Key words
|
| |
| Adrenoceptor, Nor-adrenaline , Reverse Transcription-Polymerase Chain Reaction (RT-PCR), Ruminal artery. |
| |
INTRODUCTION
|
| |
| Vascular postjunctional α1-adrenoceptors play a primary role in the maintenance of peripheral vascular resistance and therefore, in control of systemic arterial pressure. It is now well accepted that there are three functional α1-adrenoceptor subtypes α1A, α1B, and α1D corresponding to the three cloned α1-adrenoceptors, designated as α1a, α1b, and α1d [1]. These three subtypes are characterized by high affinity for prazosin in functional and radioligand binding studies. α1-adrenoceptors with low affinity for prazosin (pA2 < 9) have also been identified in functional studies and classified as either α1L- or α1N-based on either a low affinity or high affinity to HV723, respectively [2,3]. Pathophysiology and pharmacology of the sympathetic nervous system have been conducted on laboratory animals, human beings and cultured cells and as a result, only limited data on domestic animals are available. In fact, the importance of functions controlled by the sympathetic nervous system, as well as mechanisms of signal transduction (receptors, G-proteins, cellular messengers, metabolic pathways), were extensively studied and reviewed in man, cultured cells and laboratory animals during the latter two decades of the 20th century [4-13]. In view of the physiological and pathological roles played by the adrenergic system, its tissue receptor subtypes and cellular signaling mechanisms may actually be of greater importance in ruminants in identifying new treatment strategies in ruminal disorders. |
| |
| The rumen is the first stomach of cattle, sheep and goats. It enables these animals to digest high fiber plant materials unsuitable to non-ruminants. The rumen evolved in the way it has done in order to benefit from the fiber digesting activities of the microbes. The end product, volatile fatty acids are absorbed across the rumen wall and used for energy and protein synthesis. The rumen is therefore a highly efficient organ in the context of evolution of a herbivore subsisting on poor pasture. Anatomically the rumen is supplied with left and right branches (left and right ruminal artery) of celiac artery which is a common vasculature supplying to both rumen and non-ruminant stomach. The basic knowledge on vasoreactivity of the ruminal arteries during the normal ruminal environment and ruminal disorders is far from clear. |
| |
| The regulation of sodium pump in goat ruminal artery is partly mediated by K+channel and ouabain sensitive Na+, K+ ATPase[14]. The role of α1- adrenoceptor subtypes in vasoconstriction of ruminal artery has not been reported in any ruminant animal. With a long term aim to generate the basic physiological data in determining the 'normal' response of healthy vascular tissue from goat ruminal artery with respect to α1-adrenoceptors and further translate it to ruminal medicine. In the present study an attempt has been made to characterize the α1-adrenoceptor subtypes on the basis of functional and RT-PCR study in the goat ruminal artery. |
| |
MATERIALS AND METHODS
|
| |
| Ruminal artery of freshly slaughtered goats (2.0-2.5 years old) of either sex weighing between 12.5 to 15.0 Kg obtained from local slaughter house, were employed in the present study. |
| |
|
Drugs and chemicals
|
| |
| Nor-adrenaline, Diethyl pyrocarbonate (DEPC), Prazosin hydrochloride and TRI reagent (Sigma- Aldrich, USA), RNaseZap® (Ambion, USA), Taq- DNA polymerase (New England Biolabs Inc, USA), Gene Ruler™ (MBI Fermantas, USA) and Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, USA) were used. |
| |
| Functional studies |
| |
| The right ruminal artery was traced from main celiac artery supplying to the right ventral and dorsal sac of rumen. Ruminal artery (4-5 cm long) was carefully dissected out from the ruminal wall towards the anterior end before its bifurcation as per anatomical description of Wesley and Alvin [15]. The ruminal artery was immediately placed in aerated ice-cold Modified Krebs’s solution and brought to the laboratory. Arteries were cleared of fat and connective tissue and cut into rings of about 2-2.5 mm in length. The arterial ring was then mounted between two stainless steel L-shaped hooks made of 28 gauge stainless steel wire and kept under resting tension of 2 g in a thermostatically controlled (370 ± 0.50C) automatic organ bath (Pan Lab, spain ) of 20 ml capacity, containing MKHS and was aerated continuously with air. The arterial rings were equilibrated for 1.5 h before recording the muscle tension. During this period, the bathing fluid was changed every 15 min. The change in tension was measured by a highly sensitive isometric force transducer (Model: MLT 0201, AD instruments, Australia) and recorded in a PC using chart 6.0 Pro software. After equilibrating the arterial rings in modified KHS, NA (10ηM – 300 µM) was added to bath in a cumulative manner to obtain concentration-related contractile response (CRCs). Threshold concentration to maximal contractile response (Emax) was recorded. Then, the rings were washed by modified KHS at 15 min interval. After 45 min incubation period, again NA (10ηM – 300 µM) was added to the bath cumulatively to get concentration-related contractile response. Further, the arterial rings were incubated with prazosin (0.01 or 0.1 or1 ηM) for 15 min period prior to addition of NA (10ηM – 300 µM) to bath in a cumulative manner to examine the blockade of α1- adrenoceptor. |
| |
| Statistics |
| |
| The data were expressed as percentage of the maximum response to agonist obtained in the absence of antagonist (control) and analysed by the interactive non-linear regression through the computer program GraphPad Prism (GraphPad Prism Software, San Diego, CA, USA). The pD2 and EC50, concentration that produces 50 % of agonist maximum response, was determined. |
| |
|
mRNA expression studies
|
| |
| Isolation of total RNA- Ruminal artery of goat was collected immediately after slaughter and transported under ice to the laboratory for further processing. The ruminal artery was dissected in DEPC (0.01 %) treated PBS (pH 7.4) and collected in 1 ml of TRI reagent™ for each 100 mg of tissue. To minimize the effect of ribonuclease activity, properly DEPC-treated and sterilized laboratory wares were used. Total RNA was isolated using TRI reagent following the manufacturer’s instructions. The purity of isolated RNA was checked by A260/A280 ratio and the RNA quantity was determined on the basis of 40 µg of RNA having one O.D. at 260 nm. cDNA synthesis -cDNA from total RNA as template was synthesized using Oligo dT as primer as per Transcriptor First Strand cDNA Synthesis Kit protocol and stored at -20oC. This cDNA was used for polymerase chain reaction. |
| |
| Primer designing-Specific primers were designed for α1a/d-adernoceptor from the published sequence in GenBank, USA (Accession No: L31772) using online Primer 3 software. Forward (5’- GTGCGCCACTCACTCAAGTA-3’)and Reverse (5’-GAGCACACG GAGGAGAAGAC-3’) primers were procured from OPERON Biotechnologies, Germany. |
| |
| PCR protocol-The PCR amplification was carried out in automated thermal cycler (MJ Research, USA) with following programme with one cycle of initial denaturation at 94°C for 5 min followed by 25 cycles of denaturation at 94°C for 10 sec; annealing at 60°C for 30 sec; Extension 72°C for 30 sec than one cycle of final extension at 72°C for 7 min and suddenly cooled at 4°C for 10 min. After completion of thermal cycle program the PCR products were stored at -20°C . |
| |
| Confirmation of RT- PCR product-The amplified PCR product was confirmed for their size in 1.5 % (w/v) agarose gel stained with ethidium bromide with 100 bp DNA ladder. The electrophoresis was carried out in a horizontal submarine gel electrophoresis apparatus at 100 V for 1 h and visualized under UV light in a Gel documentation apparatus. |
| |
Results
|
| |
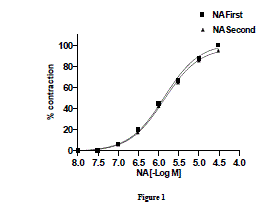
| Effect of noradrenaline on goat ruminal artery rings-Nor-adrenaline (10ηM–300 µM) added cumulatively induced a concentration dependent contractile response on goat ruminal artery. The threshold concentration and concentration for maximal response (Emax) were 0.3 and 30 µM, respectively. In order to examine the desensitization to subsequent response to NA, the tissues were allowed for 30 min washing with modified KHS. Subsequent addition of NA to bath elicited a concentration-related response with threshold concentration and concentration for maximal response (Emax) at 0.3 and 30 µM, respectively (Fig.1). Two consecutive NA-induced concentration-related response curves did not differ significantly from each other. The EC50 were 0.729 and 0.726 µM, respectively. |
| |
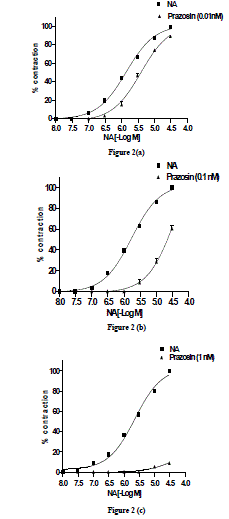
| Effect of prazosin (0.01ηM) on NA-induced concentration-related contractile response-The threshold concentration and concentration for maximal response (Emax) were 0.3 and 30 µM. After complete relaxation, in presence of prazosin (0.01ηM), NA also elicited a concentration- related response curve with threshold concentration and concentration for maximal response (EBmax) occurring at 0.3 and 30 µM, respectively (Fig. 2a). The EC50 of the NA-induced concentration-related response curve in absence and presence of prazosin (0.01ηM) were 0.73 and 0.26 µM, respectively. The PD2 value was expressed as SEM (Table 1). |
| |
| Effect of prazosin (0.1ηM) on NA-induced concentration-related contractile response-NA in presence of prazosin (0.1ηM) also elicited a concentration-related response with threshold concentration and concentration for maximal response (EBmax) at 0.3 and 30 µM , respectively (Fig. 2b). There was a parallel rightward-shift of NA-induced concentration-related response curve in presence of prazosin (0.1ηM) with a significant (P <0.001) increase in EC50 value. The EC50 obtained from NA-induced concentration-related contractile response in absence and presence of prazosin was 0.59 µM and 2.5 µM, respectively. The pD2 value was expressed as SEM (Table1). |
| |
| Effect of prazosin (1ηM) on NA-induced concentration- related contractile response-NA in presence of prazosin (1ηM) also elicited a concentration- related response curve with threshold concentration and concentration for maximal response (EBmax) at 10 µM and 30 µM, respectively (Fig. 2c). In presence of prazosin (1ηM) the EBmax did not attain 35 % maximal response; the NAinduced concentration-related response curve in presence of prazosin (1ηM) was expressed as percentage of inhibition 91.2 ± 0.17 %. The pD2 value was expressed as SEM (Table 1). |
| |
|
mRNA expression studies
|
| |
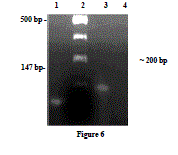
| Identification of α1a/d-adrenoceptor mRNA by RTPCR- α1a/d-adrenoceptor was amplified by RT-PCR with total RNA from goat ruminal artery (Fig. 3), Lane 1 shows a 147 bp PCR product which resulted from the control RNA and specific primers provided with the kit. DNA 100 bp ladder (Lane 2). The primer designed from sequence coding for α1a/dadrenoceptor (Accession no: L31772) was able to amplify cDNA from ruminal artery (Lane 3) with a PCR product of predicted size (~200 bp). Similarly, no PCR product was detected when RNA was used instead of cDNA in PCR, demonstrating that amplified products were from cDNA and not from genomic DNA (Lane 4). |
| |
Discussion
|
| |
| The process by which cellular sensitivity to hormone, neurotransmitter and drug stimulation becomes attenuated over time is termed desensitization [16]. Different mechanisms for desensitization have been described for adrenergic receptors: functional uncoupling of the receptor, physical sequestration of the receptor away from the cell surface and down-regulation of the total number of receptors [17,5,18,19].The time frames over which these processes occur range from seconds (phosphorylation) to minutes (endocytosis) and hours (down-regulation). The underlying biochemical mechanisms in process of desentization are regulated by second messenger-regulated kinase (e.g. protein kinase A or PKA and protein kinase C or PKC), G-protein coupled receptor kinase (GPCRK) (e.g. β-adrenergic receptor kinase or βARK) and the arrestins (visual and non-visual)[18]. |
| |
| Further classification on the basis of degree of desensitization of α1A, α1B and α1D receptor subtypes suggested that α1A is moderately desensitized, α1B is highly desensitized and α1D is least desensitized [20]. |
| |
| In the goat ruminal artery , as reported in bovine mammary artery [21], there was no significant difference in the curve fit parameters after repeating concentration related response curve to NA at interval of 45 min, suggesting that the adrenergic receptor present in this tissue exhibits least evidence of desensitization or tachyphylaxis which could be due to predominant presence of α1D receptor subtype as suggested by Piasick and Perez [20]. |
| |
| The sensitivity to NA has been reported from bovine species while characterizing α1 receptor subtypes through pEC50 or PD2 values in different vascular smooth muscles. A higher PD2 value in rat testicular capsule for NA was 7.9 ± 0.11 [22] and has been confirmed that this tissue expresses both α1A and α1B but not α1D receptor using molecular techniques. PD2 value in bovine mammary artery was 5.97± 0.07 and suggested major involvement of α1B subtype in contraction of bovine mammary artery which is similar to human internal mammary artery [21]. Similarly, the PD2 value of NA in spleenic artery strips of pigs (6.94) [23] ,bovine oviduct artery (5.67)[24], calf digital artery (5.92)[25] and bovine intra-mammary artery (6.87)[26] represents a greater variation in the sensitivity to adrenergic receptor agonists basing on tissue type. In term of rank order, potency of agonist by pEC50 values, there was great difference between the potency of NA in different vascular smooth muscles within ruminant species. So, it may be suggested that there is a significant difference in potency order in different vascular smooth muscles obtained from same species. Further, it cannot be correlated with the PD2 values of α1 selective agonist obtained from pharmacological data with level of expression of this receptor in the same tissue using molecular techniques. In our present study, we observed that the PD2 value for NA was 5.86 ± 0.02 which is at lower potency range and close to the PD2 values for NA in bovine mammary artery and calf digital artery. |
| |
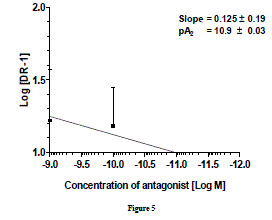
| pA2 value is unique for that antagonist acting on a specific receptor and is a measure of the affinity of the drug-receptor complex. The pA2 values of prazosin against NA reported in different vascular smooth muscles of different species vary from a low of 11.2 in rat aorta [27] to a high of 7.2 in rabbit renal artery [28]. Similarly, in bovine caudal median and oviductal artery pA2 for prazosin was 8.74 and 9.38, respectively[29] which concluded that α1- adernoceptor is mainly involved in such contraction. pA2 values for prazosin against NA in different tissues of rat were 8.8 in caudal artery[30], 9.2 in epididymal vas deferens, 9.3 in spleen vein and 9.4 in portal vein[31] 9.8 in aorta[32]. Similarly, pA2 for prazosin in perfused kidney (9.3), in perfused mesentery (9.5), in aortic rings (9.6)[33] and seminal vescicle (9.52) [34] in rat suggests a great variability in the affinity of α1-adrenoceptor to prazosin. Prazosin inhibited phenylephrine-induced contraction with pA2 value of 9.47 in bovine tail artery and it has been concluded that α1A and α1Dadernoceptors may mediate contraction in this tissue [35]. More than one α1 subtype, with predominant α1B is involved in contraction of bovine intra-mammary artery where the pA2 value of prazosin against norepinephrine was 10.46[26]. Gow et al.[21] reported pA2 value for prazosin in bovine mammary artery as 8.7 and they concluded that α1A is involved in contraction but, the role of α1B cannot be excluded. Thus, it can be easily predicted that in bovine vascular tissues, the pA2 value for prazosin ranges from 9-10 in general, against NA. Only exception found was with bovine intra-mammary artery where it is 10.46. There was no clear cut correlation of the pA2 value of prazosin against nor-epinephrine with expression of different α1-subtypes. In goat ruminal artery, pA2 value (10.9 ± 0.03) of prazosin is consistent for α1 -adrenoceptors subtypes and falls within the range of pA2 values (7.2-11.2) so far reported. Keeping in view the ranges pA2 values reported in several vascular smooth muscles and its relationship with the presence of different α1- subtypes, we hypothesize that α1a/d may be possible subtype which mediate ruminal vasoconstriction. |
| |
| Studies in animal blood vessels have indicated the correlation between mRNA or protein expression levels of α1-AR subtypes and the functional roles of these receptors in vasoconstriction[36,37]. In most cases, although all α1-AR subtypes are expressed at the protein level [38] or mRNA level[39], the correlation between protein or mRNA expression of one α1-AR subtype and the functional roles of these receptors in vasoconstriction has been elusive [40,41].The result of mRNA expression studies of α1- adrenoceptor subtype in ruminal artery agrees with the result of functional studies. RT-PCR studies confirmed that α1a/d transcript is present in goat ruminal artery. Functional studies along with RTPCR technique in which α1a/d-adrenoceptor transcript has been detected indicates that α1a/d - adrenoceptor subtype may be involved in the contractile response produced by NA in the goat ruminal artery. Although, the level of expression of receptor mRNA in a given tissue may not always correlate directly with the levels of receptor protein, taking our observation into consideration, it is necessary to sequence the mRNA transcript. Combining two observations that the least degree of desensitization against NA and pA2 value (10.9 ± 0.03) of prazosin in this tissue it could be concluded that the GRA expresses a major population of α1dadrenoceptor subtype. Functional studies using selective agonist and antagonist would further support the involvement of proposed α1- adrenoceptorr subtypes in mediating contractile response in goat ruminal artery. |
| |
Acknowledgment
|
| |
| The authors are thankful to Orissa University of Agriculture and Technology, Bhubaneswar for financial support in form ICAR development grant during the study. |
| |
Conflict of Interest
|
| |
| NONE |
| |
Source of Support
|
| |
| NIL |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |










