Key words
|
| |
| Angelica Archangelica Linn., Physicochemical parameters, Phytochemical analysis, successive solvent extraction. |
| |
Introduction
|
| |
| Peoples of all ages in both developing and undeveloped countries use plants in an attempt to cure various diseases and to get relief from physical sufferings. For centuries, plant and plant products have been used for treating various illnesses. Today, several medicinal plants and their products are still being employed as home remedies, over the counter drugs as well as raw materials for the pharmaceutical industry and they represent a substantial proportion of the global drug market. However a key obstacle, which has hindered the acceptance of the alternative medicines in the developed countries, is the lack of documentation and stringent quality control. There is a need for documentation of research work carried out on traditional medicines. Therefore it has become extremely important to make an effort towards standardization of the plant material to be used as medicine. The process of standardization can be achieved by stepwise pharmacognostic studies [1]. |
| |
| Archangelica comes from the Greek word ‘arkhangelos’ (=arch-angel), due to the myth that it was the angel Gabriel who told of its use as a medicine. Synonyms: Garden Angelica, Archangelica officinalis Hoffm., Archangelica officinalis var. himalaica C.B. Clarke. Ayurvedic names: Chandaa, canda, Chandaamshuka, Kathachoraa, Sanskrit: Laghu Coraka, Hindi: Choraka bheda, Dudhachoraa, Nature: Biennial and perennial herbal plant. |
| |
| During its first year it only grows leaves, but during its second year its fluted stem can reach a height of two meters (or six feet). Its leaves are composed of numerous small leaflets, divided into three principal groups, each of which is again subdivided into three lesser groups. The edges of the leaflets are finely toothed or serrated. The flowers, which blossom in July, are small and numerous, yellowish or greenish in color, are grouped into large, globular umbels, which bear pale yellow, oblong fruits. Angelica only grows in damp soil, preferably near rivers or deposits of water. Angelica Archangelica Linn. is a herb distributed in tropical and subtropical regions of the world. Angelica is largely used in grocery trade as well as for medicine. The herb is traditionally used for the treatment of leukoderma, nervous headaches, fever, skin rashes, wounds, rheumatism, toothaches, gastric ulcers, anorexia, and migraine, bronchitis, chronic fatigue, menstrual and obstetric complaints and for dental preparation [2]. |
| |
Materials and methods
|
| |
| Collection of plant material and authentication: The plant specimen of Angelica archangelica Linn. was collected from the forest of Gulmarg Hill, Srinagar, Jammu and Kashmir, India. The plant was identified and authenticated from Department of Pharmaceutical sciences, University of Kashmir as Specimen Voucher number - KUAA01 by Sr. Asst. Prof. (Dr) Zulfiqar Ali Bhat, Srinagar- 190006, India. |
| |
| Drying and size reduction of plant: The whole plant material of Angelica archangelica Linn. was subjected to shade drying for about 10 weeks. The dried plant material was further crushed to powder and the powder was passed through the sieve mesh 40 and stored in air tight container for further analysis. |
| |
|
Organoleptic study of plant material
|
| |
| In some cases, general appearance of the herb is similar to related species. Thus, detailed study of the morphological characters can be helpful in differentiating them. The organoleptic study of a drug includes its visual appearance to the naked eye along with its characteristics likes odour, taste, texture etc. For each particular organoleptic group, a particular systemic examination can be carried out [3]. |
| |
|
Determination of physicochemical constants of plant materials [4]:
|
| |
|
Extractive Values
|
| |
| Cold extractive values: The air-dried coarse drug powder (4g) was macerated separately with solvents (Petroleum ether, chloroform, ethyl acetate, methanol and water) of volume 100 ml in a closed flask for 24 hours, shaken frequently during six hours and allowed to stand for 24 hours. It was filtered rapidly, taking precaution against loss of solvent, the filtrate evaporated to dryness in a tarred flat bottom dish and dried on water bath, to constant weight and weighed. |
| |
| Hot extraction values: The powdered material of the drug (100g) was packed in a Soxhlet apparatus separately for each solvent like petroleum ether, chloroform, ethyl acetate and methanol but in case of water extract drug was prepared by decoction method. Each extract was evaporated to dryness and constant extractive value recorded. |
| |
| Successive extractive values: The dried and coarsely powdered material (100g) was subjected to successive extraction in a Soxhlet apparatus with different solvents like petroleum ether, chloroform ethyl acetate, methanol and in case of water solvent, drug was prepared by decoction method. The extracts were evaporated to dryness and their constant extractive values recorded [5]. |
| |
|
Ash values
|
| |
| Total ash: The ground drug (1 g) was incinerated in a silica crucible at a temperature not exceeding 450°C until free from carbon. It was then cooled and weighed to get the total ash content. |
| |
| Acid insoluble ash: Ash was boiled with 25 ml dilute HCl (2N) for five minutes. The insoluble matter collected on an ashless filter paper, washed with hot water and ignited at a temperature not exceeding 450°C to a constant weight. |
| |
| Water-soluble ash: Ash was dissolved in distilled water and the insoluble part collected on an ashless filter paper and ignited at 450oC to constant weight. By subtracting the weight of insoluble part from that of the ash, the weight of soluble part of ash was obtained [6]. |
| |
| Foreign matter: Foreign matter in the crude drugs can be due to faulty collection or deliberate mixing. It was separated from the crude drug and percentage calculated. |
| |
| Loss on drying: The powdered drug sample (10gm) without preliminary drying was placed on a tarred evaporating dish and dried at 105 ºC for 6 hours and weighed. The drying was continued until two successive reading matched each other or the difference between two successive weighing was not more than 0.25%. Constant weight was reached when two consecutive weighings after drying for 30 minutes in a desicator, showed not more than 0.01 gm difference. |
| |
| Swelling index: Specified quantity of plant material (3gm) previously reduced to the required fineness and accurately weighed was taken into 25ml glass stoppered measuring cylinder. 25 ml of water was added and the mixture was shaken thoroughly every 10 minutes for 1 hour. It was allowed to stand for 3 hours at room temperature and readings recorded. The mean value of the individual determinations was calculated related to 1gm of plant material [7]. |
| |
| Foaming index: About 1gm of the plant material was reduced to a coarse powder, weighed accurately and transferred to a 500 ml conical flask containing 100 ml of boiling water. It was maintained at moderate boiling for 30 min., cooled and filtered into 100 ml volumetric flask. Sufficient water was added through the filter paper to dilute to volume. The decoction was poured into 10 stoppered test tubes in successive portions of 1 ml, 2 ml, 3 ml etc. upto 10 ml. The volumes in these test tubes were made up with water upto 10 ml. The test tubes were stoppered and shaken in lengthwise motion for 15 sec., 2 shakes per sec. It was allowed to stand for 15 min. and height of the foam measured and foam index calculated [8]. |
| |
|
Determination of pH
|
| |
| pH 1% solution: Dissolved an accurately weighed 1gm of the drug in accurately measured 100 ml of distilled water, filtered and pH of the filtrate checked with a standardized glass electrode. |
| |
| pH 10% solution: Dissolved an accurately weighed 10gm of the drug in accurately measured 100 ml of distilled water, filtered and pH of the filtrate checked with a standardized glass electrode [9]. |
| |
| Powdered drug reaction with different reagents: The powdered drug was treated separately with different reagents and acids like, picric acid, hydrochloric acid, nitric acid, iodine, ferric chloride, and sodium hydroxide. The colour shown after the treatment was noted [10]. |
| |
|
Extraction of Plant materials
|
| |
| Whole Plant material was successively extracted by continuous hot extraction (Soxhlet) method: Whole Plant material was dried in shadow and powdered. The powdered material was passed through sieve no. 40 mesh, weighed & then used for extraction. The weighed powder was successively extracted with Petroleum ether, chloroform, ethyl acetate, methanol in soxhlet apparatus and final marc was extracted with water using decoction method. The resulting extracts were concentrated under reduced pressure using rotary vacuum evaporator to get the syrupy viscous masses. The viscous masses were transferred in porcelain dishes and dried. The amount of extract was weighed and stored in amber colored airtight bottle at 5-7°C [11]. |
| |
| Florescence analysis: Many herbs show fluorescence when cut surface or powder is exposed to UV light and this can be useful in their identification. The fluorescence character of the plant powders (40 mesh) was studied both in daylight and UV light (254 and 366 nm) and after treatment with different reagents like sodium hydroxide, picric acid, acetic acid, hydrochloric acid, nitric acid, iodine, ferric chloride etc. [12-13]. |
| |
| Phytochemical investigation: After collection and authentication, the plant material was shade dried and powdered. It was passed through sieve no. 40 and subjected to extraction. Weighed quantity of plant material was extracted separately with petroleum ether, chloroform, ethyl acetate, methanol and water by Cold extraction method. the plant material was also successively extracted with different solvents like petroleum ether, chloroform, ethyl acetate, methanol in soxhlet apparatus while water extract was prepared by decoction. The extracts were evaporated to dryness under reduced pressure and controlled temperature (40- 50 ºC) [14]. Total methanol extract was also prepared from the drug material by continuous hot extraction method. The extracts were subjected to preliminary phytochemical investigation for the detection of following compounds; Carbohydrates, Protein, amino acids, fats and oils, Sterols and steroids, Glycoside, coumarins, flavonoids, Alkaloids, tannins and phenolic compounds, Acidic compounds, saponins, terpenes and terpenoids, Mucilage Resins, Lipids/ Fats etc. [15-16]. |
| |
| Determination of fat content: Weighed quantity of sample (3gm) was extracted with anhydrous ether in a continuous extraction apparatus for six hours, the extract was filtered into a clean dry weighed flask. The extraction flask was rinsed with small quantity of ether, filtered and added to the weighed flask. The solvent was evaporated and dried to constant weight at 105ºC [7]. |
| |
| Flavonoid determination: 10 g of crude drug powder was extracted repeatedly with 100 ml of 80% aqueous methanol at room temperature. The whole solution was filtered through Whatman filter paper no. 42 (125 m). The filtrate was later transferred into a crucible and evaporated to dryness and weighed to a constant [17]. |
| |
| Saponin determination: 20 g of crude drug was put into a conical flask and 100 cm3 of 20% aqueous ethanol was added. The sample was heated over a hot water bath for 4 h with continuous stirring at about 55ºC. The mixture was filtered and the residue reextracted with another 200 ml of 20% ethanol. The combined extracts were reduced to 40 ml over water bath at about 90ºC. The concentrate was transferred into 250 ml separatory funnel and 20 ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated. 60 ml of n-butanol was added. The combined n-butanol extracts were washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath to remove the solvent. After evaporation, the sample was dried in the oven to constant weight and the saponin content calculated [18]. |
| |
|
Thin layer chromatography (TLC Profile) [19]:
|
| |
| Preparation of the plates: The adsorbent used for thin layer chromatography was silica gel G. About 25 g of silica gel G was taken in a glass mortar and 35 ml of distilled water was added to it. The mixture was stirred with glass rod until it became homogeneous. This mixture was then allowed to swell for about 15 minutes. Then an additional 15 ml of distilled water was added to it with stirring. The suspension was then transferred to a 150 ml flask fitted with a plastic stopper, and was shaken vigorously for about 2 minutes. This suspension was then spread immediately on thin layer chromatographic plates with the help of a thin layer chromatography (TLC) applicator (SUPERFIT of Continental Instruments, Bombay). |
| |
| Drying and storage of the plates: The freshly coated plates were then air dried until the transparency of the layer had disappeared. The plates were then stacked in a drying rack and were heated in an oven for 30 min. at 110°C. The activated plates were kept in a desicator, till required for further use. |
| |
| Application of the sample: For applying test samples on TLC plate, glass capillaries were used. The spots were applied with the help of a transparent template, keeping a minimum distance of 1 cm between the two adjacent spots. The spots of the samples were marked on the top of the plate to know their identity. |
| |
| Chromatographic chamber, conditions of saturation and the development of TLC plates: Chromatographic rectangular glass chamber (16.5 cm x 29.5 cm) was used in the experiments. To avoid insufficient chamber saturation and the undesirable edge effect, a smooth sheet of filter paper approximately of 15 x 40 cm size was placed in the chromatographic chamber in a ‘U’ shape and was allowed to be soaked in the developing solvent. After being thus moistened, the paper was then pressed against the walls of the chamber, so that it adhered to the walls. The chamber was allowed to saturate for 24 hours before use. The experiments were carried out at room temperature in diffused daylight. |
| |
| Developing solvent system: Number of developing solvent systems were tried, for each extract, and the satisfactory resolution system were noted down. |
| |
| Detector/spraying equipment: Compressed air sprayer with a fine nozzle was used to detect the different constituents present on TLC plates. Air compressor was attached to a glass sprayer. The sprayer was filled with about 50 ml of the detecting reagent and then used. After each spray, the sprayer was washed separately with water, chromic acid, and distilled water and then with acetone. UV chamber was also used for the substances exhibiting fluorescence. The results are observed at 254nm and 366 nm of UV light and in day light. |
| |
|
Thin layer chromatography profile [20]:
|
| |
|
Detection of Steroids/triterpenoids and their glycosides
|
| |
| Solvent system used for Detection of Steroids and their glycosides: |
| |
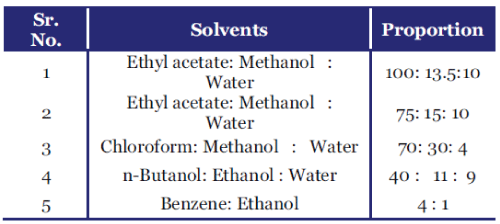 |
| |
|
Spraying reagents:
|
| |
| (i) Vanillin – Sulphuric acid (VS) reagent: |
| |
| Solution I: 5% methanolic sulphuric acid |
| |
| Solution II: 1% methanolic vanillin |
| |
| The developed TLC plate was sprayed with 10ml of solution 1, followed immediately by 5 – 10 ml of solution II, and then heated for 5 – 10 minutes at 110° C under observation. Steroids/triterpenoids and their glycosides give blue, blue-violet or pink colored spots. |
| |
| (ii) Vanillin – Phosphoric acid (VPA) reagent: Solution a: 1 gm vanillin dissolved in 100ml of 50% phosphoric acid. |
| |
| Solution b: 2 parts 24% phosphoric acid and 8 parts 2% methanolic vanillic acid. |
| |
| After spraying with either solution a or b, the plate was heated for10 minutes at 100° C. Red- violet colour indicates the presence of steroids/ triterpenoids and their glycosides. |
| |
| (iii) Antimony (III) chloride reagent: |
| |
| 20% solution antimony (III) chloride in chloroform. The developed TLC plate was sprayed with reagent and then heated for 5 – 6 minutes at 100° C. Red – violet colour in visible light; red – violet, blue and green fluorescence in UV at 365 nm indicates the presence of steroids/triterpenoids and their glycosides. |
| |
| (iv) Anisaldehyde – sulphuric acid reagent: |
| |
| 0.5 ml of anisaldehyde was mixed with 10 ml glacial acetic acid, followed by 85 ml of methanol and 5 ml of concentrated sulphuric acid, in that order. The developed TLC plate was sprayed with reagent, heated at 100° C for 5 – 10 minutes. |
| |
| Steroids /triterpenoids and their glycosides give blue, blue – violet or pink coloured spots. |
| |
|
Detection of Flavanoids and their Glycosides
|
| |
| Solvent system used for Detection of Flavanoids and their glycosides |
| |
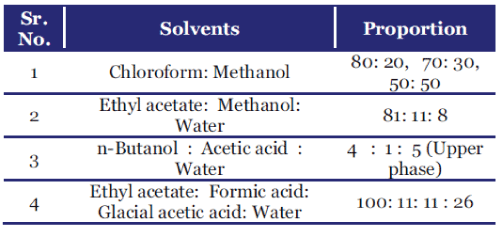 |
| |
| Detection: The developed TLC plate was observed in visible light and in UV at 365 nm Flavanoids and their glycosides appears as yellow, dark blue, orange zones /spots. The colour gels intensified on exposure of the plates to ammonia vapours. |
| |
|
Detection of Alkaloids
|
| |
| Solvent system used for Detection of Alkaloids |
| |
 |
| |
| Detection: Dragendroff’s reagent: The developed TLC plate was sprayed with reagent and then heated for 5- 6 minutes at 100°C. |
| |
|
Detection of Saponins
|
| |
| Solvent system used for Detection of Saponins |
| |
 |
| |
| Detection: Anisaldehyde – sulphuric acid reagent: The developed TLC plate was sprayed with reagent, heated at 100° C for 5 – 10 minutes. |
| |
Results
|
| |
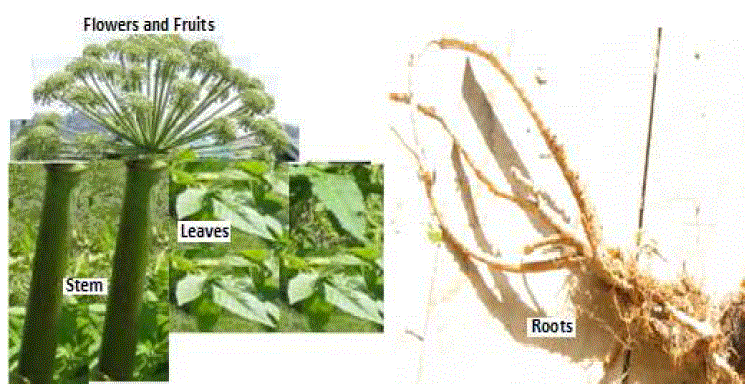
| Pharmacognostical studies of Angelica archangelica Linn.: It is Holy Angel which is used for the treatment of various diseases. The plant is shown in Figure 1. |
| |
| Organoleptic study of Angelica archangelica Linn.: The organoleptic characters of Angelica archangelica Linn. shown in Figure 2 and Table 1. |
| |
|
Physicochemical constants:
|
| |
|
Extractive value
|
| |
| Cold extractive values: The cold extractive values of whole plant material in different solvents (individual) are shown in Table 2 and Figure 3. |
| |
| Hot extractive values: The hot extractive values of whole plant material in different solvents (individual) are shown in Table 3 and Figure 4. |
| |
| Successive extractive values: The successive extractive values of whole plant material in different solvents (individual) are shown in Table 4 and Figure 5. |
| |
| Ash value: The ash values of Angelica archangelica Linn. like Total ash, Acid insoluble ash, Water soluble ash are shown in Table 5. |
| |
| Foreign matter analysis: The foreign matter in the whole plant material of Angelica archangelica Linn. is shown in Table 6. |
| |
| Foaming index: Foaming index was calculated to be more than100. |
| |
| Loss on drying: Loss on drying is depicted in Table 7. |
| |
| Swelling index: Swelling index was calculated as 2.5. |
| |
| pH Values: The pH values of 1% and 10% are shown in Table 8. |
| |
| Powdered drug reaction with different reagents: The powdered drug was reacted with reagents and the results are shown in Table 9. |
| |
| Fluorescence analysis: The powder of the whole Plant of Angelica acharengelica (mesh size 40) was examined under daylight and UV light. The observations are shown in Figure 6 and Table 10. |
| |
|
Phytochemical screening
|
| |
| Phytochemical screening of successive extracts: The phytochemical screening of successive extracts of Angelica archangelica Linn. are shown in Table 11. |
| |
| Phytochemical screening of cold extracts: The phytochemical screening of cold extracts of Angelica archangelica Linn. are shown in Table 12. |
| |
| Phytochemical screening of Total methanol extract: The whole plant of Angelica archangelica Linn. was extracted with methanol using soxhlet apparatus and subjected for phytochemical screening (Table 13). |
| |
| Fat content: The fat content of Angelica archangelica Linn. was determined (Table 14). |
| |
| Flavonoid and saponin content: The flavonoid and saponin content of Angelica archangelica Linn. were determined (Table 15). |
| |
| Thin layer chromatography profile: The TLC of extracts and successive extracts of Angelica archangelica Linn. showed the presence of compounds (Table 16 and Table 17 respectively). |
| |
Disscusion
|
| |
| During the past decade, the indigenous or traditional system of medicine has gained importance in the field of medicine. In most of the developing countries, a large number of populations depend on traditional practitioners, who in turn are dependent on medicinal plants to meet their primary health care needs. Although modern medicines are available, herbal medicines have retained their image for historical and cultural reasons. As the usage of these herbal medicines has increased, issues and the motto regarding their quality, safety, and efficacy in industrialized and developing countries have cropped up [21]. Increasing interest has forced the researcher to scientifically screen various traditional claims. There is a need for screening the traditional claims because in this scientific era, everyone is interested in the scientific support before using traditional drugs. Therefore, at present, both common users and healthcare professionals seek updated, alterative information toward the safety and efficacy of any recommended medicinal plant as a drug, prior to its use [2]. The relevance of pharmacognosy in standardization of herbal drugs was long been stressed. The process of standardization can be achieved by stepwise pharmacognostic studies. These studies help in identification and authentication of the plant material. Medicinal plant materials are categorized according to sensory, macroscopic and microscopic characteristics. An examination to determine these characteristics is the first step towards establishing the identity and degree of purity of such materials. The extractive value is used to determine the amount of active constituents. Ash values are used to determine the extraneous matter eg. Sand and soil adhering to the plant surface. Fluorescence is an important phenomenon exhibited by various chemical constituents present in plant material. Some constituents show fluorescence in the visible range in many natural products (e.g., alkaloids like berberine), which do not visibly fluoresce in day light. If the substances themselves are not fluorescent, they may often be converted into fluorescent derivatives by applying different reagents hence some crude drugs are often assessed qualitatively in this way and it is an important parameter of pharmacognostical evaluation [22]. In present investigation various standardization parameter such as organoleptic, physico- chemical paramenter, fluorescence analysis, powdered drug reaction with different reagents, phytochemical screening and TLC analysis. Saponin, fat and flavonoid contents were carried out which could be helpful in authentification of Angelica archangelica Linn. The result of present study will also serve as reference material in preparation of monograph. |
| |
Acknowledgments
|
| |
| Dinesh Kumar, ZA Bhat, and MY Shah would like to thank the Department of Pharmaceutical Sciences, University of Kashmir, Jammu and Kashmir, India for providing lab facilities and UGC – New Delhi for providing financial assistance for the present research work. |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
 |
 |
 |
 |
 |
| Table 6 |
Table 7 |
Table 8 |
Table 9 |
Table 10 |
 |
 |
 |
 |
 |
| Table 11 |
Table 12 |
Table 13 |
Table 14 |
Table 15 |
 |
 |
| Table 16 |
Table 17 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
|
| |
















