YESUFU, HB1. , BASSI*, PU2. KHAN, IZ1. ABDULRAHAMAN, FI1 and MOHAMMED, GT3
1Chemistry Department, Faculty of Sciences, University of Maiduguri, P.M.B 1069, Borno State.,Nigeria
2Department of Clinical Pharmacology and Therapeutics, College Of Medicine2 University Of Maiduguri, P.M.B 1069, Borno State,Nigeria
3Department of Pharmaceutics and Pharmaceutical Microbiology,Faculty of Pharmcy, University of Maiduguri PMB 1069, Borno state, Nigeria”
*Corresponding Author:
Dr Peter U Bassi MBBS,MSc
FMCP[Nig] Dept. of clinical Pharmacology and Therapeutics College of Medical Sciences
PMB 1069, University of Maiduguri Borno State, Nigeria
Tel: +2348034945067
E-mail: pubassi@gmail.com
Key words
Sarcocephalus latifolius, aqueous extract, phytochemicals, liver function, hepatoprotective, rats.
Introduction
The plant African peach [S.latifolius] is of the family Rubiaceae. It is a multistemmed tree or shrubs up to 12m found in undisturbed fringing forest and close savannah woodland [1]. Its generic name is derived from the Greek word sarco [fleshy] and cephalus [headed] in reference to the flowers. The specific epithet is derived from the latin word lati [broad] and folius [leaved] [2]. A hermaphrodite tree flowering from April-June and fruits ripen from July-September [3]. The grey baboon [Papio anubis] disperses its seed [4]. “Earlier reports on the various medicinal uses of this plant have been reported by many traditional medicine practitioners to be effective in the treatment and management of many ailments such as febrile illnesses, stomach disorder, cough, malaria fever and jaundices [5]. Others includes constipation, dysmenorrhoea, abscesses, vomiting and threatened abortions [6].Clinically S.latifoliues has been shown to paralyses Trichostrongylus columbriformis larvae in a concentration dependent manner [7], lower blood glucose level in normal and alloxan induced rats [8].It has also demonstrated high anti fungal activity against Macrophomina phaseolina, the causal agent of dry root decay in pawpaw[9]”.
Material and Methods
Sample collection and preparation: The plant material was collected from Gyella, Mubi South Local Government Area of Adamawa State and authenticated by Prof. S.S. Sanusi of the Department of Biological Sciences, University of Maiduguri, Borno State, Nigeria. The sample was washed, air-dried and pulverized using mortar and pestle. The pulverized sample [250g] was boiled with distilled water [2 litres] for one hour. The extract obtained was concentrated in vacuo at 40ºC and stored at 40ºC until used.
Phytochemical Analysis
Phytochemical test was carried out to determine the presence of carbohydrates, tannins, saponins, alkaloids, steroidal glycosides and flavonoids by simple qualitative standard methods [10], [11],[12].
Animals
White albino rats of mixed sexes weighing 200-230g were obtained from the Department of Human Anatomy Animal House, University of Maiduguri. They were housed in standard cages and fed with growers mash [Sander Nigeria Ltd] and water ad libitum.
Experimental Procedure
The rats were weighed and assigned into five groups of five animals each. Group A was orally fed with a single dose of normal saline [5ml/kg body weight] daily and served as control. Animals in group B, C and Dwere orally fed with 100mg/kg, 200mg/kg and 300mg/kg aqueous root bark extract respectively. This was administered to the rats daily for 21 days using feeding tube. Group E was introduced on the last day of treatment and injected intraperitoneally alongside other groups [, B, C, D and E] with equal dose of [3mg/kg body weight] carbon tetrachloride [CCl4] to induce liver toxicity. The rats were sacrificed by humane decapitation 24hrs after injecting the toxin [CCl4]. Blood was allowed to clot for 30 minutes and then centrifuged at 2500 rpm for 15 minutes and serum harvested [13]. Liver function tests for alanine transferase [ALT], aspartate transaminase [AST], total and direct bilirubin were done calorimetrically using reagent kits [Randox lab. Ltd] [14].
Statistical Analysis
Data collected from the biochemical parameters assayed were summarized as Mean ± S.E.M. Difference between individual groups was assessed by the student t-test. P value less or equal to 0.05 was considered statistically significant [15].
Results
Phytochemical analysis of the ethanol extract of S.latifolius, showed that tannins are present at high concentrations, while flavonoids, alkaloids, carbohydrates are present at moderate concentration and saponins, Cardiac glycosides, and Terpenoids/ steroids were present in low concentration. The results obtained are shown in Table 1. The aqueous root bark extract of S.latifolius was found to significantly [P<0.05] inhibit the effect of CCl4 on the liver and the protection was dose dependent. For aspartate transaminase [Table 2] percentage protection were 0.67, 18.33 and 44.33% for groups treated with 100mg/kg, 200mg/kg and 300mg/kg of the aqueous extract and injected intraperitoneally with toxin [CCl4]. For alanine transferase [Table 3] percentage protection were 0.5, 5.0 and 8.1% for group treated with 100mg/kg, 200mg/kg and 300mg/kg of the aqueous extract and injected intraperitoneally with toxin [CCl4]. Percentage protection for total bilirubin [Table 4] were 3.67, 18.60 and 37.00% for groups treated with 100mg/kg, 200mg/kg and 300mg/kg of the aqueous extract and injected intraperitoneally with toxin [CCl4]. Percentage protection for conjugated bilirubin [Table 5] were 19.0, 20.4 and 47.6% for groups treated with 100mg/kg, 200mg/ kg and 300mg/kg of the aqueous extract and injected intraperitoneally with toxin [CCl4].
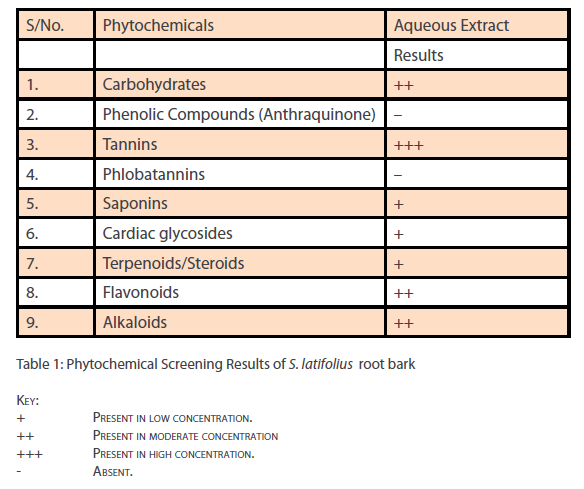
Table 1: Phytochemical Screening Results of S. latifolius root bark
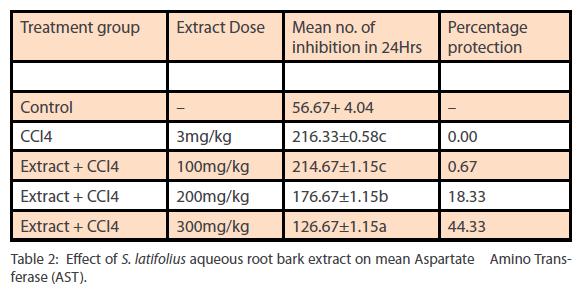
Table 2: Effect of S. latifolius aqueous root bark extract on mean Aspartate Amino Transferase (AST).
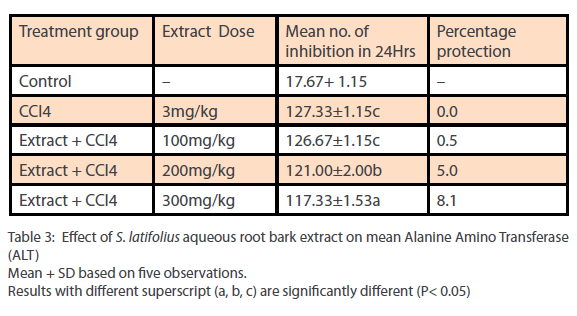
Table 3: Effect of S. latifolius aqueous root bark extract on mean Alanine Amino Transferase (ALT) Mean + SD based on five observations. Results with different superscript (a, b, c) are significantly different (P< 0.05)
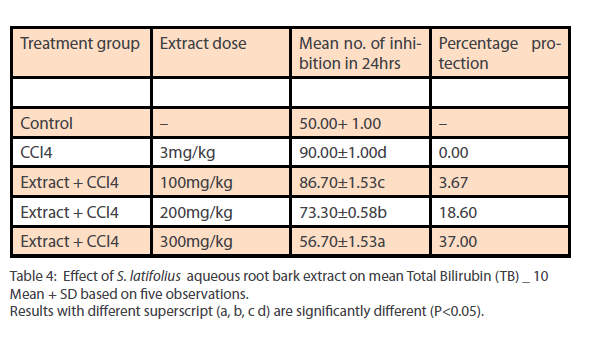
Table 4: Effect of S. latifolius aqueous root bark extract on mean Total Bilirubin (TB) _ 10 Mean + SD based on five observations. Results with different superscript (a, b, c d) are significantly different (P<0.05).
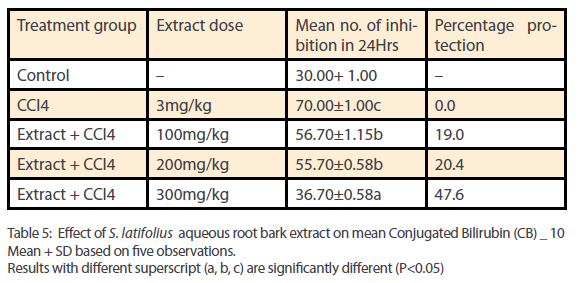
Table 5: Effect of S. latifolius aqueous root bark extract on mean Conjugated Bilirubin (CB) _ 10 Mean + SD based on five observations. Results with different superscript (a, b, c) are significantly different (P<0.05)
Discussion
Carbon tetrachloride [CCl4] is one of the most commonly used hepatotoxins in experimental studies of liver diseases [16]. The first metabolite of CCl4; trichloromethyl free radical, is believed to initiate the biochemical processes leading to oxidative stress, which is the direct cause of many pathological conditions such as diabetes mellitus, cancer, hypertension, kidney damage, liver damage and death[17 – 19]. These activated radicals bind to micromolecules and reduce lipids peroxidative degradation of polyunsaturated fatty acids. This leads to the formation of lipid peroxides which in turn gives products like malonylaldehyde that cause damage to membranes [20]. This lipid peroxidative degradation of biomembranes is one of the main causes of hepatotoxicity by CCl4. This is usually evidenced by a rise in the serum marker enzymes of the liver namely ALP, AST and ALT.
In this study, the aqueous root bark extracts of S.latifolius, demonstrated protection against liver toxicity induced by CCl4 in a dose dependent manner. The protection observed could be linked to the type of phytochemicals present. The terminal event in the attack on the liver by CCl4 is the production of highly reactive radicals leading to lipid peroxidation and inhibition of calcium pump of the microsome giving rise to liver lesions [21]. However, antioxidants inactivate free radical reactions initiated by CCl4 by either blocking the initiation of free radicals or scavenge free radicals and terminate free radical damage[22]. Photochemical such as flavonoids are potent antioxidant because of their ability to scavenge hydroxyl radicals, superoxide and lipid peroxy radicals [23, 24].
In the study, an elevation in the levels of the end products of lipids peroxidation of the liver of the rats treated with CCL4 was observed. The increase in AST and ALT suggests enhanced lipid peroxidation giving rise to liver damage and failure of the antioxidant defense mechanism to prevent formation of excessive free radicals.
The group treated with S.latifolius root bark inhibits the changes [p<0.05] at high concentrations [300mg/kg > 200mg/kg >100mg/kg body weight respectively], compared to CCl4 treated group, hence it is possible that the mechanism of hepatoprotection of S latifolius is due to its antioxidant effect.
Histopthological studies showed normal integrity of the hepatocytes in the control group. The liver of rats treated with 100mg/kg body weight of aqueous extract plus CCl4, showed severe congestion, necrosis, calcification of hepatocytes, mononuclear cell infiltration, with areas of vacoulation and interstitial haemorrhage; while rats treated with 300mg/kg body weight of the aqueous extract plus CCl4 shows minimal blood congestion, reduction in steatosis, fatty degeneration and peripheral hyalinazation of the hypatoctes of the liver tissue. This adds credence that physiologic recovery preceded obvious histological changes and the results may suggest that diets supplemented with S.latifolius will improve hepatoprotection against oxidative liver damage.
Total and conjugated bilirubin levels were significantly increased in the CCl4 treated rats as compared to control. Administration of the extracts [100mg/kg, 200mg/kg and 300mg/kg body weight] leads to a significant reduction [P < 0.05] in their levels. Report from Tirkey et al [25] also showed a marked rise in bilirubin levels after CCl4 administration.
Bilirubin, is a major product of haemoglobin breakdown which rises when there is liver injury or damage; leading to the discolouration of the skin and eyes known as jaundice [26].
Elevation of total bilirubin which results from decreased uptake and conjugation of bilirubin by the liver is caused by liver cell dysfunction, while increased levels of direct or conjugated bilirubin is due to decreased secretion from the liver or obstruction of the bile ducts [26]. Reduction of CCl4 induced increases in total and conjugated bilirubin by S. latifolius extract further show its protective effect against CCl4 induced liver toxicity. The extract perhaps protects the liver cell from damage, thereby enhancing bilirubin uptake and conjugation by the liver and subsequent secretion into the bile ducts. These reports from our study show that the ethanol extract of S. latifolius possess antihepatotoxic activity as demonstrated by its reduction of CCl4 induced elevations in the levels of ALT, AST, total and conjugated bilirubin. This hepatoprotective effect suggests that the plant may also possess antioxidant properties that helped to combat the CCl4-induced oxidative stress in the liver. The ability of natural compounds to attenuate carcinogen – induced hepatotoxicity is believed to be related to their intrinsic antioxidant properties [27]. Phytochemical result from this study revealed the presence of flavonoids, which has been reported to protect against toxicity induced by environmental toxicants [28] such as CCl4. Alkaloids present in plants are known to have numerous beneficial pharmacological effects[10,29]. Some bioflavonoids have been reported to possess antioxidant properties which help to combat free radical induced oxidative stress [24, 29]. The chemoprotective activities of flavonoids are related to their ability to inhibit peroxidative damage caused by environmental toxicants.
Several secondary metabolites have been shown to have wide ranges of antimicrobial activities [30]. In a study, by Kouassi Maximin et al[ 2007], S. latifolius was one of sixty-four extracts assayed from twenty-one plants used in the Malian traditional medicine that were found to be significantly active against the intracellular forms of Leishmania major [31]. Similarly Abreu et al [2001], showed that S.latifolius displayed antiplasmodial activity against P. falciparum [32].
Interestingly, phytochemical screening of the current investigation has revealed that extracts from the root extract possess at least three to four of the following classes of secondary metabolites: flavonoids, terpenoids, tannins, alkaloids and saponin, hence may not only be hepatoprotective but useful as chemotherapeutic agents and need to be tested for protection against hepatic pathogens.
Conclusion
The results of this study show that the aqueous extracts of S. latifolius root park have hepatoprotective actions and suggest that flavonoids present in S. latifolius root bark may have a major role in this action. Future studies are planned to carry out quantitative analyses of the phytochemical parameters in order to have a more accurate picture about the possible mechanism of action of the components of these extracts. In addition, assaying for more enzymes such as gamma-glutamyltransferase [GGT], alkaline phosphatase and lactate dehydrogenase 5 [LDH5] isoenzymes would provide more clarification on the hepatoprotective effects of this plant.
281
References
- Hutchison, F. and Datziel, M.D. [1963]. Flora of West Tropical Africa [2ndedition] Whitefriars press, London. Pp. 164-165.
- Arbonnier, M. [2002]. Trees, shrubs and lianas of West African dry zones. [2ndEdn.].Cirad, Margraf, Netherlands.P. 463.
- Keay, R.W.J. [1st Edition.1989]. Trees of Nigeria. Clarendon press, Oxford, United Kingdom of America. Pp. 413-421.
- Lieberman, D. [1979].Seed dispersal by baboons in the Shai Hills, Ghana. Ecology, 60[1]: 65-75.
- Gammaniel, K., Wambebe, C., Amupitan, J., Hussaini, I.M., Amos, S., Awodoan, A., Dunah, A., Ekuta, W.J., Akeju, E.M.O., Usman, H. and Eweren, N. [1997]. Active column fractions of N. latifolia of P. berghei and rabbit ileum. J Pharm R Dev. 2: 44-47.
- Abbiw, D.K. [1990]. Useful plants of Ghana. Intermediate technology and the Royal botanical garden. Kew, London. Pp. 127-196.
- Asuzu, U.I and Njoku, C.J. [1996]. The anthelminitic effects of Alstoniabooneibark and Nauclealatifolius leaf aqueous extracts on Trichostrongylus infective larvae. Fitoterapia. 67[3]:220-272.
- Gidado, A., Ameh, D.A., and Atawodi, S.E., [2005]. Hypoglycemic activity of Nauclealatifolia in lowering glucose level in rats.Afri J Biotech., 4:91- 93.
- Oluma, H.O.A and Elaigwu, M. [2006]. Antifungal activity of some medicinal plants against macrophominaphaseolina [tassi] gold.Nig J Bot., 19 [1]: 124-125.
- Harborne, J.B. [1973]. Phytochemical methods: A guide to modern techniques of plant analysis. Chapman & Hall, London. Pp. 279-282.
- Trease, G.E. and Evans, W.C. [2002]. A Text book of Pharmocognosy,[15th edition]. Elsevier Company, Philadelphia, USA.Pp.191-418.
- Mishra, A. K, Mishra, A., Kehri H. K., Sharma, B.AndPandy, A.K. [2009].Inhibitory activity of Indian spice plant Cinnamomumzeylanicumextract against AlternariasolaniandCurvularialunata, the pathogenic dematiaceousmoulds. Annals of clinical Microbiology and Antimicrobials, 8:9, doi: 10.1186/1476 – 0711 -8-9.
- Turner, R.A. [1965]. Screening methods in Pharmacology [1st edition], Academic press, New York. 299 p.
- Reitman, S. and Frankel, S. [1957]. Determination of Glutamate-Pyruvate transaminase [ALT] and Aspartate Aminotransfrase [AST]. J. Clin. Path.28:56.
- Mead, R. and Cumow, R.N.[Editors] [1983]. A simple experiment. In: Statistical Methods in Agriculture and Experimental Biology.Chapman Hall, London. Pp33-46.
- Uma M.M, RaoP.G.M [2005]. Antihepatotoxic effects of Cotton seed oil in Rats Ind J Pharmaco 37:180.
- Guven, A., GuvenA. andGulmez, M. [2003]. The effect of kefir on the activities of GSH-PX, GST, CAT, GSH, and LPO levels in carbontetrachloride – induced mice tissues. J. Vet Med B Infect Dis Vet Public Health. 50:412-416.
- Ahmed, F.F., Cowan, D. L. and Sun, A Y. [1987]. Detection of free radical formation in various tissue after acute carbontetrachloride administration in gerbil. Life Sci. 41:2469–2475.
- Pohl, L., Schuliek, R. and George, J. [1984]. Reductive oxygenation mechanism of metabolism of carbontetrachloride phosgene by cytochrome p-450.MolPharmacol. 25: 318–324.
- Ulicna O, Greskshek M, Vancovao O, Zlator I, Bocek P [2003]. Hepatoprotectiveeffect of Rooibos tea [Aspalathuslinearis] on CCl4 induced liver damage PhysiolRes 52: 461-466.
- Herfindal, E.T. and Gourley, D.R. [2002]. Text book of therapeutics.Drug and Disease Management [7th edition] Williams and Wilkins, Philadelphia, U.S.A. Pp. 74-79.
- Gamzi, S.C., Vinay, K.andTuekar, C. [1999]. Robbins pathologic basis of disease [6th edition] W.B. Saunder Company. Philadelphia, U.S.A. Pp. 846-868.
- Litito, S.B and Frei, B [2006]. Comsumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon ? Free Radic. Biol. Med. 41[12]:1727-1746.
- Farombi, E. O., Akanni, O. O. and Emerole, G. O. [2001]. Antioxidant and Scavanging activities of flavonoid extract [kolaviron] of Garcinia kola seeds in vitro. Pharmaceutical Biology.47:3163-3168.
- Tirkey, Pukhwal, S., Kuhad, A. and Chopra, K. [2005]. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbontetrachloride in rat liver and kidney. BMC Pharmacol. 5: 2-6 doi 10.1186/1471–2210–5–2.
- Sanjiv, C. [2002]. The liver book: A comprehensive guide to diagnosis, treatment and recovery. Atria Jimcafe Company.
- Farombi, E. O. [2003]. locally derived natural antioxidant substances in Nigeria:Potential role as new chemotherapeutic agents. In: Molecular and Therapeutic Aspects of Redox Biochemistry. TheeshanBahorun and AmeenahGurib-Fakim [eds.], pp 208-225. OICA International [UK] Limited.
- Mishra, A.K ,Mishra,A, Kehri,H.K, Sharma,B and Pandey, A.K[2009]. Inhibitory activity of Indian spice plant Cinnamomumzeylanicum extracts against Alternariasolani and Curvularialunata, the pathogenic dematiaceousmoulds. Ann ClinMicrobiolAntimicrob. 2009; 8: 9[7].doi: 10.1186/1476 0711-8-9.
- Chioma A. A, Uchenna B. U, and Ogechi N,[2008]. Effect of ethanol extract of Pyrenacanthastaudtii leaves on carbontetrachloride induced hepatotoxicity in rats. NigSocExperimatal Bio: 20[1], PP. 17-22
- Cordell G.A, Quinn-Beattie M.L, Farnsworth N.R[2001]. The potential of alkaloids in drug discovery.Phytother Res.; 15:183–205. doi: 10.1002/ptr.890
- Kouassi M. A, Jean-Robert I, KarineN.I, Drissa D, Jacques M and Kurt H. [2007].Antileishmanial activities associated with plants used in the Malian traditional medicine. J Ethnopharm;110[1]: 99-104
- Abreu P, Pereira A [2001]. New Indole alkaloids from Sarcocephaluslatifolius.Nat Prod Lett;15[1]:43 – 8.










