Key words
|
| |
| Melatonin, Hyperalgesia, Allodynia, Oxidative stress |
| |
Introduction
|
| |
| Peripheral nerve injury is often followed by the development of severe neuropathic pain. Nerve degeneration accompanied by inflammatory mediators is thought to play a role in generation of neuropathic pain (19). Neuropathic pain is one of the chronic painful conditions (49). Neuropathic pain is a severe and debilitating condition which affects approximately 4 million people in the India alone (9). Neuropathic pain represents pathological change in the peripheral and central nervous system. It may also result nerve damage caused by a variety of insults including traumatic nerve injury, metabolic diseases, viral infections, and stroke etc. A combination of positive and negative sensory symptoms often occurs in neuropathic pain patients. These can be paresthesias (numbness or tingling), dysesthesias (electric shock phenomenon), hyperesthesia (increased sensitivity to mild painful stimuli), hyperalgesia (increased sensitivity to normally painful stimuli), hyperpathia (pain produced by subthreshold stimuli), spontaneous pain and allodynia (pain produced by normally nonpainful of stimuli) (43). |
| |
| On other hand, neuropathic pain is thought to arise from abnormal physiology of peripheral or central nervous system and unrelated to ongoing tissue damage or inflammation (13). Recent study indicates that inflammatory events initiated by nerve injury play a central role in the pathogenesis of neuropathic pain. These involve inflammatory cells (eg, macrophages, the production of cytokines/interleukins and nerve growth factor) (19). Studies on the role of cytokines in neuropathic pain have only recently begun, mostly in animal model systems that involve nerve injury (7). Involvement of oxidative damage has also been reported in the pathophysiology of neuropathic pain and its related complications (39, 40). However, little is known about the role of inflammation and oxidative damage in neuropathic pain and related complications. |
| |
| Treatment and management of neuropathic pain is a challenge to clinicians. Several tricyclic antidepressants, anticonvulsants, antioxidants and neuroprotective are being tried with a limited success (8). The potent opioids, on other hand are effective only in some conditions of neuropathic pain such as postherpetic neuralgia and diabetic neuropathy (5). Involvement of nitric oxide mechanism has been suggested in the pathophysiology of neuropathic pain (16, 21, 22). It has further been hope that inhibition of NO synthase could be used as effective drug target to enhance the clinical efficacy of these therapeutic agents against neuropathic pain (20). Role of Larginine- NO guanosine 3’:5’-cyclic monophosphate (cyclic GMP) pathway in central and peripheral nociceptive processing has been well documented (38). |
| |
| Melatonin (N-acetlye-5-methoxytryptamine), the chief neurosecretory product of the pineal gland, is a potent antioxidant in biological system (27). Numerous studies have demonstrated the antioxidant potentials of melatonin against oxidative related cascades (17). Melatonin is well known free radical scavenger and decrease nitric oxide synthase activity (32, 2, 30, 33, and 34). Melatonin inhibits post traumatic polymorph nuclear infiltration and stimulates superoxide dismutase, glutathione peroxidase reductase (35) suggesting the potential therapeutic option of melatonin against peripheral nerve injuries (36). It is interesting to note that melatonin has been shown to protect the sciatic nerve from ischemia-reperfusion injury by attenuating neural lipid peroxidation (37). Melatonin has been demonstrated to be involved in numerous physiological processes through activating its receptor, including circadian entrainment (10), blood pressure regulation (39), etc. In some other studies, melatonin has been proved to produce significant analgesic effects in behavioral nociceptive tests in both rats and without any obvious toxic or side reaction (47). Melatonin has also been reported to induce analgesia (48). Recently, melatonin was reported to possess centrally mediated antiinflammatory and antinociceptive action, suggesting that melatonin could be a potent drug to treat inflammation hyperalgesia and neuropathic pain (49). |
| |
| Therefore study was designed to investigate the role of nitric oxide and its modulators in the protective effect of melatonin against sciatic nerve ligation induced behavioral and biochemical alterations in rats. |
| |
Materials and Methods
|
| |
|
Animals
|
| |
| Male Wistar rats (Central Animal House of the facility of the Panjab University, Chandigarh) weighing 180–200 g were used at the start of the surgery. Animals were acclimatized to laboratory conditions prior to experimentation. Following surgery, the animals were kept under standard conditions of light and dark cycle with food and water ad libitum in groups of single in plastic cages with soft bedding. All the experiments were carried out between 09:00 and 15:00 h. The protocol was approved by the Institutional Animal Ethics Committee and carried out in accordance with the Indian National Science Academy Guidelines for the use and care of animals. |
| |
|
Induction of peripheral neuropathy by sciatic nerve ligation
|
| |
| Peripheral neuropathy was induced by sciatic nerve ligation (CCI) in rats. In brief, rats were deeply anesthetized with thiopental sodium (40mg/kg i.p). The hair of rat’s lower back and thigh were shaved. The skin of the lateral surface of the right thigh was incised and a cut was made directly through the biceps. Expose the sciatic nerve and two ligatures (silk 4-0), were placed around the nerve. After performing the ligation, muscular and skin layer was immediately sutured with thread and topical antibiotic was applied. Nociceptive threshold was assessed at weekly intervals on 7, 14, and 21st after the surgery (4, 8). |
| |
| Drugs and treatment schedule – Animals were divided into eleven groups (6 animals in each). First, second and third group were treated as naïve (vehicle treated), sham group (expose the sciatic nerve but not ligated) and control (CCI ligated animals) respectively. Melatonin (2.5 and 5 mg/kg, ip), Larginine (100 mg/kg, ip) and L-NAME (5 mg/kg, ip) were treated as groups 4–7, respectively. L-arginine (100 mg/kg, ip) or L-NAME pretreatments with melatonin (2.5 and 5 mg/kg, ip) were treated as groups 8–11, respectively. L-arginine and L-NAME were freshly prepared in distilled water and administered intraperitoneally 15 minutes before melatonin treatment. Melatonin was mixed with one drop of DMSO and then diluted with distilled water. Melatonin (2.5 and 5 mg/kg) treatment was given for the duration of 3 weeks. Doses of melatonin, Larginine and L-NAME were selected based on reported literature and laboratory reports. |
| |
|
Behavioral Examinations
|
| |
| Hot plate test Thermal hyperalgesia was assessed by placing individual animal on a hot plate (Eddy’s Hot Plate) maintained at 55°C on weekly intervals on (7th, 14 and 21st day) after CCI The latency to first sign of paw licking or jumping response to avoid thermal pain was taken as an index of pain threshold. A cut off time of 15 sec was maintained throughout the experimental protocol (48). |
| |
| Cold allodynia Cold allodynia was assessed by measuring paw (both ipsilateral and collateral) withdrawal latency (PWL), when dipped in water bath maintained at 4°C ± 2°C on weekly intervals on (7th, 14 and 21st days) after SNI (42). A cut off 15 sec was sec was maintained throughout the experimental protocol (31). |
| |
|
Biochemical estimations for oxidative stress determination
|
| |
| Dissection and homogenization: On 21st day, animals were sacrificed by decapitation immediately after behavioral assessments. The complete sciatic nerve and brains were removed and 10% (w / v-1) tissue homogenates were prepared in 0.1 M phosphate buffer (pH 7.4). Homogenate were centrifuged for 20 minutes at 15000 rpm and supernatant was used for estimation of lipid peroxidation and reduced glutathione levels. The post nuclear fractions for catalase assay are obtained by centrifugation of the homogenate at 1000 × g for 20 min, at 4°C and for other enzyme assays centrifuged at 12,000 × g for 60 min at 4°C. |
| |
| Lipid peroxidation assay The quantitative measurement of lipid peroxidation was perfomed according to the method of Wills et al. The amount of malondialdehyde (MDA) was measured by reaction with thiobarbituric acid 532 nm using Shimadzu Spectrophotometer. The values were calculated using molar extinction coefficient of chromophore (1.56 × 105 M-1cm-1) and expressed as percentage of control (46). |
| |
| Estimation of reduced glutathione Reduced glutathione was estimated according to the method described by Ellman et al(11)1 ml supernatant was precipitated with 1ml of 4% sulfosalicylic acid and cold digested at 4°C for 1h. The sample was centrifuged at 1200 × g for 15 min at 4°C to 1ml of this supernatant, 2.7 ml of phosphate buffer (0.1M, pH 8) and 0.2 ml of 5,5-dithiobis (2-nitrobenzoic acid)(DTNB). The yellow color developed was read immediately at 412 nm using Shimadzu Spectrophotometer. Results were calculated using molar extinction coefficient of chromophore (1.36 × 104 M-1cm-1) and expressed as percentage of control. |
| |
| Estimation of nitrite The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide (NO) was determined with a colorimetric assay with Greiss reagent (0.1% N- (1-napthyl) ethylenediamine dihyrochloride, 1% sulfanilamide and 2.5% phosphoric acid) (15). Equal volumes of supernatant and Greiss reagent were mixed, the mixture was incubated for 10 min at room temperature and the absorbance was measured at 54° nm using Shimadzu Spectrophotometer. The concentration of nitrite in the supernatant was determined from a standard curve and expressed as percentage of control. |
| |
|
Protein Estimation
|
| |
| The protein content was measured according to the method of (24) using bovine serum albumin as standard. |
| |
|
Estimation of Catalase
|
| |
| Catalase activity was assayed by method of Luck (25) where in the breakdown of H2O2 was measured at 240 nm. Briefly, the assay mixture consisted of 3 ml of H2O2 phosphate buffer (1.25 × 10-2 M H2O2) and 0.05ml of supernatant of the brain homogenate (10%), and the change in absorbance was recorded at 240 nm using the Shimadzu Spectrophotometer. Enzyme activity was calculated using the millimolar extinction coefficient of H2O2 (0.07). The result was expressed as micromoles of H2O2 decomposed per minute per milligram of protein. |
| |
|
Statistical analysis
|
| |
| All data are expressed as mean ± SEM. Data were analyzed by analysis or variance (ANOVA) followed by turkey test values of p< 0.05 were considered to be significant. |
| |
Results
|
| |
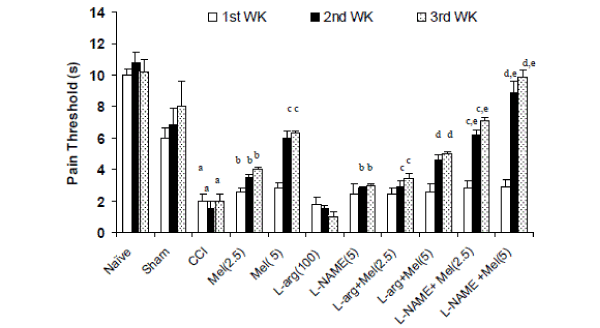
| Effects of melatonin and its modulation by LNAME and L-arginine on thermal hyperalgesia in CCI rats- Sciatic nerve ligation (CCI) significantly caused hyperalgesia as reflected by shorten pain threshold (Jump or licking time latency) after 2nd and 3rd week intervals as compared to naïve and sham group. Melatonin (2.5mg/kg and 5.0 mg/kg, ip) pretreatment significantly reversed CCI induced hyperalgesia as compared to control (CCI) after 1st, 2nd and 3rd week interval (Figure 1). Further, L-NAME (5mg/kg) pretreatment with melatonin (2.5mg/kg and 5.0 mg/kg, ip) significantly potentiated the protective effects of melatonin on 2nd and 3rd week which was significant as compared to their effect per se (Figure 1). Similarly, L-arginine (100 mg/kg) pretreatment with melatonin (2.5mg/kg and 5.0 mg/kg, ip) significantly reversed the protective effect of melatonin and caused hyperalgesic response after 1st, 2nd and 3rd week (Figure 1). L-arginine (100 mg/kg) treatment per se did not produce any significant effect on hyperalgesic response even after 3rd week. |
| |
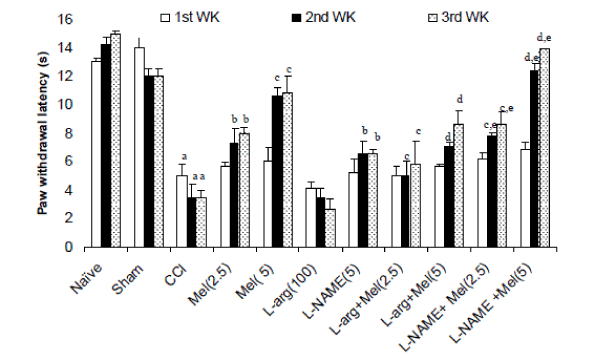
| Effects of melatonin and its modulation by LNAME and L-arginine on cold allodynia in CCI rats- Sciatic nerve ligation (CCI) significantly caused cold allodynia as reflected by decreased paw withdrawal latency after 2nd and 3rd week intervals as compared to naïve and sham group. Melatonin (2.5mg/kg and 5 mg/kg, ip) treatment significantly delayed withdrawal latency as compared to control (CCI) after 1st, 2nd and 3rd week (Figure 2). L-NAME (5mg/kg) pretreatment with melatonin (2.5mg/kg and 5.0 mg/kg, ip) caused potentiation in melatonin’s antiallodynic effect after 1st, 2nd and 3rd week interval which was significant as compared their effect per se. Further, L-arginine (100mg/kg) per se pretreatment did not produce significant antiallodynic like behavior as compared to control (P < 0.05). However, L-arginine (100 mg/kg) pretreatment with melatonin (2.5mg/kg and 5.0 mg/kg, ip) significantly reversed the antiallodynic effect of melatonin after 1st, 2nd and 3rd week intervals (Figure 2). |
| |
|
Effects of melatonin and its modulation by LNAME and L-arginine on oxidative damage in CCI rats
|
| |
| Sciatic nerve ligation significantly caused oxidative damage as indicated by increased lipid peroxidation, nitrite concentration, depletion of reduced glutathione level and catalase activity in sciatic nerve as compared to naïve or sham treated animals. Treatment with melatonin (2.5mg/kg and 5.0 mg/kg, ip) significantly caused attenuation of lipid peroxidation, nitrite concentration, restoration of depleted reduced glutathione and catalase activity as compared to the control (CCI) in sciatic nerve P<0.05) (Table 1 and Table 2). |
| |
| L-NAME (5mg/kg) pretreatment with melatonin (2.5mg/kg and 5 mg/kg, ip) caused potentiation in the melatonin effect in sciatic nerve which was significant as compared their effect per se (Table 1 and Table 2). Further, L-arginine (100 mg/kg) pretreatment with melatonin (2.5mg/kg and 5 mg/kg, ip) significantly reversed the antioxidant effect of melatonin treatment in sciatic nerve (Table 1 and Table 2). L-arginine (100 mg/kg, per se) did not produce any significant effect on oxidative stress parameter as compared to CCI group in sciatic nerve. |
| |
Discussion
|
| |
| Neuropathic pain is a debilitating disease afflicting wider population now days. Peripheral nerve injury produces a persistent neuropathic pain state characterized by spontaneous pain, allodynia and hyperalgesia. In present work, we studied unilateral sciatic nerve ligation induced behavioral and biochemical alterations in rats and it was found that unilateral ligation of the sciatic nerve in rats produced an ipsilateral cold allodynia, thermal hyperalgesia and oxidative damage in sciatic nerve. In the present study, sciatic nerve ligation (CCI) caused thermal hyperalgesia as well as cold allodynia in response to non-noxious cold stimulus in animals that was more significant after 2nd and 3rd week of CCI. Studies have also reported that partial ligation of sciatic nerve induced neuropathic pain causes hyperalgesia and cold allodynia like behaviors in animals (27). Reports further suggest the mechanism of hyeralgesic action could be due to sensitization of primary afferent nerves (24). The molecular targets associated with this mode of action, however, have only recently been identified where both direct and indirect mechanisms of the hyperalgesic action are involved. In the present study, sciatic nerve ligation caused significant oxidative damage in sciatic nerve as indicated by raised lipid peroxidation, nitric concentration and depletion of reduced glutathione and catalase activity. |
| |
| Melatonin, the secretory product of the pineal gland, has potent antioxidant properties. The perception of pain sensation (threshold), whether local or central, is altered by inflammatory processes. Antiinflammatory drugs block this effect by raising the pain threshold and by reducing the inflammatory process (30). Melatonin is claimed to have antiinflammatory activity in animal models of acute and chronic inflammation. However, it is not known whether melatonin can reverse the hyperalgesia that is secondary to the inflammation. The pain modulating properties of melatonin are generally well recognized. In the present study, melatonin treatment significantly reversed thermal hyperalgesia, cold allodynia in sciatic nerve ligated animals suggesting its therapeutic potential in the effective treatment and management of chronic painful conditions. Results of our study further coincided with the earlier works which proved the antinociception activity of melatonin in rats (31). These findings suggest that melatonin plays an important role in pain regulation at central and peripheral level. Raghavender et.al reported the therapeutic potentials of melatonin against hyperalgesia and related states (32). Tu and its coworkers suggest that activation of the endogenous melatonin system in the spinal cord can reduce the generation, development and maintenance of central sensitization, with a resultant inhibition of capsaicininduced secondary mechanical allodynia and hyperalgesia (40). Wang and its team recently reported the involvement of opoidergic system in the antihyperalgesic action of melatonin (43). However the entire mechanism against such a painful conditions is not fully understood. |
| |
| Melatonin is known to reduce the harmful effects of free radical in the central nervous system either by free radical scavenging or decreasing nitric oxide synthase activity. In present study, melatonin treatment significantly attenuated oxidative damage in sciatic nerve. Melatonin treatment significantly reduced rise in lipid peroxidation, nitrite level and restored the reduced glutathione and catalase activity, suggesting its antioxidant like action against CCI. Although, pathogenesis of neuropathic pain is not clear so far. Recent evidences suggested that antioxidant agents could be used to reverse peripheral nerve injuries. The primary pathway for peripheral nerve injury is now believed to be free radical-induced lipid peroxidation along neural fibers [1, 19, and 26]. As peripheral nervous system is composed primarily of lipids, they are potentially vulnerable to lipid peroxidation. Moreover, neural tissue does not contain highly active oxidative defense mechanisms. Hence, lipid peroxidation is potentially damaging because it may alter the fluidity and permeability of neuronal membranes and therefore, affect the functional and structural integrity of membrane-bound receptors and enzymes. It could also be possible that reactive oxygen and nitrogen species activate second messenger related to central sensitization, a mechanism involving hyperalgesia perpetuation based on neurochemical adaption in the sciatic nerve. NO is an important neuromodulator, involved in the several disease pathologies. NO modulators plays an important role in neuropathic pain conditions, however its exact role is still not clear (13). In the present study, L-NAME pretreatment with melatonin caused potentiation of antihyperalgesic, antiallodynic and antioxidant like effect of melatonin, suggesting the possible involvement of NO pathway in its protective action. Further, L-arginine per se, nitric oxide precursor caused hyperalgesia and allodynia behaviors, indicating that NO may be a pain producing substance. However, effect was not significant as compared to SNL treated group. Besides, NO reacts with reactive oxygen species quickly enough to avoid the action antioxidant system, forming peroxynitrite anion (ONOO−). Further, Larginine pretreatment with melatonin reversed the protective effect of melatonin confirm the involvement of NO mechanism in the protective action. |
| |
| In summary, nitric oxide mechanism could be involved in the protective action of melatonin against CCI induced neuropathic pain and oxidative damage. Study further suggests the therapeutic potential of melatonin in effective management of neuropathic pain and related conditions. |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |








