Key words
Migraine; vestibular function; vertigo; electronystagmography; caloric testing.
Abbreviations
MA, migraine with aura; MoA, migraine without aura; ENG, electronystagmography; PTA, pure-tone audiometry; UW, unilateral caloric weakness; DP, directional preponderance; ABR, auditory brainstem response; IPL, interpeak latency
Introduction
Migraine is a common chronic presenting complaint with an estimated prevalence of 17.2% in women and 6% in men (1). Heritability in migraine was estimated to be between 40- 60% (2). Migraine is a complex disease that includes neurologic, gastrointestinal and autonomic symptoms, although headache is most common feature. Moderate to severe recurrent attacks of migraine headache usually last for 4–72 hours. The common migraine or migraine without aura (MoA) is often unilateral, pulsating (throbbing), may be associated with nausea and vomiting. In the classic migraine or migraine with aura (MA), headache is preceded by transient neurological symptoms as photophobia and phonophobia (3).
In some patients, migraine headache is often associated with vestibular manifestations during the attacks or in the headache-free periods, but the highest incidence occurring during the headache attacks (3-7). Vestibular symptoms include: dizziness, light headedness, giddy sensation, passing out episodes, imbalance, head motion intolerance, spontaneous attacks of vertigo or rotational-type vertigo, positional vertigo and nystagmus (4-20). Motion sensitivity with bouts of motion sickness occurs in about two thirds of patients with migraine (8). Episodes of vertigo occur in about one fourth of patients (11, 12, 14, 16, 18, 19). Vertigo and dizziness are frequent co-existing symptoms in patients with migraine and was reported in nearly 10% of all dizzy patients (20). In some, vertigo is the only symptom (so-called “migraine equivalent”) (21). The terms migraine-associated vertigo, benign recurrent vertigo, migraine-associated dizziness, migraine-related vestibulopathy and vestibular migraine, all have been applied to the commonly syndrome called migrainous vertigo (18) which affects more than 1 % of the general population and nearly represents 10 % of patients in dizziness and at least 9 % of patients in migraine clinics. Although migrainous vertigo is not included in the current classification of International Headache Society (22), yet its specific criteria have been proposed and utilized in clinical trials. Migrainous vertigo has female preponderance and is characterized by attacks of spontaneous or positional vertigo lasting seconds to days and typically accompanied by imbalance and nausea. Nearly half of the cases occur without an association with headache, phonophobia, photophobia or auras are common but not mandatory and most of cases are completely free of dizziness between attacks (17,23). A central or peripheral vestibular damage may occur in patients with migrainous vertigo (24) particularly during the attacks as central spontaneous or positional nystagmus and, less commonly, unilateral vestibular hypofunction (25,26). Auditory manifestations may also be associated with migraine during and in between the attacks but are less frequent than vestibular manifestations and mostly mild and non-progressive. Audiologic symptoms include: pitch distortion, phonophobia (5), tinnitus (6) and increased sensitivity to light and sound (25,26) which increased during headache attacks. Also fluctuating hearing loss, sudden deafness (26) and permanent hearing loss may occur in small percentage of patients (4,27). Some studies reported abnormalities in audiometric, auditory brainstem response (ABR) and caloric testing but in general, their results are few and controversial (6,25,28,29,30).
The pathophysiology of vestibular and manifestations associated with migraine is still incompletely delineated, however, the following have been hypothesized as possible mechanisms: 1) subcortical dysfunction with disruption of central sensory processing mechanisms and hypersensitivity of the labyrinth (25,30,31,32), 2) spontaneous and aberrant neural activity at any level along the auditory axis, with abnormal reorganization processes in the auditory cortex, central sensitization, cortical hyperexcitability (33,34), malfunctioning of the vascular activity of the cochlea (35), and 3) migraineinduced vasospasm and ischemia of the labyrinth, cochlea and cerebral vasculature (4,5,27,32,36,37). The flood of information from different studies is important to determine the exact pathologic spectrum of migraine. This also will lead to improved diagnosis and better prophylactic and treatment for migraine and its long-term consequences in the future.
Aim of work
The aims of the study were: 1) to evaluate the function of the vestibular system using electronystagmography (ENG) in patients with migraine in between the attacks of headache, and 2) to verify the possibility of peripheral or/and central lesion(s) in the vestibular pathways.
Materials and methods
This study included 58 adults (male = 7; female = 51) with migraine and 40 healthy subjects matched for age, sex (male = 10; female = 30), socioeconomic status and educational level (as controls for comparison). Headache was classified according to the criteria of International Headache Society (2nd edition) (22). Patients were studied during the attack-free periods with at least 3 days since the last attack. Patients were recruited from the out-patient headache clinic of the department of Neurology and Psychiatry of Assiut University Hospital (tertiary referral center), Assiut, Egypt. The protocol of the study was approved by the ethical committee and subjects included gave informed consents before participation. Excluded were subjects with: previous history of otological or labyrinthine disorders, history of ear discharge, otosclerosis, history of head or ear trauma, presence of systemic or metabolic diseases associated with hearing loss (as renal insufficiency, gout, diabetes mellitus, hypertension, ischemic heart disease, hypercholesterolemia/dyslipidemia, hypothyroidism or active gastrointestinal disease), reported exposure to unsafe noise, use of ototoxic drugs, and family history of hearing loss or clinical evidence of postural hypotension.
All patients and control subjects went through a detailed medical, physical, otolaryngological, and neurological history and examination.
Neurological evaluation
This included monitoring the frequency of migraine attacks per month. Accordingly, patients were divided into two groups: a) high frequency: ≥4/month. b) low-frequency: <4/ month. The neurological testing for spontaneous and positional nystagmus. The integrity of the posterior column was assessed by applying the Romberg’s and tandom Romberg’s testing.
Audiological evaluation
All patients and control subjects underwent: 1) basic audiological evaluation which included: a) initial otoscopic examination, standard pure-tone audiometry (PTA), speech audiometry and tympanometry and, d) acoustic reflex to exclude pathologic conditions of the external or middle ear (38). 2) Speech audiometry was used to identify the hearing level at which subjects understood and repeated at least 90% of a set of 10 monosyllables. Immittance testing was done to determine middle ear function using the Amplaid Model 720 immittance bridge (Amplaid, Milan, Italy). Pure tone audiometric thresholds were measured from 0.25-8 kHz (0.25, 0.5, 1.0, 2.0, 4.0 and 8.0 kHz) and pure average thresholds for the right and left ears were obtained (audiometer Madsen OB822). Air conduction hearing threshold levels were measured for octave frequencies between 250-8000 Hz. Low frequency tympanometry and acoustic reflex thresholds were elicited contralaterally using interacoustic AZ 7 immitancemeter. Hearing thresholds were determined in decibel hearing level (dB HL). The examined ears were defined as normal if no absolute threshold level > 20 dB was measured over the whole frequency range. Threshold shifts in PTA were considered to be significant if they showed at least 10 dB change in more than two consecutive frequencies, or if a threshold greater than 20 dB was observed in any audiometric range.
Vestibular evaluation
This was done by Electronystagmography (ENG) (Micromedical tech, Meta 4, software version 4.5). Before ENG testing, all medications were stopped for at least 2-3 days, food intake was limited prior to examination and arrangement for transportation was done after the examination. Essentially, ENG test consists of 3 parts: a) Oculomotor evaluation: calibration, gaze, fixation, sacchade, tracking (pursuit) and optokinetic nystagmus; b) positioning/positional testing; and c) Caloric stimulation: bithermal caloric testing was done using otocalorimeter (Bithermic caloric stimulator McA Biomedica) as proposed by Dix-Hallpike. Caloric testing was done by irrigating each ear with 250 mL of saline for 40 seconds at 44°C and 30°C. For ENG test, we analyzed various nystagmus types as spontaneous nystagmus , gaze nystagmus, positional and positioning nystagmus with headshaking and head-thrust (Halmagyi) tests, optokinetic, and postcaloric (in Bruning’s proof, using Jongkees’ formula for evaluation of unilateral weakness (UW) or labyrinthine preponderance (LP) and directional preponderance (DP) (39, 40). We also assessed eye-tracking (sacchade and pursuit) with caloric modification. Data were interpreted in terms of UW and DP. UW was considered significant if asymmetry was > 25% and the nerve or end-organ lesion was in the side of the weakness. DP was considered significant with spontaneous nystagmus >20-30%. Positional tests were done within minimum of 20-30 seconds in normal individuals with mental tasking infrared goggles or with the patient’s eyes closed. It was done with head hanging, supine, supine and head right, supine and lateral head lift (right or left). Positional tests were considered abnormal if nystagmus: a) exceeded 60 per second, b) with change direction in a single position, c) persist in at least 3 different positions, and d) intermittent in all positions. The presence of vestibular peripheral lesion was considered if nystagmus: 1) was present with eyes open and enhanced by eye closure; 2) increased when the patient gazes in the direction of the fast phase but decreased or decreased when the gaze is in the direction of slow phase (Alexander’s law); and 3) direction was fixed and with geotropic direction changing in different positions with positional tests. The presence of vestibular central lesion was considered in the presence of: 1) enhancement of nystagmus with ocular fixation and reduced by eye closure. Normally nystagmus occurs only with eccentric gaze in one direction. Unilateral and bilateral gaze-paretic nystagmuses are consistent with central nervous system (CNS) pathology. They are elicited in the direction of the gaze (i.e. right-beating nystagmus with right gaze and left-beating nystagmus with left gaze); 2) positional geotropic or apogeotropic nystagmus is bilateral, direction changing, showing no latency, low frequency, lack of fatigability and habituation and without concomitant vertigo; 3) disorganized pursuit or asymmetrical pursuit gain; 4) under or overshoot of saccades or asymmetrical latency or velocity; 5) bilateral hyperreflexia; 6) rebound nystagmus which is defined as bursts of nystagmus which begins when the eyes are returned to center gaze and lasts for 5 seconds. Rebound nystagmus indicated brainstem or cerebellar lesions; and 7) nystagmus occasionally increased when the patient gaze is in the direction of the fast phase but decreased or disappeared when the gaze was in the direction of slow phase.
Auditory Brainstem Response (ABR)
Recording was done by (Nicolet Spirit) OS/2 version 3. ABR was performed using alternating clicks at 0.1 seconds, time window was 10 millisecond and filter settings were 150 Hz-3 kHz. The stimuli were delivered at 90 dBHL with repetition rate of 11.1-51.1 pulse/second. Each response reflected an average of 1500 stimuli presentations. The absolute latencies of waves I, III and V and I-III, III-V and I-V interpeak latencies (IPLs) were recorded from both ears. Radiologic examination including magnetic resonance imaging (MRI) was done in suspected patients with retrocochlear lesion.
Statestical analysis
Calculations were done with the statistical package SPSS, version 12.0. Data were presented as mean ± SD (Standard Deviation). Kolmogorov-Simirnov test was used to test the parameter distributed. Student’s t test was used for comparison of means of normally distributed parameters while Mann- Whitney U test was used for comparison of non-normally distributed parameters. Chi square test was applied to assess differences between norminal variables. Pearson’s r was used to assess correlations as the analyzed variables were contiguously distributed. For all tests, values of p<0.05 were considered statistically significant.
Results
This study included 58 patients (116 ears examined) with migraine with mean age of 31.60±9.17 years and duration of illness of 8.33±4.47 years. Forty healthy subjects matched for age (30.25±5.24), sex, socioeconomic status and educational level were also included for statistical comparisons. The majority of patients (79.3%) had MoA while 20.7% had MA. Table (1) showed the demographic and clinical characteristics of the studied groups. None of the patients with migraine had manifest hearing impairment.
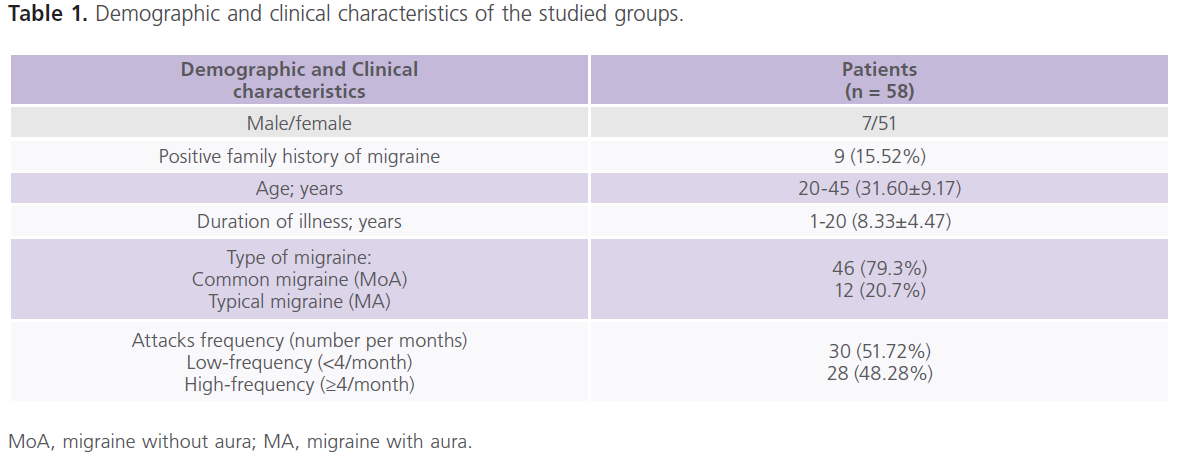
Table 1: Demographic and clinical characteristics of the studied groups.
Basic audiological examination (table 2) revealed that few patients had evidence suggesting conduction hearing problems. Normal middle ear status was confirmed by otoscopy and standard aural immittance procedures. According to the standard dichotomous aural criteria, all patients’ ears (except 7 right ears and 5 left ears) fell in the normal ear category with auditory thresholds better than 20 dB at 0.25, 0.5, 1.0, 2.0, 4.0 and 8.0 kHz. All patients’ ears had type (A) tympanograms (except 2 right and 3 left ears) with normal middle ear pressures and normal static compliance. Acoustic reflexes were present bilaterally (but absent in 8 right ears and 6 left ears) at 1 and 2 kHz threshold levels which did not exceed 100 dB HL.
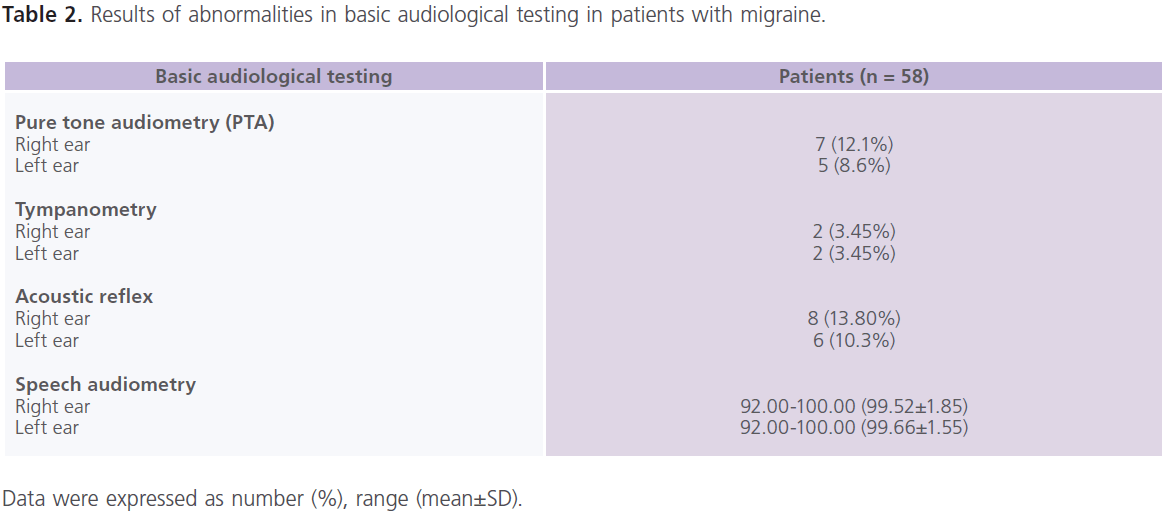
Table 2: Results of abnormalities in basic audiological testing in patients with migraine.
Table (3) showed the frequency of vestibular and audiological symptoms in the studied group. In the control group, they were present in 22.5% (n = 9) of individuals. In between the attacks of migraine, patients reported the following vestibular manifestations which included: dizziness (61.90%), rotatory vertigo (28.57%), sense of imbalance (19.05%) and positional vertigo (14.29%). While phonophobia (23.81%) and tinnitus (19.05%) were the reported auditory manifestations. Dizziness was more frequent in patients with MoA while sense of imbalance was more with MA. All patients with phononphobia and/or tinnitus had dizziness and/or vertigo, abnormal PTA and ENG findings.
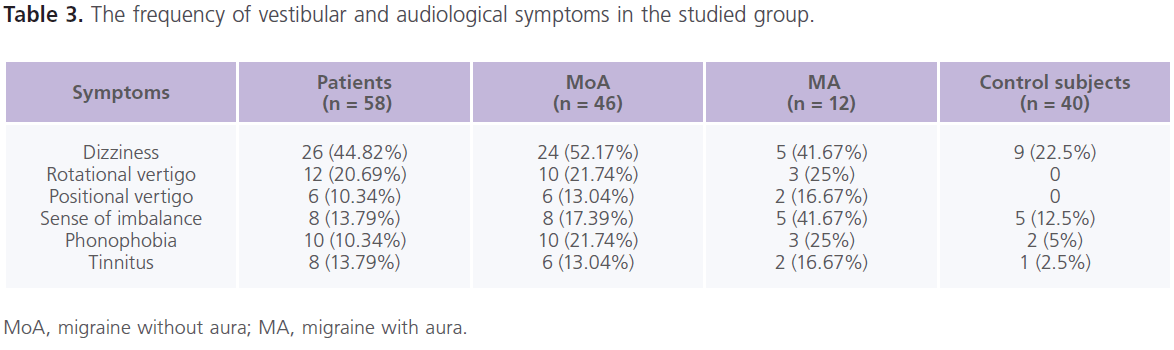
Table 3: The frequency of vestibular and audiological symptoms in the studied group.
Table (4) The frequency of electronystogmographic (ENG) abnormalities in the studied group. Forty three (74.14%) patients with migraine at lease one abnormality in ENG. In the control group, abnormalities in ENG examination were present in 15% (n = 6) of individuals. In between attacks of migraine, dizziness (44.82%), rotatory vertigo (20.69%), positional vertigo (10.34%) and sense of imbalance (13.79%), post head shaking (31.03%) positioning and positional test (20.67%), optokinetic nystagmus (24.14%), unilateral caloric weakness (17.24%), pursuit (13.79%) and saccadic (8.62%) eye tracking, gaze nystagmus (10.34%), spontaneous nystagmus (5.17%) and directional preponderance (6.9%). Both peripheral and central vestibular lesions were recorded in patients with migraine Patients with MA reported higher frequencies of ENG abnormalities compared to MoA.
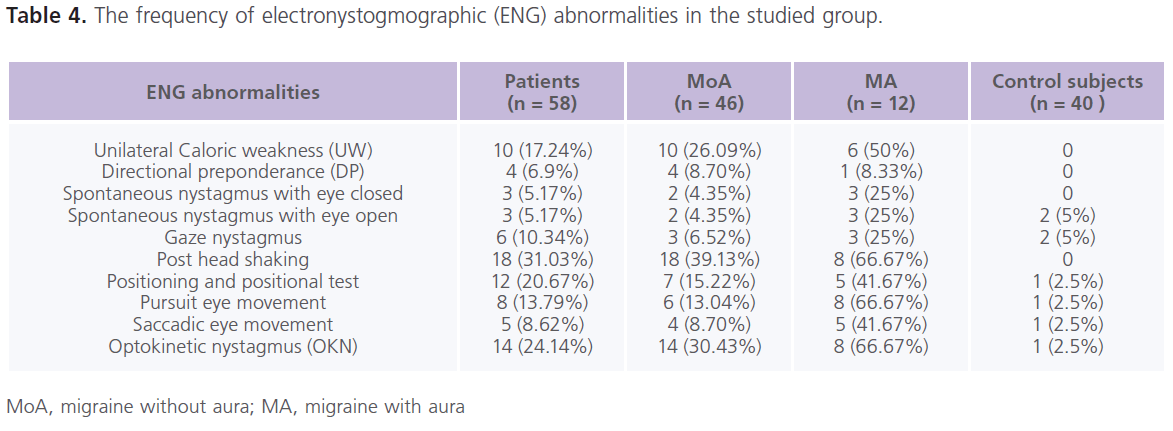
Table 4: The frequency of electronystogmographic (ENG) abnormalities in the studied group.
Table (5 and 6) showed that 28% of patients had one or more ABR abnormalities in the form of: prolonged absolute latency of wave III and I-III, III-V and I-V IPLs. In general and compared to control subjects, patients with migraine reported prolonged absolute latency of wave III and I-V IPL (particularly in MoA). No gender differences were identified in ABR parameters. significant positive correlations between (1) age and absolute latency of right wave I at low repetition frequency rate (0.321; P = 0.018) and left I-V IPL at high repetition frequency rate (0.269, P = 0.054), (2) the frequency of migraine and ENG abnormalities (0.457; P = 0.012) and left I-V IPL at high frequency repetition rate (0.296, P = 0.045), (3) duration of illness and ENG abnormalities (0.308; P = 0.042) and right I-V IPL at high repetition frequency rate (0.349; 0.01), left absolute latency of wave III (0.269; P = 0.047) and left I-V IPL (0.287; P = 0.036).
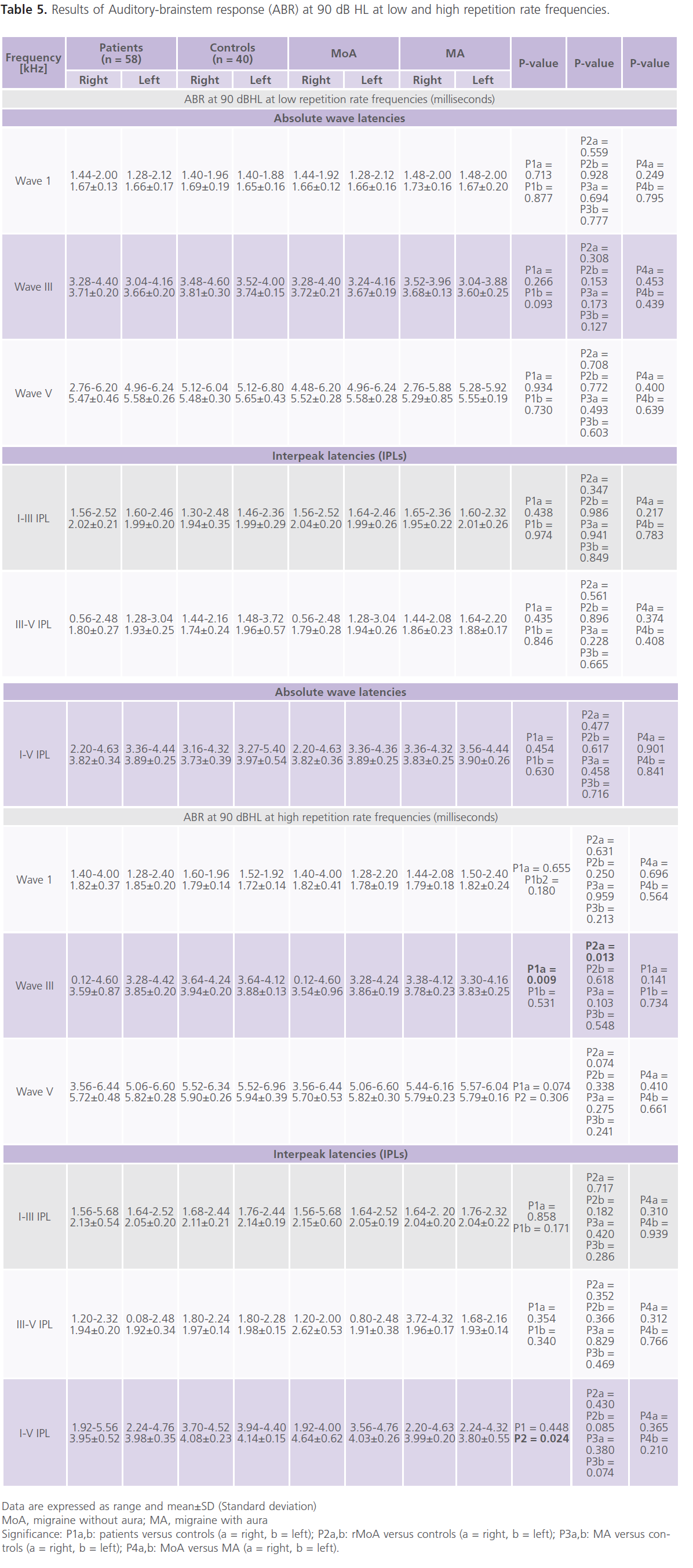
Table 5: Results of Auditory-brainstem response (ABR) at 90 dB HL at low and high repetition rate frequencies.
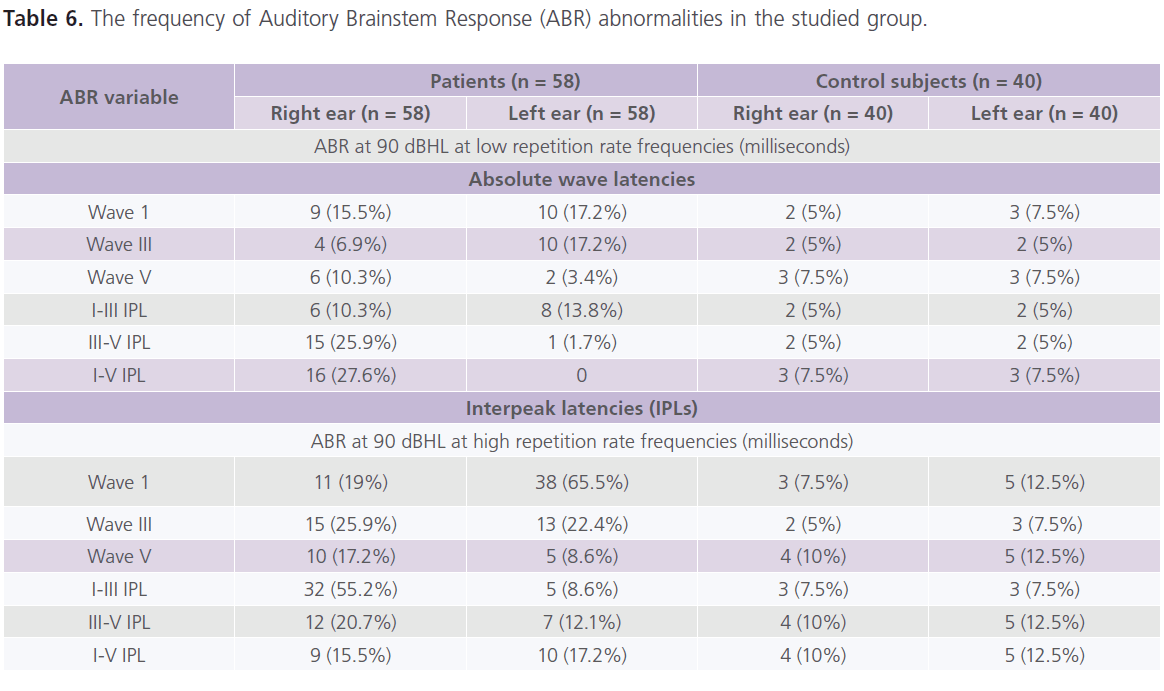
Table 6: The frequency of Auditory Brainstem Response (ABR) abnormalities in the studied group.
Discussion
Vestibular as well as auditory symptoms and signs are not uncommon with migraine and may occur prior or during the headache attacks or during a headache free interval (41). However, little is known about the pathogenesis of these manifestations.
In the present study, we have found a higher frequency of symptoms of migraine-related-vertigo as dizziness (61.91%), rotatory and positional vertigo (42.86%) and unsteadiness (19.05%). In the literature vestibular symptoms were reported in two-thirds of patients in which migraine related vertigo was reported in 55% and 76% (5). Kayan and Hood (4) reported that approximately half of patients with migraine had vertigo while the other half had only giddy sensation. Cutrer and Baloh (10) reported that 35% of patients with migraine had vertigo and similar percentage had non-vertiginous dizziness (severe imbalance, nausea and sensitivity to motion). Cass et al. (42) reported dysequilibrium, light headedness and imbalance in 79% and vertigo in 21% of patients with migraine. Whitney et al. (13) found migraine related vestibulopathy in 64%, among were 56% had symptoms of space and motion disorders. Bayazit et al. (6) reported dizziness in 30% followed by vertigo (25%) and tinnitus (20%). Neuhauser and Lempert (16) found 45.45% of patients with vertigo had regularly migrainous headache, 48.48% and 6.06% headache and vertigo never occurred together. In general population, both migraine and vertigo are common with lifetime prevalence of about 16 % for migraine and 7 % for vertigo. Therefore, a concurrence of the two conditions by chance alone can be expected in about 1.1 % of the general population. However, recent epidemiological evidences suggest that the actual comorbidity is higher, (3.2%). This can be explained by the fact that several dizziness and vertigo syndromes occur more frequently in patients with migraine than in controls including benign paroxysmal positional vertigo, Meniere’s disease, motion sickness, cerebellar disorders and anxiety syndromes (3,16). The differences between our results and others in the percentage of vestibulopathy may be attributed to the criteria used for the selection of the studied populations or may be related to methodological differences between laboratories.
In the present study, 74.14% patients with migraine had abnormalities in at least one of ENG tests as post head shaking, positioning and positional test, OKN, UW, pursuit and saccadic tracking eye movements, gaze nystagmus, spontaneous nystagmus and DP, indicating the presence of peripheral and central lesion(s) in any of the vestibular pathway. The frequency of migraine attacks and the duration of illness were identified as important confounders associated with ENG abnormalities. Togli et al. (9) reported abnormal vestibular finding in 80% of patients with MoA. Kayan and Hood (4) identified ENG abnormalities in of 77.5% of patients with migraine. Cass et al. (41) reported abnormal results in at least one of the vestibular function tests in 73% with migraine related vertigo. The authors observed abnormal caloric response in 55%, rotational vestibular test dysfunction in 42%, oculomotor test dysfunction in 29% and abnormal positional test results in 19%. Dieterich and Brandt (12) reported that 66% of patients with migraine showed mild central ocular motor signs such as vertical (48%) and/or horizontal (22%) saccadic pursuit, gaze-evoked nystagmus (27%), moderate positional nystagmus (11%), and spontaneous nystagmus (11%) in the attack free periods of migraine. Whitney et al. (13) reported abnormal responses in ocular motor, rotational caloric and positional testing in 92% of patients with migraine related vestibulopathy and in 75% of migraine without vestibulopathy. In accordance, Bir et al. (14) reported that 58% of the patients with migraine and 55% of the patients with migraine and vertigo had abnormal ENG findings. While Bayazit et al. (6) reported lower percentage of ENG abnormalities. the authors reported positive positional test (Hallpike maneuver) was reported in 2 (10%) and bithermal caloric testing canal paresis in 3 patients (15%).
In the present study, patients with MA had more vestibular abnormalities compared to patients with MoA. In accordance, Savundra et al. (11) observed that patients with MA and presented with vertigo commonly had both central and peripheral vestibular findings. Harno et al. (15) detected ENG abnormalities in 29% with MoA and with 33% in MA, on the other hand, 17% patients with MoA had peripheral abnormalities while central abnormalities were more common in MA and MoA.
In the present study, ABR showed abnormalities were reported in 28% of patients in the form of prolonged absolute latency of wave III and I-III, III-V and I-V IPLs. In general and compared to control subjects, patients with migraine reported prolonged absolute latency of wave III and I-V IPL (particularly with MoA). The frequency of migraine attacks and the duration of illness were identified as important confounders associated with ABR abnormalities. Some authors reported prolongation of the absolute latencies of waves I, III and V and I-III, III-V and I-V IPLs in 35% of patients with migraine in between attacks (6). In the study of Dash et al. (42), the authors observed that all patients with vertigo had abnormalities the ABR as prolonged absolute latencies or/and IPLs. Bayazit et al. (6) reported that 35% of their patients with migraine had abnormal ABR results; 20% of them had peripheral pathway involvement in the form of prolonged absolute latencies of waves I, III and V but normal I-III, III-V and I-V IPLs, while 15% of them had central pathway affection in the form of prolonged absolute wave latencies and IPLs. While in contrast, Kochar et al. (43) reported significant prolongation in ABR absolute and IPLs at the time of acute attack of migraine and disappeared after 7 days from the attacks indicating reversible pathological changes in different areas of the brain and brainstem. Some authors reported prolonged absolute latency of wave V and I-V IPL during the headache attach indicating transient impairment of the auditory brainstem function (44).
In the present study, phonophobia (23.81%) and tinnitus (19.05%) were the auditory symptoms reported in patients with migraine but none of the patients had manifest hearing impairment. All patients with phononphobia and/or tinnitus had dizziness and/or vertigo, ENG findings and abnormal pure tone audiometry (PTA). Similarly several investigators reported nearly the same results. Baloh (5) reported that photophobia was the most common auditory symptoms but fluctuating and acute permanent hearing loss occurs in a small percentage. Kayan and Hood (4) reported sensorineural hearing loss in 18%. Another two studies on migraine related vestibulopathy reported that hearing loss is rare and it is difficult to be related to migraine alone (10,42). Harno et al. (15) found that only 5.56% of their patients with various types of migraine had sensorineural hearing loss. Battista (28) reported normal audiometric findings in patients with migraine associated dizziness.
The exact pathogenesis of vestibular and auditory involvement in migraine remains unknown, however possibilities include: First: This and other studies indicate that migraine process may involve any level of the vestibular peripheral or/ and central (cortical, subcortical and brainstem) pathways or systems and this is responsible for the observed ictal and interical vestibular and auditory manifestations with migraine (4, 5, 25, 27, 30, 31, 32, 33, 34, 36, 37), in support: a) a cortical and possibly subcortical dysfunction of which the hallmark is deficient habituation were observed with MoA and MA in electrophysiologic studies (as evoked cortical potential, nociceptive blink reflex studies and during repetitive electrical stimulation), indicating interictal abnormal processing of repeated stimuli in the reflex circuit or labyrinthine or cortical hyperexcitability (25,30). In addition, excitability changes in visual, parietal and temporal cortices were also observed with transcranial magnetic stimulations (TMS) (29, 34). Furthermore, b) It is well known that the vestibular nuclei receive noradrenergic fibers from the locus coeruleus (40) and serotonergic afferents from the dorsal raphe nucleus (45). Thus it is likely that activation of these pathways, could also activate central vestibular processing (46), and c) The reciprocal connection of vestibular nuclei and trigeminal nucleus caudalis may provide a close link between vestibular and vasculartrigeminal processing during migraine attacks and trigeminal inner ear sensory innervation may explain the peripheral vestibular and auditory manifestations in patients with migraine(47). Second: The calcium channels are primarily expressed in the brain and inner ear and thus their defect could lead to reversible hair cell depolarization, leading to auditory and vestibular symptoms (5). The recent discovery of a mutation in a brain calcium-channel gene in families with hemiplegic migraine and in families with episodic vertigo and ataxia suggests a possible mechanism for vestibular and auditory symptoms in patients with more common varieties of migraine. In addition, neurophysiological methods have revealed subclinical abnormalities of cerebellar function and neuromuscular transmission, which may improve the genetic and therapeutic studies in phenotyping of patients with migraine (30). Third: It has been observed that migraine prophylactic treatment shows encouraging results in patients with, 69.3% had satisfactory control of symptoms (sum of complete resolutions and substantial controls) and 81.8% had at least a 50% reduction of the vertiginous episodes frequency (47). Topiramate (TPM) (an antiepileptic medication) is a safe, effective, and well-tolerated drug which shows reduction of both migraine and migraine-related vertigo manifestations (48, 49) and as prophylaxis of migraine and migraine-related vertigo and other vestibular symptoms (50). Topiramate blocks voltage- sensitive sodium channels and voltage-activated calcium channels, inhibits glutamate release, and increases GABA levels (51-53). Forth: It has been suggested that short duration vertigo may be related to vasospasm-induced ischaemia of the labyrinth and repeated episodes may lead to irreversible damage (38). This is further supported by the association of other neurologic phenomena with migraine as amaurosis fugax, hemiplegia, facial pain, chest pain, and visual aura suggesting that vasospasm of the cochlear vasculature is the cause of the sudden hearing loss in patients with migraine (4, 5, 27, 32, 37).
To summarize, the results of this study indicate that: 1) In between the attacks of migraine, migraine related vertigo (as dizziness, vertigo and sense of imbalance) are more frequent symptoms compared to the less common auditory symptoms (as phonophobia and tinnitus). Also patients with migraine have frequent abnormalities in ENG (as post head shaking, positioning and positional test, OKN, UW, pursuit and saccadic tracking eye movements, gaze nystagmus, spontaneous nystagmus and DP) and ABR (as prolonged absolute wave latencies and IPLs) compared to the less common abnormalities in basic audiological examination, 2) The frequency of migraine attacks and the duration of illness were identified as important confounders associated with ENG and ABR abnormalities, and 3) The beginning of vestibular and auditory symptoms after the beginning of migraine headache suggests that the disease process involves any level of the vestibular peripheral or/and central pathways or systems and over a period of time the chronicity of the disease process may be responsible for changing the reversible to irreversible lesions of the vestibular peripheral or/and central pathways or systems.
However and despite the importance of the results of this study, there are some limitations which include: 1) relatively small number of patients, and 2) due to the cross-sectional design of this study, the temporal relationship between appearance of vestibular dysfunction and migraine attacks is unknown.
Conclusions
Vestibular dysfunctions are frequently associated with migraine in between the headache attacks. The frequency of migraine and duration of illness are important confounders. Our results indicate that chronic migraine may result in irreversible damage at any level of the vestibular peripheral and/ or central pathways or systems.
Authors disclosures
None.
6460
References
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001; 41(7):646-657.
- Russell MB, Olesen J. Increased familial risk and evidence generic factor in migraine. BMJ 1995; 311(7004):541-544.
- Olesen J. The International Classification of Headache Disorders, 2nd edition: application to practice. Funct Neurol 2005; 20(2):61-68.
- Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984; 107 (Pt 4):1123-1142.
- Bayazit Y, Yilmaz M, Mumbuç S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Rev Laryngol Otol Rhinol (Bord). 2001; 122(2):85-88.
- Casani AP, Sellari-Franceschini S, Napolitano A, Muscatello L, Dallan I. Otoneurologic dysfunctions in migraine patients with or without vertigo. Otol Neurotol 2009; 30(7):961-967.
- Kuritzky A, Ziegler DK, Hassanein R. Vertigo, motion sickness and migraine. Headache. 1981; 21(5):227-231.
- Toglia JU, Thomas D, Kuritzky A. Common migraine and vestibular function: electronystagmographic study and pathogenesis. Ann Otol Rhinol Laryngol 1981; 90(3 Pt 1):267-271.
- Cutrer FM, Baloh RW. Migraine associated dizziness. Headache 1992; 32(6):300-304.
- Savundra PA, Carroll JD, Davies RA, Luxon LM. Migraine associated vertigo. Cephalalgia 1997; 17(4):505-510.
- Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999; 246(10):883-892.
- Whitney SL, Wrisley DM, Brown KE, Furman JM. Physical therapy of migraine related vestibulopathy and vestibular dysfunction with history of migraine. Laryngoscope. 2000; 110(9):1528-1534.
- Bir LS, Ardic FN, Kara CO, Akalin O, Pinar HS, Celiker A. Migraine patients with or without vertigo: comparison of clinical and electronystagmographic findings. J Otolaryngol 2003; 32(4):234-238.
- Harno H, Hirvonen T, Kaunisto MA, Aalto H. Subclinical vestibulocerebellar dysfunction in migraine with and without aura. Neurology. 2003; 61(12):1748-1752.
- Neuhauser HK and Lempert T. Vertigo and dizziness related to migraine: a diagnostic challenge. Cephalgia 2004; 24(2):83-91.
- Troost BT. Vestibular migraine. Curr Pain Headache Res 2004; 8(4):310-314
- Crevits L, Bosman T. Migraine related vertigo: towards a distinctive entity. Clinical Neurology and Neurosurgery 2005; 107(2):82-87.
- Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006; 116(10):1782-1786.
- Rzeski M, Stepien A, Kaczorowski Z. Evaluation of the function of the vestibular system in patients with migraine. Neurol Neurochir Pol 2008; 42(6):518-524.
- Harker LA, Rassekh C. Migraine equivalent as a cause of vertigo. Laryngoscope. 1988; 98(2):160-164.
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004; 24 Suppl 1:9-160.
- Marcus DA, Kapelewski C, Rudy TE, Jacob RG, Furman JM. Diagnosis of migrainous vertigo: validity of a structured interview. Med Sci Monit 2004; 10(5):CR 197 -201.
- Brantberg K, Trees N, Baloh RW. Migraine-associated vertigo. Acta Otolaryngol. 2005; 125(3):276-279.
- Sand T, Vingen JV. Visual, long-latency auditory and brainstem auditory evoked potentials in migraine: relation to pattern size, stimulus intensity, sound and light discomfort thresholds and preattack state. Cephalalgia 2000; 20(9):804-820.
- Bernard PA, Stenstrom RJ. Fluctuating hearing losses in children can be migraine equivalents. Int J Pediatr Otorhinolaryngol 1988; 16(2):141- 148.
- Viirre ES, Baloh RW. Migraine as a cause of sudden hearing loss. Headache. 1996; 36(1):24-28.
- Battista RA. Audiometric findings of patients with migraine-associated dizziness. Otol Neurotol. 2004 Nov; 25(6):987-992.
- Schoenen J, Ambrosini A, Sándor PS, Maertens de Noordhout A. Evoked potentials and transcranial magnetic stimulation in migraine: published data and viewpoint on their pathophysiologic significance. Clin Neurophysiol 2003; 114(6):955-972.
- Schoenen J. Neurophysiological features of the migrainous brain. Neurol Sci. 2006; 27 (Suppl 2):S77-81.
- Woodhouse A, Drummond PD. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia 1993; 13(6):417- 421.
- Bolay H, Bayazit YA, Gündüz B, Ugur AK, Akçali D, Altunyay S, Ilica S, Babacan A. Subclinical dysfunction of cochlea and cochlear efferents in migraine: an otoacoustic emission study. Cephalalgia 2008; 28(4):309-317.
- Volcy M, Sheftell FD, Tepper SJ, Rapoport AM, Bigal ME. Tinnitus in migraine: an allodynic symptom secondary to abnormal cortical functioning? Headache. 2005; 45(8):1083-1087.
- Hamed SA. A migraine variant with abdominal colic and Alice in Wonderland syndrome: a case report and review. BMC Neurol 2010 6; 10:2.
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine-current understanding and treatment. N Engl J Med 2002; 346(4):257-270.
- Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol. 2000; 57(11):1631-1634.
- Buisseret-Delmas C, Compoint C, Delfini C, Buisseret P. Organisation of reciprocal connections between trigeminal and vestibular nuclei in the rat. J Comp Neurol 1999; 409(1):153-168.
- American Speech and Hearing Association Committee on audiometric evaluation, 1988 Guidelines for manual pure-tone threshold audiometry, 20:297-301.
- Perez P, Llorente JL, Gomez JR, Del Campo A, Lopez A, Suarez C. Functional significance of peripheral head-shaking nystagmus. Laryngoscope 2004; 114(6):1078-1084.
- Schubert MC, Tusa RJ, Grine LE, Herdman SJ. Optimizing the sensitivity of the head thrust test for identifying vestibular hypofunction. Phys Ther 2004; 84(2):151-158.
- Cass SP, Furman JM, Ankerstjerne K, Balaban C, Yetiser S, Aydogan B. Migraine related vestibulopathy. Ann Otol Rhinol Laryngol 1997; 106(3):182-189.
- Dash AK, Panda N, Khandelwal G, Lal V, Mann SS. Migraine and audiovestibular dysfunction: is there a correlation? Am J Otolaryngol 2008; 29(5):295-299.
- Kochar K, Srivastava T, Maurya RK, Jain R, Aggarwal P. Visual evoked potential & brainstem auditory evoked potentials in acute attack & after the attack of migraine. Electromyogr Clin Neurophysiol 2002; 42(3):175-179.
- Firat Y, Ozturan O, Bicak U, Yakinci C, Akarcay M. Auditory brainstem response in pediatric migraine: during the attack and asymptomatic period. Int J Pediatr Otorhinolaryngol 2006; 70(8):1431-1438.
- Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience 2006; 140(3):1067-1077.
- Furman JM, Marcus DA, Balaban CD. Migrainous vertigo: development of a pathogenetic model and structured diagnostic interview. Curr Opin Neurol 2003; 16(1):5-13.
- Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006; 116(10):1782-1786.
- Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005; 1039:517-520.
- Teggi R, Fabiano B, Recanati P, Limardo P, Bussi M. Case reports on two patients with episodic vertigo, fluctuating hearing loss and migraine responding to prophylactic drugs for migraine. Menière’s disease or migraine-associated vertigo? Acta Otorhinolaryngol Ital. 2010; 30(4):217.
- Silberstein SD, Feliu AL, Rupnow MF, Blount AC, Boccuzzi SJ. Topiramate in migraine prophylaxis: long-term impact on resource utilization and cost. Headache 2007; 47(4):500-510.
- Bradford HF. Glutamate, GABA and epilepsy. Prog Neurobiol 1995; 47(6):477-511.
- Taverna S, Sancini G, Mantegazza M, Franceschetti S, Avanzini G. Inhibition of transient and persistent Na+ current fractions by the new anticonvulsant topiramate. J Pharmacol Exp Ther 1999; 288(3):960- 968.
- Zhang X, Velumian AA, Jones OT, Carlen PL. Modulation of highvoltage- activated calcium channels in dentate granule cells by topiramate. Epilepsia 2000; 41:(S1):S52-S60.











