Keywords
Fungal peritonitis; End stage renal disease; Continuous Ambulatory Peritoneal Dialysis (CAPD); Predictors; Candida albicans; Non-albicans Candida species; Dimorphic fungi
Introduction
Peritonitis is a common complication of Peritoneal Dialysis (PD) and Fungal Peritonitis (FP) is almost 3-30% of all peritonitis [1-5]. The majority of patients could not resume PD [1,2], and the patients FP-related mortality was very high [4,5]. Predictors for developing FP could not be clearly determined. Numerous situations were listed which play an important role in the appearance of the mycotic infection. The strongest predictors for FP in PD patients were previous bacterial peritonitis, prolonged use of antibiotics, prolonged time in the dialysis program, prolonged time with the peritoneal catheter inserted, use of immunosuppressive agents, hospitalization and co-existence of an extra peritoneal fungal infection [6].
Most FP cases were caused by yeasts with Candida species, accounting for 70% - 90% in adults and 80% - 100% in the paediatric population. Filamentous fungi (moulds) such as Aspergillus, Penicillium, and the other yeasts were much less common, and together represent about 10% cases. In most centres, C. albicans were more predominant as a single pathogen; however, four other major Candida species-C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei became increasingly recognized cause of FP [7,8]. Thus the significance of the presence of non-albicans Candida species in the pathogenesis of PD associated peritonitis was required to be reconsidered. Therefore, this retrospective study was carried out to assess the frequency of Candida species as a single pathogen, its association with the predictors and its impact on the outcome of the patients.
Methods
It is a retrospective study involving patients undergoing Continuous Ambulatory Peritoneal Dialysis (CAPD) at our centre who developed peritonitis over a period of 16 years, from Jan 2000 to August 2015. Out of 402 patients, 65 End Stage Renal Disease (ESRD) patients developed fungal peritonitis. Aerobic, Anaerobic, mycobacterium and polymicrobial peritonitis were excluded from the analysis due to their different outcomes.
As per the peritoneal dialysis related infection recommendations published by ISPD in 2010 [9], the patient’s exchange bags, containing effluent dialysate were received in the microbiology laboratory for macro level examination, microscopic examination and culturing simultaneously. From these bags, 100 ml of fluid was withdrawn with a sterile needle and syringe under aseptic conditions. The fluid was centrifuged in sterile tubes at a rate of 3000 g for 15 minutes and supernatant was discarded, leaving 0.5 ml. In the centrifuged deposit, 10 ml of sterile distilled water was added together and the mixture was shaken vigorously on vortex for 30 sec. This mixture was then divided into 4 parts of 1 ml, 3 ml, 3 ml and 3 ml each. 1 ml was further divided for staining characteristic like gram stain, Z.N. stain, and lacto phenol cotton blue film, while 3 ml in FA bottle for isolation of aerobes and fungi, 3 ml in FN bottle and remaining 3 ml in MP bottle for the isolation of anaerobes and mycobacterium respectively. These three inoculated bottles were further incubated in BactAlert 3D system following standard protocols. The isolated fungi were reexamined microscopically to ensure the staining and morphologic characteristic. Each positive specimen was inoculated on Sabouraud Dextrose agar (M286) and Sabouraud Cycloheximide Chloramphenicol agar (M664).
Cultures were routinely incubated at 25°C and 37°C and examined daily for a long period of four weeks. The identification of individual fungi was based on standard methods such as microscopy, morphology, colonial characterization, pigment production, rate of growth while yeast identification was done by Vitek-2 (Biomeurix, France). Here it is important to note that what took a long time in the above process, it took comparatively very short spell of time for the same e.g. microscopy became available within 3 to 5 hrs and identification along with antibiogram of Candida species and yeast was made available within 48 hrs dimorphic fungi was identified within 4-6 days after the clinical diagnosis was made.
The definitions used in the article are as follows. Predictors have been defined as predictors for developing FP. Previous bacterial peritonitis episode suggests that fungal peritonitis appears after the episodes of bacterial peritonitis. Prior antibiotic use means the use of antibiotic for a suspected infectious disease in a period of 30 days, 3 months, or 6 months prior to the development of FP. Prolonged time in the dialysis program means prolonged use of catheter for several years. Prolonged time with the peritoneal catheter inserted means maintaining the catheter after detecting the fungal infection. Use of immunosuppressive agents referred to steroid or immunosuppressive use for at least 2 weeks prior to the diagnosis of FP. Hospitalization means a risk when an infection of nosocomial origin occurs in the 30 days, 3 months, or 6 months prior to development of FP. Co-existence of an extra peritoneal fungal infection illustrates when a patient is suffering from extra peritoneal fungal infection which causes a fungal peritonitis through a haematogenic pathway [6]. De novo means those cases of FP which occur due to direct contamination of dialysis during the exchange procedure, underlying intestinal pathology such as diverticulosis in the host and environmental contaminations [10,11].
Statistical analysis was performed using chi square test and contingency coefficient. Data were expressed as mean ± standard deviation. Statistical significance was defined at a p value of 0.05
Results
During the period from Jan 2000 to June 2015, 402 ESRD patients were initially on CAPD. The total no of episodes of fungal peritonitis during the entire period was 65. The average rate of fungus peritonitis was 2.6 episodes/CAPD year. Their base line and demographic data were described as follows. Out of total FP population males were 89.2% and females were 10.8%. The mean age of the study population was 58.29 ± 8.845 and the mean duration on CAPD before development of fungal infection was 18.26 ± 8.080 months. Predominant cause of ESRD in this group was diabetic nephropathy (52.3%), glomerulonephritis (32.3%), hypertension (13.9%) and others (1.5%).
In our case series fungal peritonitis was presented with abdominal pain, sub-acute intestinal obstruction, nausea, vomiting, occasional fever and cloudy dialysate.
Microscopic examination of 65 episodes of FP of the dialysate pellet with lacto phenol cotton blue film revealed conidia, blastoconidia, pseudohyphae and in few cases occasional mycelia like structures and culture examination revealed 89.3% Candida species, 1.5% yeast and 9.2% dimorphic fungi. On analyzing the data of Candida species 78.5% were non-albicans and10.8% were C. albicans. Among non-albicans Candida species C. tropicalis (13.8%) was the most frequent fungi isolated. It is worth mentioning here that it is for the first time when thirteen species of Candida were isolated from the cases of PD associated peritonitis. The spectrum of Candida and its species is depicted in (Figure 1).
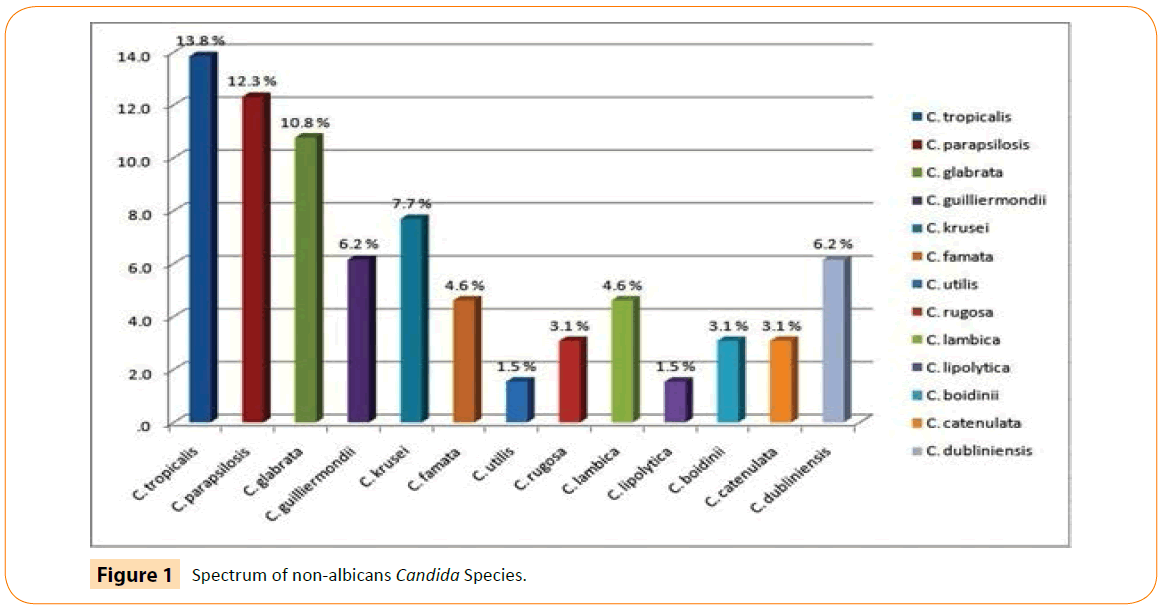
Figure 1: Spectrum of non-albicans Candida Species.
Association between predictors and pathogen reveals 30.8% de novo episodes and remaining 60.2% episodes of FP predisposed by different predictors. Among de novo non-albicans were 23.1 %. The strongest predictor to predispose the fungal infection was previous bacterial peritonitis episode (16.9%) as shown in Figure 2. It was first time when analysis was done between predictors and pathogen and was found significant at 0.05 levels (Table 1).
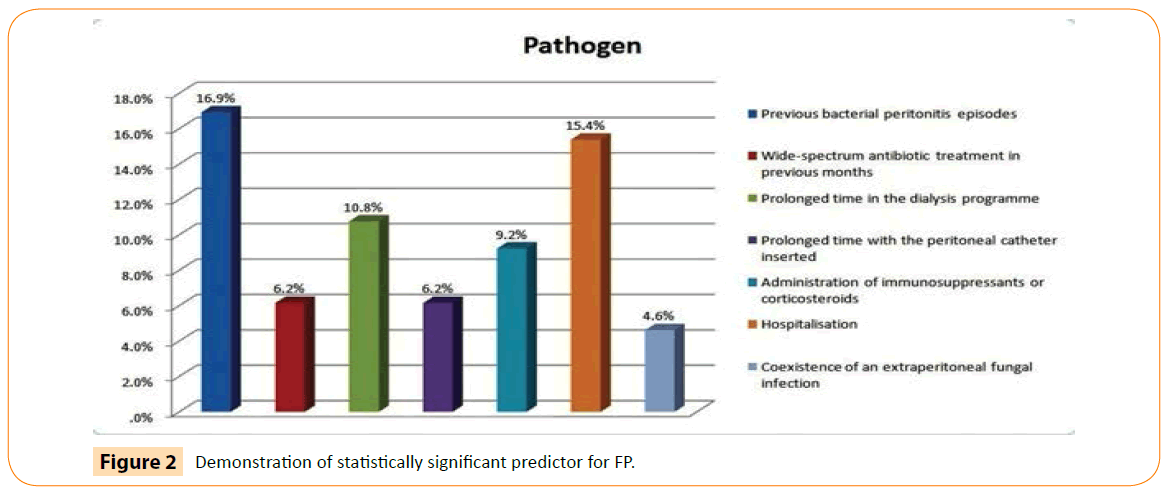
Figure 2: Demonstration of statistically significant predictor for FP.
| Predictors |
Fungi |
Total |
df |
Pearson Chi-square |
Contingency Coefficient |
| Candida albicans |
Non albicans |
Yeast |
Di morphic fungi |
| Previous bacterial peritonitis episodes |
0
0.0% |
7
10.8% |
1
1.5% |
3
4.6% |
11
16.9% |
21 |
51.990* |
0.667 |
| Wide-spectrum antibiotic treatment in previous months |
0
0.0% |
3
4.6% |
0
0.0% |
1
1.5% |
4
6.2% |
| Prolonged time in the dialysis programme |
0
0.0% |
7
10.8% |
0
0.0% |
0
0.0% |
7
10.8% |
| Prolonged time with the peritoneal catheter inserted |
4
6.2% |
0
0.0% |
0
0.0% |
0
0.0% |
4
6.2% |
| Administration of immune suppressants or corticosteroids |
0
0.0% |
6
9.2% |
0
0.0% |
0
0.0% |
6
9.2% |
| Hospitalisation |
0
0.0% |
10
15.4% |
0
0.0% |
0
0.0% |
10
15.4% |
| Coexistence of an extra peritoneal fungal infection |
0
0.0% |
3
4.6% |
0
0.0% |
0
0.0% |
3
4.6% |
| De novo |
3
4.6% |
15
23.1% |
0
0.0% |
2
3.1% |
20
30.8% |
| Total |
7
10.8% |
51
78.5% |
1
1.5% |
6
9.2% |
65
100.0% |
* Significant at p value 0.05.
Table 1: Association between predictors and pathogens.
Association among primary cause of ESRD, pathogens and outcome showed that catheter removal (32.4%) and loss of life (14.7%) was significantly more frequent in patients suffered with peritonitis due to non-albicans Candida species while in glomerulonephritis, patient required maximum hospitalization (28.6%) due to non-albicans Candida species for resolution of peritonitis. Cure rate (55.6%) was more significant in patients with hypertension. It is also noticed that de novo fungal infection was more frequent with glomerulonephritis group (Table 2). On analyzing the outcome data obtained from all 65 patients through Chi test, we inferred that non-albicans were the major cause of death in majority of patients. The similar trend was found in almost all the primary causes of End Stage renal disease test set as depicted in Figure 3, however the sample size (others) was less than the minimum number of cases to be inferred statistically sound. The Loss of life parameters was found to be more than 3 fold higher in case of non-albicans Candida species pathogen.
| Primary Diagnosis |
Pathogens |
Outcomes |
Total |
| Loss of Life |
Loss of Catheter |
Hospita-lization |
Change of Modality |
Cure |
| Diabetes |
Non albicans |
5
14.7% |
11
32.4% |
6
17.6% |
1
2.9% |
4
11.8% |
27
79.4% |
| Yeast |
0
0.0% |
1
2.9% |
0
0.0% |
0
0.0% |
0
0.0% |
1
2.9% |
| Dimorphic Fungi |
2
5.9% |
4
11.8% |
0
0.0% |
0
0.0% |
0
0.0% |
6
17.6% |
| Total |
7
20.6% |
16
47.1% |
6
17.6% |
1
2.9% |
4
11.8% |
34
100.0% |
| Hypertension |
Non albicans |
1
11.1% |
1
11.1% |
2
22.2% |
-- |
5
55.6% |
9
100.0% |
| Total |
1
11.1% |
1
11.1% |
2
22.2% |
-- |
5
55.6% |
9
100.0% |
| Glomerulo-nephritis |
Candida albicans |
0
0.0% |
0
0.0% |
0
0.0% |
-- |
6
28.6% |
6
28.6% |
| Non albicans |
2
9.5% |
5
23.8% |
6
28.6% |
-- |
2
9.5% |
15
71.4% |
| Total |
2
9.5% |
5
23.8% |
6
28.6% |
-- |
8
38.1% |
21
100.0 |
| Unknown |
Candida albicans |
-- |
-- |
-- |
-- |
1
100.0% |
1
100.0% |
| Total |
-- |
-- |
-- |
-- |
1
100.0% |
1
100.0% |
Table 2: Association among primary cause of ESRD, pathogens and outcome.
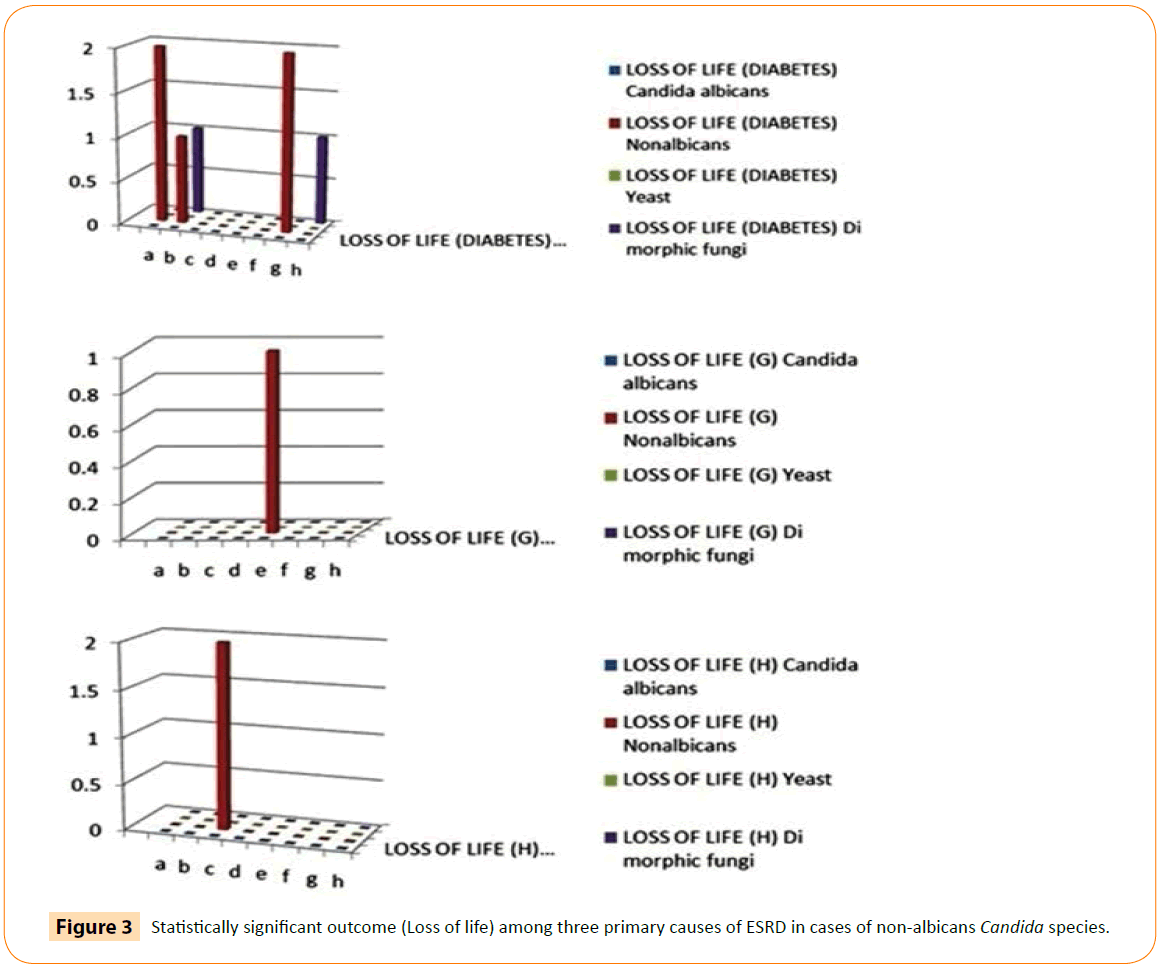
Figure 3: Statistically significant outcome (Loss of life) among three primary causes of ESRD in cases of non-albicans Candida species.
Discussion
Fungi are widely found in human environment, being part of the normal flora of the skin and mucosa, but in certain conditions, they become pathogenic. The lethality, although variable, remains very high [12] because the fungi form a biofilm on the surface of the silastic catheters that reduces the penetration of antifungal agents [13]. It penetrates the peritoneal cavity through intraluminal or periluminal pathways and cross the intestinal mucosa, or enters through the hematogenic pathway due to a distant fungal infection [6].
In our case series Candida species were isolated in 89.3% cases, yeast in 1.5% cases and dimorphic fungi were isolated in 9.2% cases. On analyzing the data of Candida species 78.5% nonalbicans and10.8% C. albicans were found. Among non-albicans C. tropicalis (13.8%), C. parapsilosis (12.3%), C. glabrata (10.8%), C. guilliermondii (6.2%), C. cruzi (7.7%), C. famata (4.6%), C. utilis (1.5%), C. rugosa (3.1%), C. lambica (4.6%), C. lipolytica (1.5%), C. boidinii (3.1%), C. catenulata (3.2%) and C. dubliniensis (6.2%) were isolated in 48 to 50 hours. Hence, here in for the first time in the world we are reporting isolation of 13 species of non-albicans from dialysate pellet which is unique in itself. This had been made possible because of the prompt identification of the isolated colonies done by Vitek -2.
Association among primary cause of ESRD, pathogens and outcome showed that non-albicans species of Candida were more complicated to treat as compared to albicans. As data revealed, due to non-albicans species of Candida , catheter removal (32.4%) and loss of life (14.7%) were significantly more frequent in diabetics while in glomerulonephritis, patient required maximum hospitalization (28.6%) for resolution of peritonitis. Cure rate (55.6%) was more significant in patients with hypertension. It was also noticed that de novo infections were more frequent with glomerulonephritis group. Statistical analysis had been calculated separately and was found significant in case of glomerulonepritis at p value of 0.05. On analyzing the outcome data obtained from all 65 patients through Chi test, we inferred that non-albicans Candida species were the major cause of death in majority of patients. The similar trend was found in almost all the primary causes of End Stage renal disease test set, however the sample size (others) was less than the minimum number of cases to be inferred statistically sound. The Loss of life parameters was found to be more than 3 fold higher in cases of non-albicans Candida species.
In Australia [3] and Turkey [14] Candida albicans predominated but in Hong Kong [5] and in USA [1] Candida parapsilosis were found to be the dominant strain. However Candida tropicalis was the most common species in Taiwan [15]. While more than 90% of FPs were caused by Candida species in a report from the United Kingdom [16], most of the reports from Eastern countries and South American countries had described the presence of non-Candida FPs, including Aspergillus and Penicillium species [3,14,17-19]. Tropical climate may increase the likelihood of non-c Candida species with reports from Australia describing up to 32% of non-Candida FPs. Seasonal variation was also reported [3,19]. An Indian study reported Candida albicans 65%, nonalbicans Candida 25%, Rhizopus species 5% and Alternaria 5% [20]. According to Levallois all nine episodes of FP were caused by Candida species. Only one episode was caused by C. albicans . Among the other eight episodes, three were caused by C. parapsilosis , three by Candida tropicalis, one by C. krusei , and again one by C. glabrata [21].
The incidence rate for other non-albicans Candida species was not well-established because the species were not identified in many cases and listed as Candida species only. Over the last decade, the number of non-albicans Candida species had been growing and their involvement had become associated with increased mortality and were intrinsically resistant to the usual antifungal used in the treatment [1,5,22-24].
Although larger series of FP in PD had already been published, antifungal susceptibility testing of isolates had been reported in two series. The first series was from Mexico and reported antifungal susceptibilities to triazoles only. Ten Candida isolates were identified resistant with various triazoles [25]. The second series was from Greece, which reported 46 episodes of fungal peritonitis and was tested for susceptibility to Amphotericin B and various triazoles [13].
Fluconazole resistance was predictable with C. krusei , itraconazole and voriconazole were resistant with C. glabrata isolate. Resistance to specific triazoles had also been described in the literature with some isolates of C. albicans , C. parapsilosis , and C. tropicalis . Of concern was the Amphotericin B MIC of 2 mg/l observed in one C. tropicalis , one C. parapsilosis , and one C. glabrata isolate. This was the first published report of in vitro resistance of Candida species isolates to amphotericin B in a PD population [21].
In recent years it is confirmed that appearance of Candida species were resistant to fluconazole (C. krusei , C. ciferrii, C. norvegensis, C. glabrata , C. famata, C. lusitaniae, C. guilliermondii and C. tropicalis ), which indicates that it is not convenient to use fluconazole in monotherapy for certain peritonitis episodes, and that its effectiveness must be evaluated for some yeast species [6].
From the results obtained in the forging paragraph, identification of species had become necessary and essential to start empirical therapy on the basis of microscopy as few species of non-albicans exhibited resistance towards triazoles which consequently increase morbidity and drug resistant.
This study has limitations. Because of the relative rarity of FP, our total number of episodes remains quite small, despite a long follow-up time. Furthermore, the analysis between predictors and pathogens and analysis among disease, pathogen and outcome were more limited due to small sample size. Despite these limitations, we believe that this study is important considering its unique description of Candida species in FP.
Thus current study concludes that in PD associated FP nonalbicans species of Candida predominates in comparison to Candida albicans. Therefore, initial empirical antifungal therapy should be based on the local epidemiology and their intrinsic behavior to different antifungal agents. Further early assessment of predictors, rapid species identification and optimum antifungal coverage can lead to reduce morbidity, allowing shorter stay in the hospital and thereby preventing further nosocomial infection, antifungal resistance and chances of treatment failure.
8448
References
- Goldie SJ, Kiernan-Tridle L, Torres C, Gorban-Brennan N, Dunne D, et al. (1996) Fungal peritonitis in a large chronic peritoneal dialysis population: a report of 55 episodes. Am J Kidney Dis 28:86-91
- Huang JW, Hung KY, Wu KD, Peng YS, Tsai TJ, et al. (2000) Clinical features of and risk factors for fungal peritonitis in peritoneal dialysis patients. J Formos Med Assoc99:544-548
- Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, et al. (2009) Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int76:622-628
- Ram R, Swarnalatha G, Neela P, DakshinaMurty KV (2008)Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single-centre experience in India. Nephron ClinPract110:207-212
- Wang AY, Yu AW, Li PK, Lam PK, Leung CB, et al. (2000) Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis 36:1183-1192
- Agudo RG, Martos PG (2009) Clinical and microbiological aspects of fungal peritonitis in peritoneal dialysis. Nefrologia 29:506-517
- Warady BA, Bashir M, Donaldson LA(2000) Fungal peritonitis in children receiving peritoneal dialysis: a report of the NAPRTCS. Kidney Int58:384-389
- Chen CM, Ho MW, Yu WL, Wang JH (2004) Fungal peritonitis in peritoneal dialysis patients: effect of fluconazole treatment and use of the twin-bag disconnect system. J MicrobiolImmunol37:115-120
- Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, et al. (2010) Peritoneal dialysis-related infections recommendations: 2010 [Update]. Perit Dial Int30:393-423
- Kuen KY, Seto WH, Ching TY, Cheung WC, Kwok Y, et al. (1992) An outbreak of Candida tropicalis peritonitis in patients on intermittent peritoneal dialysis. J Hospinfect22:65-72
- Narain U, Gupta A (2016)Role of predictors and rapid diagnosis of fungal peritonitis in CAPD patients. Int J Adv Med 3:130-135.
- Prasad N, Gupta A (2005)Fungal peritonitis in peritoneal dialysis patients. Perit Dial Int25:207-222
- Bibashi E, Memmos D, Kokolina E, Tsakiris D, Sofianou D, et al. (2003) Fungal Peritonitis Complicating Peritoneal Dialysis during an 11-Year Period: Report of 46 Cases. Clin Infect Dis 36:927-931
- Oygar DD, Altiparmak MR, Murtezaoglu A, Yalin AS, Ataman R, et al. (2009) Fungal peritonitis in peritoneal dialysis: risk factors and prognosis. Renal Failure 1:25-28
- Yang DC, Wang MC, Lin WH, Wu AB, Sung JM, et al. (2012) Peritoneal Dialysis-Related Fungal Peritonitis: Twenty- Year Experience of a Medical Center in Southern Taiwan. ActaNephrol 26:149-154
- Davenport A, Wellsted D, Pan Thames Renal Audit Peritoneal Dialysis G (2011) Does antifungal prophylaxis with daily oral fluconazole reduce the risk of fungal peritonitis in peritoneal dialysis patients? The pan thames renal audit. Blood Purif 32: 181-185
- Chang TI, Kim HW, Park JT, Lee DH, Lee JH, et al. (2011) Early catheter removal improves patient survival in peritoneal dialysis patients with fungal peritonitis: Results of ninety-four episodes of fungal peritonitis at a single center. Perit Dial Int 31: 60-66
- Nadeau-Fredette AC, Bargman JM (2013) Characteristics and Outcomes of Fungal Peritonitis in a Modern North American Cohort. Perit Dial Int35:78-84
- Baer R, Killen JP, Cho Y, Mantha M (2013) Non-Candidal fungal peritonitis in far north queensland: A case series. Perit Dial Int 33:559-564
- Kumar KV, Mallikarjuna HM, Gokulnath, Jayanthi S (2014)Fungal peritonitis in continuous ambulatory peritoneal dialysis: The impact of antifungal prophylaxis on patient and technique outcomes. Indian J Nephrol 24: 297-301
- Levallois J, Nadeau-Fredette AC, Labbe AC, Laverdiere M, Ouimet D, et al. (2012) Ten-year experience with fungal peritonitis in peritoneal dialysis patients: antifungal susceptibility patterns in a North-American center. Int J Infect Dis 16:e41-e43
- Chen KH, Chang CT, Yu CC, Huang JY, Yang CW, et al. (2006) Candida parapsilosis peritonitis has more complications than other Candida peritonitis in peritoneal dialysis patients. Ren Fail 28:241-246
- Liang CC, Fang JT, Chen KH, Hung CC, Hwang TL, et al. (2008) Candida parapsilosis peritonitis complicated with infected pancreatic pseudocysts in a peritoneal dialysis patient: a challenge for nephrologists. ClinNephrol69:461-463
- Montenegro J, Aguirre R, González O, Martínez I, Saracho R (1995) Fluconazole treatment of Candida peritonitis with delayed removal of the peritoneal dialysis catheter. ClinNephrol 44:60-63
- Manzano-Gayosso P, Hernandez F, Mendez-Tovar L, Gonzalez-Monroy J, Lopez-Martinez R (2003) Fungal peritonitis in 15 patients on continuous ambulatory peritoneal dialysis (CAPD). Mycoses 46:425-429








