K, Muluk NB, Cingi C (2015) A pastille combining myrrh tincture, peppermint oil and menthol to treat the upper airway. ENT Updates5: 128.
- Babaeian M, Naseri M, Kamalinejad M, Ghaffari F, Emadi F, et al. (2016) Park JW The efficacy of mentha longifolia in the treatment of patients with postprandial distress syndrome: A double-blind randomized clinical trial. Iran. Red. Crescent 19.
- Choi O, Cho SK, Kim J, Park CG, Kim J (2016) Antibacterial properties and major bioactive components of mentha piperita essential oils against bacterial fruit blotch of watermelon. Arch Phytopath Plant Protect49: 325-334.
- Lv J, Huang H, Yu L, Whent M, Niu Y, et al. (2012) Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem132: 1442-1450
- Alankar S (2009) A review on peppermint oil. Asian J Pharm ClinRes2: 27-33.
- Nair PS, Ramanathan HN (2012) The future of indian mint-a study to forecast the mint exports from india. JSCM1: 10.
- Stachowicz J, Krajewska Kułak E, Łukaszuk C, Niewiadomy A (2014) Relationship between antifungal activity against candida albicans and electron parameters of selected n-heterocyclic thioamides. Indian J Pharm Sci76: 287.
- Aihara Ji (1999) Weighted homo-lumo energy separation as an index of kinetic stability for fullerenes. Theor. Chem. Acc102: 134-138
- Rakhi R, Suresh CH (2016) A dft study on dihydropyrazine annulated linear polyacenes: Aromaticity, stability and homo–lumo energy modulation. Phys. Chem. Chem. Phys18: 24631-24641
- Janjua MRSA, Yamani ZH, Jamil S, Mahmood A, Ahmad I, et al. (2016) First principle study of electronic and non-linear optical (nlo) properties of triphenylamine dyes: Interactive design computation of new nlo compounds. Aust J Chem69: 467-472.
- Buyukuslu H, Akdogan M, Yildirim G, Parlak C (2010) Ab initio hartree-fock and density functional theory study on characterization of 3-(5-methylthiazol-2-yldiazenyl)-2-phenyl-1h-indole. Spectrochim Acta Mol Biomol Spectrosc75: 1362-1369.
- Fukui K (1982) Role of frontier orbitals in chemical reactions. Science218: 747-754
- Yamaguchi K, Homma T, Nomi Y, Otsuka Y (2014) Characterisation of maillard reaction products derived from lekfd – a pentapeptide found in β-lactoglobulin sequence, glycated with glucose – by tandem mass spectrometry, molecular orbital calculations and gel filtration chromatography coupled with continuous photodiode array. Food Chem145: 892-902
- Shao Y, Molnar LF, Jung Y, Kussmann J, Ochsenfeld C, et al. (2006) Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys8: 3172-3191
- Anjali J, Nardev SA (2016) Review on natural additives used in cosmetic preparations. World J Pharm Sci5: 630-648.
- Rita P, Animesh DK (2011) An updated overview on peppermint (mentha piperita l.). Int Res J Pharm2: 1-10.
- Katzer G (2016) Peppermint (mentha piperita l). Journal of Pharmacology 121.
- Hawrył M, Niemiec M, Słomka K, Waksmundzka-Hajnos M, Szymczak G (2016) Micro-2d-tlc separation of phenolics in some species of mint and their fingerprints on diol bonded polar stationary phase. Acta Chromat28: 119-127.
- Hocking GM, Edwards LD (1955) Cultivation of peppermint in florida. Economic Botany9: 78-93.
- Kavrayan D, Aydemir T (2001) Partial purification and characterization of polyphenoloxidase from peppermint (mentha piperita). Food Chem74: 147-154.
- Fitsiou E, Mitropoulou G, Spyridopoulou K, Tiptiri-Kourpeti A, Vamvakias M, et al.(2016) Phytochemical profile and evaluation of the biological activities of essential oils derived from the greek aromatic plant species ocimum basilicum, mentha spicata, pimpinella anisum and fortunella margarita. Molecules21: 1069.
- Wang J, Li R, Tan J, Jiang ZT ( 2016) Chemical composition of essential oil of grapefruit mint (mentha suaveolens×piperita) from china. J Essent Oil Bear Plant19: 1047-1050.
- Gaurav N (2016) An experimental text book on phytochemical analysis and antimicrobial activity of mentha piperita. Onlinegatha:
- Nogueira MVC, de Lima Castro SAB, de Amorim AM, Maia RM, Paulillo LCMS ( 2016) Ethnobotanical survey of plants from the caatinga with possible therapeutic uses. Int. J. Curr. Microbiol. App. Sci5: 767-772
- Çoban Ö, Baydar NG (2016) Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (mentha piperita l.) under salt stress. Ind Crops Prod86: 251-258.
- Mallick B, Sinha S, Roy D (2016) Evaluation of antioxidative potential of field grown and tissue culture derived mentha piperita l. Plants. Int J Curr Microbiol App Sci 5: 382-391.
- Gras A, Garnatje T, Bonet MÀ, Carrió E, Mayans M, et al.( 2016) Beyond food and medicine, but necessary for life, too: Other folk plant uses in several territories of catalonia and the balearic islands. J Ethnobiol Ethnomed 12: 23.
- Bokhari N, Perveen K, Al Khulaifi M, Kumar A, Siddiqui I ( 2016) In vitro antibacterial activity and chemical composition of essential oil of mentha arvensis linn. Leaves. J Essent Oil Bear Pl19: 907-915.
- Mahmoudi R, Katiraee F, Tajik H, Abbas A (2016) Inhibitory effect of mentha longifolia l. Essential oil against listeria monocytogenes using transmission electron microscopy. Int J Vet Sci Res 17: 14-23.
- Diop SM, Guèye MT, Ndiaye I, Hadji E, Fauconnier GL( 2016) Chemical composition of essential oils and floral waters of mentha longifolia (l.) huds. From senegal. Am J Essent Oils Nat Prod 4: 46-49.
- Saji N (2016) 1-vinylcyclohex-3-ene carbaldehyde and 4-vinylcyclohex-1-ene carbaldehyde for use in flavour and oral care composition. Google Patents.
- Bharti N, Barnawal D, Shukla S, Tewari SK, Katiyar R, et al.( 2016) Integrated application of exiguobacterium oxidotolerans, glomus fasciculatum, and vermicompost improves growth, yield and quality of mentha arvensis in salt-stressed soils. Ind Crops Prod83: 717-728.
- Yingying L, Haitao L, Xiaohua L, Lixia Z ( 2016) Diversity of tropical plant resources in yunnan province and its conservation. J Landsc Res 8: 90-92.
- Nair B (2000) Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. int J Toxicol20: 61-73.
- Jenner P, Hagan E, Taylor JM, Cook E, Fitzhugh O (1964). Food flavourings and compounds of related structure i.e. Acute oral toxicity. Food Cosmet Toxicol2: 327-343.
- Wraith JM, Robinson DA, Jones SB, Long DS ( 2005) Spatially characterizing apparent electrical conductivity and water content of surface soils with time domain reflectometry. Comput Electron Agric46: 239-261.
- Douhan, LI, Johnson D (2001) Vegetative compatibility and pathogenicity of verticillium dahliae from spearmint and peppermint. Plant Dis85: 297-302.
- Maffei M (1999) Sustainable methods for a sustainable production of peppermint (mentha x piperita l.) essential oil. J Essent Oil Res11: 267-282
- Clark R, Menary R(1981) Variations in composition of peppermint oil in relation to production areas. Economic Botany 35: 59-69.
- Rohloff J (1999) Monoterpene composition of essential oil from peppermint (mentha×piperita l.) with regard to leaf position using solid-phase microextraction and gas chromatography/mass spectrometry analysis. J Agri Food Chem47: 3782-3786.
- Areias F, Valentao P, Andrade P, Ferreres F, Seabra R (2001) Phenolic fingerprint of peppermint leaves. Food chem73: 307-311.
- Umezu T, Sakata A, Ito H (2001) Ambulation-promoting effect of peppermint oil and identification of its active constituents. Pharmacol. Biochem. Behav69: 383-390
- Clark R, Menary R(1980) Environmental effects on peppermint (mentha piperita l.). I. Effect of daylength, photon flux density, night temperature and day temperature on the yield and composition of peppermint oil. Funct Plant Biol7: 685-692
- Croteau R, Venkatachalam K ( 1986) Metabolism of monoterpenes: Demonstration that (+)-cis-isopulegone, not piperitenone, is the key intermediate in the conversion of (−)-isopiperitenone to (+)-pulegone in peppermint (mentha piperita). Annu Rev Phys Chem249: 306-315.
- Mascher H, Kikuta C, Schiel H (2001) Pharmacokinetics of menthol and carvone after administration of an enteric coated formulation containing peppermint oil and caraway oil. Arzneimittelforschung51: 465-469
- Park YJ, Baskar TB, Yeo SK, Arasu MV, Al-Dhabi NA, et al. ( 2016) Composition of volatile compounds and in vitro antimicrobial activity of nine mentha spp. Springer plus5: 1628.
- Gershenzon J, McConkey M.E, Croteau RB (2000) Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol 122: 205-214.
- Mahmoud SS, Williams M, Croteau R ( 2004) Cosuppression of limonene-3-hydroxylase in peppermint promotes accumulation of limonene in the essential oil. Phytochem65: 547-554
- McGarvey DJ, Croteau R (1995) Terpenoid metabolism. The Plant Cell7: 1015
- Mahmoud SS, Croteau RB (2002) Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci7: 366-373
- Rios-Estepa R, Turner GW, Lee JM, Croteau RB, Lange BM (2008) A systems biology approach identifies the biochemical mechanisms regulating monoterpenoid essential oil composition in peppermint. Proc Natl Acad Sci USA105: 2818-2823.
- Burbott AJ, Loomis WD (1969) Evidence for metabolic turnover of monoterpenes in peppermint. Plant Physiol44: 173-179.
- Tan XC, Chua KH, Ravishankar Ram M, Kuppusamy UR (2016) Monoterpenes: Novel insights into their biological effects and roles on glucose uptake and lipid metabolism in 3t3-l1 adipocytes. Food Chem 196: 242-250
- Turner GW, Gershenzon J, Croteau RB (2000) Development of peltate glandular trichomes of peppermint. Plant Physiol124: 665-680.
- McConkey ME, Gershenzon J, Croteau RB (2000) Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol122: 215-224.
- Mahmoud SS, Croteau, RB (2003) Menthofuran regulates essential oil biosynthesis in peppermint by controlling a downstream monoterpene reductase. Proc Natl Acad Sci USA100: 14481-14486.
- Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R (1999) Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol120: 879-886.
- Lange BM, Mahmoud SS, Wildung MR, Turner GW, Davis EM, et al. (2011)Improving peppermint essential oil yield and composition by metabolic engineering. Proc. Natl. Acad. Sci. USA 108: 16944-16949.
- Figueroa Pérez MG, Rocha-Guzmán NE, Mercado-Silva E, Loarca-Piña G, Reynoso-Camacho R(2014) Effect of chemical elicitors on peppermint (mentha piperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem156: 273-278.
- Morton C, Garioch J, Todd P, Lamey P, Forsyth A (1995) Contact sensitivity to menthol and peppermint in patients with intra‐oral symptoms. Contact Dermatitis 32: 281-284.
- Chaitanya K (2012) Molecular structure, vibrational spectroscopic (ft-ir, ft-raman), uv–vis spectra, first order hyperpolarizability, nbo analysis, homo and lumo analysis, thermodynamic properties of benzophenone 2, 4-dicarboxylic acid by ab initio hf and density functional method. Spectrochim. Acta Mol Biomol Spectrosc86: 159-173.
- Endo A, Sato K, Yoshimura K, Kai T, Kawada A, et al. ( 2011) Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl Phys Lett 98: 083302
- Mills NS, Levy A, Plummer BF (2004) Antiaromaticity in fluorenylidene dications. Experimental and theoretical evidence for the relationship between the homo/lumo gap and antiaromaticity. J Org Chem69: 6623-6633.
- Cunha JD, Lavaggi ML, Abasolo MI, Cerecetto H, González M (2011) 2d‐and 3d‐quantitative structure‐activity relationship studies for a series of phenazine n, n’‐dioxide as antitumour agents. Chem Biol Drug Des78: 960-968.
- Vennila P, Govindaraju M, Venkatesh G, Kamal C (2016) Molecular structure, vibrational spectral assignments (ft-ir and ft-raman), nmr, nbo, homo-lumo and nlo properties of o-methoxybenzaldehyde based on dft calculations. J Mol Struct1111: 151-156.
- Prabavathi N, Nilufer A, Krishnakumar V (2012) Molecular structure, vibrational, uv, nmr, hyperpolarizability, nbo and homo–lumo analysis of pteridine2, 4-dione. Spectrochim. Acta Mol Biomol Spectrosc99: 292-302.
- Zhou Z, Parr RG (1990) Activation hardness: New index for describing the orientation of
- Ruiz-Morales Y (2002) Homo-lumo gap as an index of molecular size and structure for polycyclic aromatic hydrocarbons (pahs) and asphaltenes: A theoretical study. I. J. Phys. Chem. A 106: 11283-11308.
- Im H, Kim S, Park C, Jang SH, Kim CJ et al. (2010) High performance organic photosensitizers for dye-sensitized solar cells. Chem Commun 46: 1335-1337.
- Nakagawa T, Ku SY, Wong KT, Adachi C (2012) Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor–acceptor structure. Chem Commun 48: 9580-9582.
- Goswami S, Aich K, Das AK, Sarkar D, Panja SA (2013) red fluorescence ‘off–on’molecular switch for selective detection of al 3+, fe 3+ and cr 3+: Experimental and theoretical studies along with living cell imaging. Chem. Commun 49: 10739-10741.
- Nolen IH, Friend DR (1994) Menthol-β-d-glucuronide: A potential prodrug for treatment of the irritable bowel syndrome. Pharm Res 11: 1707-1711.
- Froehlich O, Shibamoto T (1990) Stability of pulegone and thujone in ethanolic solution. J Agri Food Chem 38: 2057-2060.
- Wandtke CM, Lübben J, Dittrich B (2016) Molecular electrostatic potentials from invariom point charges. Chem. Phys. Chem 17: 1-10.
- Kumar A, Gadre SR (2016) Exploring the gradient paths and zero flux surfaces of molecular electrostatic potential. J. Chem. Theory Comput 12: 1705-1713
- Politzer P, Laurence PR, Jayasuriya K (1985) Molecular electrostatic potentials: An effective tool for the elucidation of biochemical phenomena. Environ Health Perspect61: 191.
- Arabi AA, Matta CF (2016) Electrostatic potentials and average electron densities of bioisosteres in methylsquarate and acetic acid. Future Med. Chem 8: 361-371.
- Tao Y, Han L, Li X, Han Y, Liu Z (2016) Molecular structure, spectroscopy (ft-ir, ft-raman), thermodynamic parameters, molecular electrostatic potential and homo-lumo analysis of 2, 6-dichlorobenzamide. J Mol Struct 1108: 307-314.
- Balachandran V, Santhi G, Karpagam V, Revathi B, Karabacak M (2015) Spectroscopic investigation, natural bond orbital analysis, homo–lumo and thermodynamic functions of 2-tert-butyl-5-methyl anisole using dft (b3lyp) calculations. Spectrochim. Acta Mol. Biomol. Spectrosc136: 451-463.
- Politzer P, Truhlar DG (2013) Chemical applications of atomic and molecular electrostatic potentials: Reactivity, structure, scattering, and energetics of organic, inorganic, and biological systems. Springer Science & Business Media.
- Naray-Szabo G, Ferenczy GG (1995) Molecular electrostatics. Chem Rev 95: 829-847.
- Becke A, Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules: From solid state to DNA and drug design. John Wiley & Sons.
- Honig B, Nicholls A (1995) Classical electrostatics in biology and chemistry. Science 268: 1144.
- Nawawi AA, Nakamura N, Hattori M, Kurokawa M, Shiraki K (1999) Inhibitory effects of indonesian medicinal plants on the infection of herpes simplex virus type 1. Phytother Res 13: 37-41.
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D et al. (2008) Antiviral effects of glycyrrhiza species. Phytother Res 22: 141-148.
- Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME et al. (1986) Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med 315: 77-81.
- Vijayan P, Raghu C, Ashok G, Dhanaraj S, Suresh B (2004) Antiviral activity of medicinal plants of nilgiris. Indian J Med Res 120: 24.
- Reichling J, Schnitzler P, Suschke U, Saller R (2009) Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties–an overview. Forsch 16: 79-90.
- Schuhmacher A, Reichling J, Schnitzler P (2003) Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 10: 504-510.
- Brand YM, RoaLinares VC, BetancurGalvis LA, DuránGarcía DC, Stashenko E (2016) Antiviral activity of colombian labiatae and verbenaceae family essential oils and monoterpenes on human herpes viruses. J Essent Oil Res 28: 130-137.
- Shalayel M, Asaad A, Qureshi M, Elhussein A (2016) Anti-bacterial activity of peppermint (mentha piperita) extracts against some emerging multi-drug resistant human bacterial pathogens. J Herb Med
- Bekhit AE, Cheng VJ, McConnell M, Zhao JH, Sedcole R et al. (2011) Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chem Fleischner AM (2001) Dietary supplements for each specific blood type. Google Patents
- Moradi MT, Rafieian-Kopaei M, Karimi A (2016) A review study on the effect of iranian herbal medicines against in vitro replication of herpes simplex virus. Avicenna J Phytomed 20: 1-11.
- Santoyo S, Jaime L, García-Risco MR, Rodríguez A, Reglero G (2014) Antiviral properties of supercritical co2 extracts from oregano and sage. Int J Food Prop 17: 1150-1161.
- Geuenich S, Goffinet C, Venzke S, Nolkemper S, Baumann I (2008) Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-hiv-1 activity by increasing the virion density. Retrovirology 5: 1-16.
- Shaikh S, Yaacob HB, Rahim ZHA (2014) Prospective role in treatment of major illnesses and potential benefits as a safe insecticide and natural food preservative of mint (mentha spp.): A review. Asian J Biomed Pharm 4: 1.
- Arora R, Chawla R, Marwah R, Arora P, Sharma R (2010) Potential of complementary and alternative medicine in preventive management of novel h1n1 flu (swine flu) pandemic: Thwarting potential disasters in the bud. J Evid Based Complementary Altern Med 10: 1-16.
- Singh R, Shushni MM, Belkheir A (2015) Antibacterial and antioxidant activities of mentha piperita l. Arabian J. Geosci 8: 322-328.
- Reichling J, Nolkemper S, Stintzing FC, Schnitzler P (2008) Impact of ethanolic lamiaceae extracts on herpesvirus infectivity in cell culture. Forschende Komplementärmedizin/Research in Complementary Medicine 15: 313-320.
- Kunnumakkara AB, Chung JG, Koca C, Dey S (2009) Mint and its constituents. In Molecular targets and therapeutic uses of spices: Modern uses for ancient medicine 9: 373-401.
- Edris AE (2007) Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother Res 21: 308-323.
- Shaaban HA, Ghorab AH, Shibamoto T (2012) Bioactivity of essential oils and their volatile aroma components: Review. J ESSENT OIL RES 24: 203-212.
- Mahboubi M, Kazempour N (2014) Chemical composition and antimicrobial activity of peppermint (mentha piperita l) essential oil SJST 20: 36.
- Almajano MP, Carbó R, Jiménez JAL, Gordon MH (2008) Antioxidant and antimicrobial activities of tea infusions. Food Chem 108: 55-63.
- Mucciarelli M, Camusso W, Maffei M, Panicco P, Bicchi C (2007) Volatile terpenoids of endophyte-free and infected peppermint (mentha piperita l): Chemical partitioning of a symbiosis. Microb Ecol 54: 685-696.
- Bohnert T, Patel A, Templeton I, Chen Y, Lu C (2016) Evaluation of a new molecular entity as a victim of metabolic drug-drug interactions-an industry perspective. Drug Metab Dispos dmd 115: 690-696.
- Rodrigues F, Dupret JM (2002) 3d model of human arylamine n-acetyltransferase 2: Structural basis of the slow acetylator phenotype of the r64q variant and analysis of the active-site loop. Biochem. Biophys. Res. Commun 291: 116-123.
- Sun Z, Wang H, Wang J, Zhou L, Yang P (2014) Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of mentha piperita grown in china. PloS one 9: e114-767.
- Saeed S, Naim A, Tariq P (2006) In vitro antibacterial activity of peppermint. Pak J Bot 38: 869.
- Oumzil H, Ghoulami S, Rhajaoui M, Ilidrissi A, Faid M et al.(2002) Antibacterial and antifungal activity of essential oils of mentha suaveolens. Phytother Res 16: 727-731.
- Bansod S, Rai M (2008) Antifungal activity of essential oils from indian medicinal plants against human pathogenic aspergillus fumigatus and a. Niger. World J Med Res 3: 81-88.
- Goga M, Antreich SJ, Bačkor M, Weckwerth W, Lang I (2016) Lichen secondary metabolites affect growth of physcomitrella patens by allelopathy. Protoplasma 20: 1-9.
- Shah AN, Iqbal J, Ullah A, Yang G, Yousaf M (2016) Allelopathic potential of oil seed crops in production of crops: A review. Environ Sci Pollut Res 20: 1-14.
- Mahdavikia F, Saharkhiz MJ (2016) Secondary metabolites of peppermint change the morphophysiological and biochemical characteristics of tomato. Biocatal Agric Biotechnol 7: 127-133.
- Ashurst PR (1991) Food flavorings. Springer Science & Business Media 19: 151.
- SKRZYPEK E, REPKA P, STACHURSKA SA, BARABASZ KB, MOŻDŻEŃ K (2015) Allelopathic effect of aqueous extracts from the leaves of peppermint (mentha×piperita)on selected physiological processes of common sunflower (helianthus annuus l). Not Bot Horti Agrobot Cluj Napoca 20: 43-48.
- Eccles R (1994) Menthol and related cooling compounds. J Pharm Pharmacol 46: 618-630.
- Eccles R (2003) Menthol: Effects on nasal sensation of airflow and the drive to breathe. Curr Allergy Asthma Rep 3: 210-214.
- Haghighi AB, Motazedian S, Rezaii R, Mohammadi F, Salarian L, et al. (2010) Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double‐blind, placebo‐controlled, crossed‐over study. Int J Clin Pract 64: 451-456.
- Patel T, Ishiuji Y, Yosipovitch G (2007) Menthol: A refreshing look at this ancient compound. J Am Acad Dermatol 57: 873-878.
- Khanna R, MacDonald JK, Levesque BG (2014) Peppermint oil for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. J Clin Gastroenterol 48: 505-512.
- Watson EC, Koenig MN, Grant ZL, Whitehead L, Trounson E, (2016) Apoptosis regulates endothelial cell number and capillary vessel diameter but not vessel regression during retinal angiogenesis. Development 143: 2973-2982.
- Rasouli H, Mansouri K, Mahamed-Khosroushahi L (2014) Anti-angiogenic/inflammatory behavior of mushroom ganoderma lucidum extract could be effective for treatment of corneal neovascularization: A hypothesis. J Rep Pharm Sci 3: 14-18.
- Rasouli H, Parvaneh S, Mahnam A, Rastegari-Pouyani M, Hoseinkhani Z, et al. (2017) Anti-angiogenic potential of trypsin inhibitor purified from cucumis melo seeds: Homology modeling and molecular docking perspective. Int J Biol Macromolec 96: 118-128.
- Ku CM, Lin JY (2013) Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating th1/th2 cytokine secretion profiles using murine primary splenocytes. Food Chem 141: 1104-1113.
- Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB (2010) Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2: 2428-2466.
- Kaefer CM, Milner JA (2008) The role of herbs and spices in cancer prevention. J Nutr Biochem 19: 347-361.
- Kale A, Gawande S, Kotwal S (2008) Cancer phytotherapeutics: Role for flavonoids at the cellular level. Phytother Res 22: 567-577.
- Liu X, Sun Z-L, Jia A-R, Shi Y-P, Li R-H, et al. (2014) Extraction, preliminary characterization and evaluation of in vitro antitumor and antioxidant activities of polysaccharides from mentha piperita. Int J Mol Sci 15: 16302-16319.
- Lin J-P, Li Y-C, Lin W-C, Hsieh C-L, Chung J-G (2001) Effects of menthol on arylamine n-acetyltransferase activity in human liver tumor cells. Am J Chin Med 29: 321-329.
- Deguchi T, Mashimo M, Suzuki T (1990) Correlation between acetylator phenotypes and genotypes of polymorphic arylamine n-acetyltransferase in human liver. J Biol Chem 265: 12757-12760.
- Payton M, Mushtaq A, Yu TW, Wu LJ, Sinclair J, et al. (2001) Eubacterial arylamine n-acetyltransferases–identification and comparison of 18 members of the protein family with conserved active site cysteine, histidine and aspartate residues. Microbiology 147: 1137-1147.
- Upton A, Johnson N, Sandy J, Sim E (2001) Arylamine n-acetyltransferases–of mice, men and microorganisms. Trends Pharmacol Sci 22: 140-146.
- Nissen L, Lau E (2016) Old drug new indication: Antihistamine for the pain in your stomach? Australian Pharmacist 35: 32-35.
- Sadraei H, Asghari G, Alipour M (2016) Anti-spasmodic assessment of hydroalcoholic extract and essential oil of aerial part of pycnocycla caespitosa boiss. & hausskn on rat ileum contractions. Res Pharm Sci 11: 33-37.
- Harris LA (2016) Treating irritable bowel syndrome: A fresh and minty approach to an old therapy. Dig Dis Sci 61: 334-336.
- Jain PK, Das D, Jain P, Jain P (2016) Pharmacognostic and pharmacological aspect of bacopa monnieri-a review. Innovare J Ayurvedic Sci 1: 7-11.
- Chey WD, Kurlander, J, Eswaran S (2015) Irritable bowel syndrome: A clinical review. Jama 313: 949-958.
- Kline RM, Kline JJ, Di Palma J, Barbero GJ (2001) Enteric-coated, ph-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr 138: 125-128.
- Rees W, Evans B, Rhodes J (1979) Treating irritable bowel syndrome with peppermint oil. BMJ 2: 835-836.
- Saha L (2014) Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol 20: 6759-6773.
- Korterink JJ, Rutten JM, Venmans L, Benninga MA, Tabbers MM (2015) Pharmacologic treatment in pediatric functional abdominal pain disorders: A systematic review. J Pediatr 166: 424-431.
- Egan M, Connors EM, Anwar Z, Walsh JJ (2015) Nature’s treatment for irritable bowel syndrome: Studies on the isolation of (−)-menthol from peppermint oil and its conversion to (−)-menthyl acetate J Chem Educ 92: 1736-1740.
- Sagduyu K (2002) Peppermint oil for irritable bowel syndrome. Psychosomatics 43: 508-509.
- Tate S (1997) Peppermint oil: A treatment for postoperative nausea. J Adv Nurs 26: 543-549.
- Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L (2007) Peppermint oil (mintoil®) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Dig Liver Dis 39: 530-536.
- Akehurst R, Kaltenthaler E (2001) Treatment of irritable bowel syndrome: A review of randomised controlled trials. Gut 48: 272-282.
- Camilleri M, CHOI MG (1997) Review article: Irritable bowel syndrome. Aliment Pharmacol Ther 11: 3-15.
- Madisch A, Holtmann G, Plein K, Hotz J (2004) Treatment of irritable bowel syndrome with herbal preparations: Results of a doubleblind, randomized, placebo‐controlled, multicentre trial. Aliment Pharmacol Ther 19: 271-279.
- Gobel H, Schmidt G, Soyka D (1994) Effect of peppermint and eucalyptus oil preparations on neurophysiological and experimental algesimetric headache parameters. Cephalalgia 14: 228-234.
- Maliakal PP, Wanwimolruk S (2001) Effect of herbal teas on hepatic drug metabolizing enzymes in rats. J Pharm Pharmacol 53: 1323-1329.
- Wolf KK, Gufford BT, Brantley SJ, Watkins PB, Paine MF (2016) Drug metabolism, transport. pharmacogenomics 22: 626-638.
- Jancova P, Anzenbacher P, Anzenbacherova E (2010) Phase ii drug metabolizing enzymes. Biomed Papers 154: 103-116.
- Khodadust MR, Asadi GH (2015) Effects of peppermint (mentha piperita l.) alcoholic extract on carbon tetrachloride-induced hepatotoxicity in broiler chickens under heat stress condition. Poultry Sci J 3: 1-16.
- Kaushik P, Mathur M, Rawat N, Dutt P (2012) Radioprotective effect of alcoholic extract of mentha piperita (linn) on swiss albino mice exposed to whole body gamma irradiation: A preliminary study. Int J Pharm Bio Sci 3: 598-610.
- Samarth RM, Panwar M, Kumar M, Kumar A (2006) Retracted: Radioprotective influence of mentha piperita (linn) against gamma irradiation in mice: Antioxidant and radical scavenging activity. Int J Rad Biol 82: 331-337.
- Kumar A, Samarth R, Yasmeen S, Sharma A, Sugahara T (2004) Anticancer and radioprotective potentials of mentha piperita. Biofactors 22: 87-91.
- Gardiner P (2000) Peppermint (mentha piperita). Longwood Herbal Task Force 1-22.
- Nash P, Gould S, Bernardo D (1986) Peppermint oil does not relieve the pain of irritable bowel syndrome. Br J Clin Pract 40: 292-293.
- Millqvist E, Ternesten-Hasséus E, Bende M (2013) Inhalation of menthol reduces capsaicin cough sensitivity and influences inspiratory flows in chronic cough. Respir Med 107: 433-438.
- Shah PP, Mello P (2004) A review of medicinal uses and pharmacological effects of mentha piperita. Nat Prod Rad 3: 214-221.
- Mitchell AR, Crowe FJ (1996) Peppermint oil yield and composition from mini and industrial distilleries. J Herbs Spices Med Plants 4: 81-88.













