Blake T Dotta, Carly A Buckner, Robert M Lafrenie and Michael A Persinger*
Biomolecular Sciences Program, Laurentian University, Sudbury, Ontario, Canada P3E 2C6
*Corresponding Author:
Michael A Persinger
Biomolecular Sciences Program
Laurentian University, Sudbury
Ontario, Canada
Tel: 01-705-675-4824
Fax: 01-705-671-3841
E-mail: mpersinger@laurentian.ca
Received date: June 08, 2016; Accepted date: August 20, 2016; Published date: August 24, 2016
Citation: Dotta BT, Buckner CA, Lafrenie RM, et al. Photon Emissions as Differential Indicators for Different Components of Protein Kinase A (PKA) in Transfected Murine Melanoma Cells. Arch Can Res. 2016, 4: 3.
Keywords
Ultraweak photon emissions; PKA; Regulatory domain; Catalytic domain; Inhibitor domain; Melanoma cells; Intercellular communication
Introduction
There are a variety of signaling pathways involved with the induction of conditions that promote aberrant cellular division and tumor formation. Although the numbers of different types of cells that can be differentiated by histological criteria are within the hundreds, the myriad of experiments during the last half-century of molecular biology have suggested common pathways [1]. The identification of these pathways is often tedious, labour-intensive and expensive. Several recent experiments [2-4] have demonstrated that the measurement of photon emissions from malignant cells and the extraction of the spectral power densities of the amplitude-modulation of the visible band can differentiate normal and abnormal conditions. Quick measurements of photon profiles that discern components of activation from regulatory subunits of primary molecules in signaling pathways may allow alternative methods to monitor cellular states. Here we present clear evidence that transfection with different subunits of Protein Kinase A (PKA) which differentially regulates PKA activity and impact major pathways involved with cellular function produced differential magnitude and temporal patterns of ultraweak photon emissions from transfected melanoma cells.
Photons exhibit unique features that constitute properties of both waves and particles. Theoretically photons have the capacity to mediate the interface between matter whose functions are determined by spatial (molecular) structure and energy whose functions are determined by the temporal pattern of the sequences [5]. Dotta et al. [3], who employed Cosic’s model involved with recognition by molecular resonance, demonstrated a clear relationship between specific wavelengths of photon emissions from mouse melanoma cells and specific molecules that either inhibited or facilitated these emissions. During the early 20th century Gurwitch [6] had postulated that all living systems emit photons. Popp [7] extended this concept and suggested that since all energy that presently composed living systems originated from the sun, “virtual” photons might be stored within every cell. Persinger [8] showed quantitatively that the ubiquitous approximately 10-12 W·m-2 of photon flux density emitted by living system was consistent with a fraction of spontaneous ‘re-emission’ of the accumulation of solar photon energy since Life evolved. Trushin [9] Fels [10] and Bajpai et al. [11] have argued that these photon emissions from cells and bacteria may reflect the fundamental method of information transfer. Like action potentials whose energy is contained within the axonal membrane and not carried by the propagating spike, the photons between cells contain the information or “keys” that initiate the powerful forces and energies that are selfcontained with the molecular pathways.
Protein Kinase-A [1] has been described as a family of enzymes whose primary activities are dependent upon the concentrations of cyclic AMP within cells. Cell-modulating molecular structures circulating within the vasculature such as adrenalin and glucagon initiate the cascade that triggers the dynamics of PKA. Four cyclic AMP molecules activate a single PKA macromolecule that augments but does not participate directly in subsequent reactions. A pair of cyclic AMP molecules is sequestered by each of two regulatory subunits which detach from the catalytic subunits that become activated. The catalytic subunits ultimately contribute to the phosphorylation of other proteins. From some perspectives PKA markedly influences most if not all of the cellular responses associated with the cyclic AMP as well as other specific enzymes, ion channels (particular calcium channels), H1 (histones) and CREB (cyclic AMP response element binding factor) involved with promoter selections that stimulate transcription. Considering the role PKA plays in malignant and non-malignant cells and the availability of different subunits, this enzyme was considered optimal to explore photon emission correlates.
Method
All experiments and each treatment involved a minimum of three replicates and were conducted over a period of several months. B16-BL6 murine melanoma cells [12,13] were transiently transfected with PKA-CA (MGC-6169, Clontech), PKA-REG (MGC-11864, Clontech), or PKA-DN (Addgene 16716). Background readings were obtained from the vector only control mCherry vector (Clontech) condition [14]. Only one transfected condition was tested per day. The PKA-DN constructs corresponded to the regulatory domain that contained the mutations in the cAMP binding sites.
The experimentally manipulated or control melanoma cells were maintained on cell culture plates as specified [15]. The cells were grown to approximately 80-90% confluence 48hr post-transfection when the photon readings were measured (corresponding to approximately 106 cells per dish). Due to equipment availability photon measurements were obtained for only one plate per day. Each dish was placed within a blackpainted box that was covered with several layers of thick black terrycloth in a dark room. The plate was placed over sensor for the digital photomultiplier unit described previously [3]. The output was measured by a laptop computer. Sampling times, depending upon the experiment, were once every 2.5 s for the long-duration (several hours) experiments and 50 Hz (20 ms increments) for the short-term measures. All analyses involved SPSS PC 16 software.
Results
For part 1 of the study that involved the measurement of photon emissions for 22.5 hrs after removal from the incubator, the results were conspicuous as shown in Figure 1. Those cells transfected with regulatory or catalytic domains of PKA units exhibited markedly increased photon counts for the first approximately 300 s (5 min). On the other hand the typical melanoma cells (untransfected) and control vector transfected melanoma cells displayed <50 counts per s. More specifically during the first 5 min there was a factor of 200 increase in photon emissions from the PKA transfected cells compared to the control cells with PKA activity.
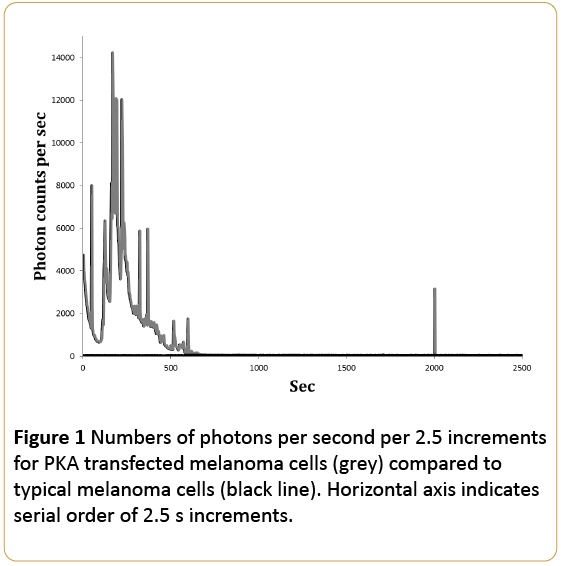
Figure 1: Numbers of photons per second per 2.5 increments for PKA transfected melanoma cells (grey) compared to typical melanoma cells (black line). Horizontal axis indicates serial order of 2.5 s increments.
On the other-hand the cells that were transfected with the dominant negative inhibitory PKA plasmid, a type of control for experimental manipulation of cell dynamics, did not display the massive increase in photon emissions. As shown in Figure 2, the photon emissions ever over 22.5 hrs was within the typical range of melanoma cells removed from incubation. The only discrepancy was the burst that occurred about 4 hrs later. However the magnitude was not comparable to the photon emissions measured during the first 5min from the cells transfected with the regulatory or catalytic PKA domains.
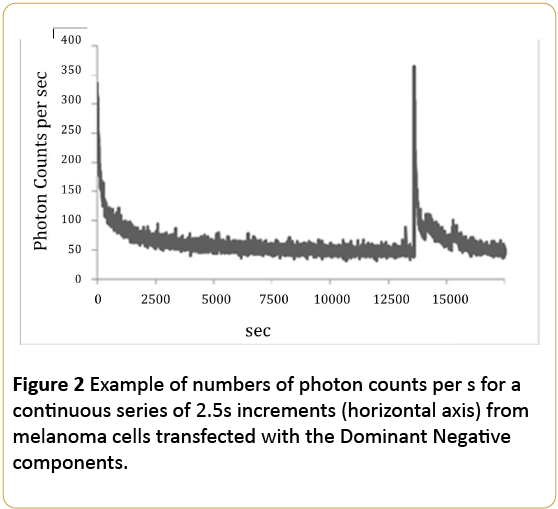
Figure 2: Example of numbers of photon counts per s for a continuous series of 2.5s increments (horizontal axis) from melanoma cells transfected with the Dominant Negative components.
The average photon counts per second for the first hour of measurement for the control (typical melanoma), PKADominant Negative (DN), PKA Catalytic Subunit (CA) and PKA Regulatory Subunit (REG) expressing plasmids are shown in Figure 3. The values for the mCherry empty plasmid control (not shown) produced no significant differences from baseline conditions (cells with no treatment). One way analyses of variance demonstrated a statistically significant difference [F(3,22)=3.20, p<0.05; eta2=0.33] in numbers of photon emissions between the groups. Stated alternatively one-third of the variance in photon emissions were explained by the experimental manipulations.
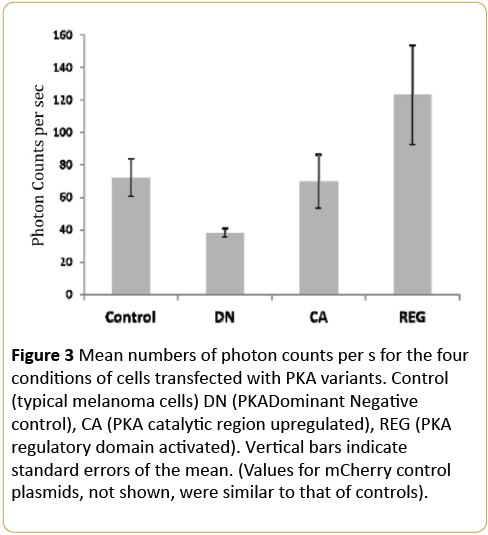
Figure 3: Mean numbers of photon counts per s for the four conditions of cells transfected with PKA variants. Control (typical melanoma cells) DN (PKADominant Negative control), CA (PKA catalytic region upregulated), REG (PKA regulatory domain activated). Vertical bars indicate standard errors of the mean. (Values for mCherry control plasmids, not shown, were similar to that of controls).
The more protracted display of photon emissions over 9, 20 min blocks as a function of the different experimental conditions as well as the effects of the chemotherapeutic agent are shown in Figure 4. Again the marked increase in average photon counts per 20 min block for the first 40 min was emitted by the enhanced regulatory unit transfection compared to all other groups. The effects of the drug increased photon counts compared to all other treatments for the first 40min, including the negative control (DN). Clearly the most significant enhancement of photon emissions occurred for the cells transfected with the regulatory subunit which was evident for at least 3 hrs.
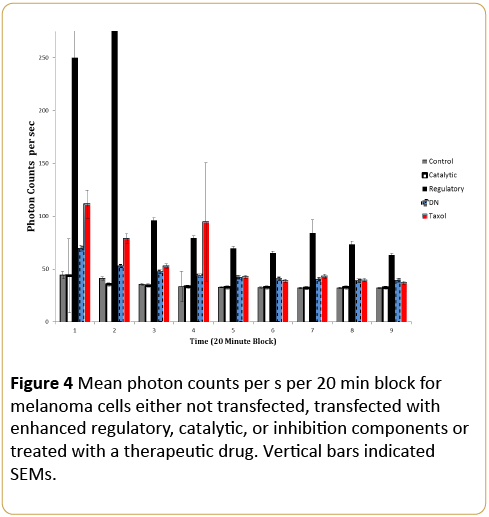
Figure 4: Mean photon counts per s per 20 min block for melanoma cells either not transfected, transfected with enhanced regulatory, catalytic, or inhibition components or treated with a therapeutic drug. Vertical bars indicated SEMs.
To demonstrate protein expression after 48 hours, a Western blot analysis was conducted and can be seen in Figure 5. This clearly illustrates the lower exposure time of Vectoronly and PKA-CA when compared to PKA-DN and PKA-REG.
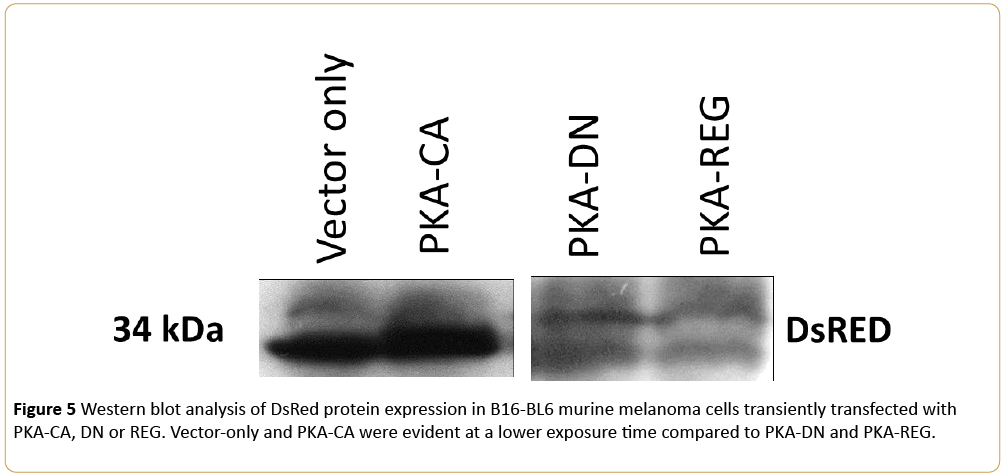
Figure 5: Western blot analysis of DsRed protein expression in B16-BL6 murine melanoma cells transiently transfected with PKA-CA, DN or REG. Vector-only and PKA-CA were evident at a lower exposure time compared to PKA-DN and PKA-REG. Archives in Cancer Research ISSN 2254-6081 Vol.4 No.3:100 2016 ©
Discussion
If photons contain the information that control the vectorial characteristics of inter-cellular or intraorganismic interactions, then the numbers of photons should be qualitatively and quantitatively correlated with those molecular components that are known to control the dynamics of a cell. We have assumed that the actual massive amounts of energy that control the molecular machinery are contained with the glucose metabolism-based chemical processes that are determined by the genetics of the cell. The photons would be the local and non-local determinants of the information that initiates or terminates these processes. One metaphor is that the energy and forces required to move a significant mass such as an automobile are substantial. However its initiation requires the small effort of turning the key in the ignition and its direction involves a small effort to turn the steering wheel.
Previously Murugun et al. [12] showed that injecting morphine into typical melanoma cells produced brief bursts of photons. The magnitude was considered sufficient to facilitate the known metastases associated with this opiate. Karbowski et al. [13] showed that only the introduction of melanoma cells into normal cells such that the former constituted only 1% of the population was sufficient to increase photon emissions. This flux density would be sufficient to affect substantial distances particularly if non-locality was involved [16]. Dotta et al. [3] had shown that during states as the melanoma cells adjusted to ambient temperatures there were predictable shifts in the peak wavelength of the photon emissions across the visible spectrum. All of these experiments support the hypothesis that photons may be the source of information that triggers the determinant pathways that lead to malignancy and its proliferation.
In the present study melanoma cells transfected with PKA demonstrated an extraordinary enhancement of photon emissions as shown in Figure 1. Assuming the average of 8000 photons per s during the first 6 to 8 min, this would be equivalent to more than 10-11 W·m-2. This is at least a factor of 10 greater than photon flux densities emitted by typical melanoma cells. On the other hand cells transfected with the PKA dominant negative vectors where PKA was inhibited by the transfection, showed photon emissions in the order of 300 photons per, or about 10-12 W·m-2 which is more typical of ultraweak photon emissions from typical melanoma cells and even from non-malignant brain slices [17].
The implications of the differential photon emissions between the regulatory and catalytic components of PKA are significant. The regulatory subunit normally interacts with the catalytic domain of PKA to inhibit activity but when it sequesters cyclic AMP it dissociates from the catalytic domain that then becomes capable of enzymatic activity. There is also strong evidence that the regulatory subunits can exhibit additional biological activity. Considering these functions the markedly increased photon emissions when cells were transfected with this component as well as the protracted emission would be expected. The catalytic subunit, the active site for PKA, which contains a domain to sequester the molecules for phosphorylation (and binds ATP) would be expected to exhibit more brief and discrete activity which would require less photon density. This inference was supported by the measurements. In summary these results indicate that the temporal profile of photon flux density might be employed as a tool to discern which component of a signaling pathway has been differentially activated.
Acknowledgements
Thanks to Dr. W. E. Bosarge, CEO of Capital Industries and to the Bosarge Family Organization for supporting this research. M7 pdnPKA-GFP was a gift from Randall Moon (Addgene plasmid #16716).
11077
References
- Albert B, Johnson A, Lewis J, Raff M, Roberts K, et al. (2002) Molecular biology of the cell. Garland Science: N.Y.
- Dotta BT, Buckner CA, Cameron D, Lafrenie RM, Persinger MA (2011) Biophoton emissions from cell cultures: biochemical evidence for the plasma membrane as the primary source. Gen Physiol Biophys 30: 301-309.
- Dotta BT, Murugan NJ, Karbowski LM, Lafrenie, RM, Persinger MA (2014) Shifting wavelengths of ultraweak photon emissions from dying melanoma cells: their chemical enhancement and blocking are predicted by Cosic’s theory of resonant recognition model for macromolecules. Naturwissenschaften 101: 87-94.
- Van Wijk R, Van Aken JM (1992) Photon emission in tumor biology. Experientia 48: 1092-1100.
- Persinger MA (2015) Thixotropic phenomena in water: quantitative indicators of Casimir-Magnetic transformations from vacuum oscillations (virtual particles). Entropy 17: 6200-6212.
- Gurwitch AG (1926) Das problem der zelltilung physiologish betrachtet. Springer: Berlin.
- Popp FA (1979) Coherent photon storage of biological systems. In Electromagnetic Bioinformation, Popp FA, Becker G, Konig HL and Peschka W (eds) Urban and Schwarzenberg: Munich 123-149.
- Persinger MA (2016) Spontaneous photon emissions in photoreceptors: potential convergence of Arrhenius reactions and the latency for rest mass photons to accelerate to Planck unit energies. Journal of Advances in Physics 11: 3529-3535.
- Trushin MV (2004) Light-mediated conversation among microorganism. Microbiol Res 159: 1-10.
- Bajpai R, Brizhik L, Del Giudice E, Finelli F, Popp FA, et al. (2010) Light as a trigger and a probe of the internal dynamics of living organisms. J Acpunct Meridian Stud 3: 291-297.
- Murugun NJ, Dotta BT, Karbowski LM, Persinger MA (2014) conspicuous bursts of photon emissions in malignant cell cultures following injections of morphine: implications for cancer treatment. Int J Cur Res 6: 10588-10592.
- Karbowski LM, Murugan NJ, Dotta BT, Persinger MA (2015) Only 1% melanoma proportion in non-malignant cells exacerbates photon emissions: implications for tumor growth and metastases. International Journal of Cancer Research and Molecular Mechanisms 1: 2.
- Zhang L, Bewick M, Lafrenie RM (2002) Role of Raf-1 and FAK in cell density-dependent regulation of integrin-dependent activation of MAP kinase. Carcinogenesis 23: 1251-1258.
- Karbowski LM, Harribance SL, Buckner CA, Mulligan BP, Koren SA, et al. (2012) Digitized quantitative electroencephalographic patterns applied as magnetic fields inhibit melanoma cell proliferation in culture. Neuroscience Letters 523: 131-134.
- Dotta BT, Persinger MA (2012) “Doubling” of local photon emissions when two simultaneous, spatially-separated, chemiluminescent reactions share the same magnetic field configurations. J Biophys Chem 3: 72-80.
- Isojima Y, Isoshima T, Nagai K, Kikuchi K, Nakagawa H (1995) Ultraweak biochemiluminesence from rat hippocampal slices. NeuroReport 6: 658-660.










