Research Article - (2022) Volume 13, Issue 11
Potential of serological markers for evaluating neurological function and progression rate of amyotrophic lateral sclerosis
Xiaowen Chen,
Junrong Li,
Yingying Lv,
Wei Zhang and
Delian Kong*
Department of Neurology, Nanjing Medical University, Nanjing, China
*Correspondence:
Dr.
Delian Kong, Department of Neurology,
Nanjing Medical University, Nanjing,
China,
Tel: 86-13813292005,
Email:
Received: 25-Apr-2022, Manuscript No. IPJNN-22-12753;
Editor assigned: 28-Apr-2022, Pre QC No. P-12753(PQ);
Reviewed: 01-Nov-2022, QC No. Q-12753;
Revised: 05-Nov-2022, Manuscript No. R-12753(R);
Published:
17-Nov-2022
Summary
Objective: The present study was to investigate the potential of creatinine, uric
acid, creatine kinase, total cholesterol, triglyceride, HCY (Homocysteine), and cystatin C for predicating neurological function and progression rate of amyotrophic lateral sclerosis.
Methods: All enrolled ALS patients were given corresponding serological tests
at the initial diagnosis. The Revised ALS Functional Rating Scale (ALSFRS-R) and
Disease Progression Rate (DPR) were evaluated. The detected indexes in blood
tests included creatinine, uric acid, creatine kinase, total cholesterol, triglycerides,
homocysteine, and cystatin C. Data analysis was performed by SPSS 22.0 statistical
software.
Results: There were significant differences in creatinine, uric acid, creatine kinase,
total cholesterol, HCY and cystatin C between the two groups (P<0.05). The levels
of uric acid and creatinine of ALS group were lower than those of the control
group, and the levels of other test indicators were higher than that in the control
group.
The results from correlation analysis demonstrated that there was a significant positive correlation between ALSFRS-R and creatinine (P<0.01, r=0.567); There
was negative significant correlation between DPR and creatinine (P<0.01, r=-
0.408). The correlations of DPR with triglyceride and total cholesterol were significantly negative correlated (P<0.05, r=-0.201,-0.210 respectively). The remaining indexes did not show any correlation with ALSFRS-R and DPR.
Conclusion: Uric acid and creatinine of ALS patients were lower than that in
healthy people. There were significant metabolic abnormalities in ALS patients 2.
Creatinine level is an independent risk factor affecting ALSFRS-R. The creatinine
and total cholesterol levels are also the independent risk factors affecting DPR.
Creatinine and total cholesterol levels could be used as reliable indicators to
evaluate the ALSFRS-R and DPR of ALS patients.
Abbrevations
ALS: Amyotrophic Lateral Sclerosis; ALSFRS-R: ALS Functional Rating Scale; DPR: Disease Progression Rate; FVC: Forced Vital Capacity; CK: Creatine Kinase; HCY: Homocysteine
INTRODUCTION
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s
disease, is a progressive neurodegenerative disorder characterized by
loss of motor neurons in motor cortex, brainstem, and spinal cord
that results in muscle weakness and atrophy, spasticity,
compromised speech, swallowing, and breathing. There is
currently no effective treatment for ALS that can reverse the
progression of the disease. The main possible treatments include drug
therapy, stem cell transplantation, momentum transplantation, gene
therapy, respiratory support, and nutritional management. The
prognosis of ALS is poor, and the rate of disease progression varies
greatly. Rapidly progressing patients may involve the respiratory
system within a few months due to respiratory failure, requiring
assisted ventilation or even death, and slower progressing ones can
even survive for 10 years or longer. Since the vast majority of ALS is
fatal, it is very important to assess the prognosis, which can help
patients plan their lives better.
Previous studies have confirmed that biological markers can be
used as indicators for early diagnosis and prognosis of ALS. The
purpose of this experiment is to explore the relationship between
serum markers and amyotrophic lateral sclerosis function score and
disease progression rate, which will play significant role in early
diagnosis and effective treatment.
Previous studies have confirmed that biological markers can be
used as indicators for early diagnosis and prognosis of ALS. The
purpose of this experiment is to explore the relationship between
serum markers and amyotrophic lateral sclerosis function score and
disease progression rate, which will play significant role in early
diagnosis and effective treatment.
Objective
Enrollment criteria: ALS patients were selected from the first confirmed
patients admitted to the neurology clinic and inpatient department
from January 2015 to August 2020. The enrolled 103
patients (60 males and 43 females) were all met the EI Escorial
amyotrophic lateral sclerosis clinical diagnostic criteria revised in
2000 by the Motor Neuron Disease Group of the World Federation of
Neurology [1]. The average age of the patients is (58.95 ± 9.98) years
old. EMG examination is performed on all patients.
The enrollment criteria are as follows: There are three conditions
that the patients must be met: Firstly, the evidence of lower motor
neuron injury must be found through clinical, electrophysiological or
neuropath logical examination secondly, evidence of upper motor
neuron lesion must be confirmed by clinical examination. Finally, the
upper and lower motor neuron lesions spread from one part to other
parts gradually based on medical history or examination. There are two conditions that must be excluded at the same time: At
first, patient suffering from other diseases that cause upper and
lower motor neuron lesions is proven through electrophysiology
or pathology. Secondly, neuroimaging proves that the patient has
other diseases lead to clinical manifestations of upper and lower
motor neuron diseases and electrical physiological changes.
Exclusion criteria:
• The patients suffering from acute cardiovascular and
cerebrovascular events, stroke, acute coronary syndrome.
• Those who also suffer from other neurodegenerative diseases,
such as multiple system atrophy, Parkinson's disease, front
temporal dementia, etc.
• Those who also suffer from renal insufficiency or gout.
The renal function is evaluated by the glomerular filtration
rate. When the glomerular filtration rate is less than 90 ml/
min, it means renal insufficiency.
• Those with mental symptoms and cognitive impairment who
cannot cooperate with the test; Patients with severe liver and
kidney disease or malignant tumors or thyroid disease or
patients who have taken drugs that affect monitoring
indicators in the past 3 months (such as lipid-lowering
drugs, diuretics, folic acid, B vitamins, etc.).
• The patients who have a confirmed ALS in their family or
similar symptoms but undiagnosed patients.
Healthy control group: 90 healthy volunteers had a physical
examination in the physical examination center of our hospital
during the same period. Patients with mental disorders, severe liver
and kidney diseases, thyroid diseases, and malignant tumors were
excluded, and those who had taken drugs that affected the
observed indicators in the past 3 months were also excluded
(such as lipid-lowering drugs, diuretics, folic acid, B vitamins,
etc.). There were 52 males and 38 females, aged 35-77 (59.34 ±
7.71) years old. There was no age and gender bias between the
control group and the ALS patient group.
Materials and Methods
Detection of serum markers: 2 ml of peripheral venous blood
was collected from all patients on an empty stomach for at least
eight hours in the morning of outpatient or admission, after
centrifugation at 1000 r/min for 5 minutes; the serum was separated and detected by the Roche modular P-900 automatic biochemical
analyzer.
ALSFRS-R scores: The ALSFRS-R revised in 2000 was used to
evaluate ALS patients function at the first visit. ALSFRS-R is a 12
items rating scale covering four functional areas: fine motor, gross
motor, bulbar and respiratory function. Each project contains five
levels (0=cannot perform task to 4=normal function). These 12
items involve activities of daily living: speech, salivation,
swallowing, writing, food preparation, dressing, bed activities,
walking, climbing stairs, breathing difficulties, sitting breathing,
and mechanical ventilation. The total score ranges from 0-48 [2].
Disease progression rate: The patient’s functional scores at the first
diagnosis and the time from the onset of symptoms to clinical
diagnosis (calculated in months) were recorded. The progression
rate of amyotrophic lateral sclerosis disease (DPR)=(48-ALSFRS-R
at the first diagnosis)/first onset to time of clinical diagnosis
(months) [3].
Statistical analysis
All data were analyzed by SPSS 22.0. The mean and standard
deviation were used to describe the uric acid level, which conforms
to the normal distribution. Two independent sample t-tests were
used to compare the two groups. The data of creatinine, creatine
kinase, triglycerides, total cholesterol, HCY, and cystatin C are not
In line with normal distribution, which were described by the
median and quartile, and the two independent sample rank sum
tests were used for comparison between the two groups; The
correlation analysis was used to analyze the correlation between
ALSFRS-R or DPR and the above mentioned serological
indicators; linear regression analysis were performed for those
with obvious correlation with ALSFRS-R and DPR.
Results
• Comparison of serum index (creatinine, uric acid, creatine
kinase, total cholesterol, triglycerides, HCY, cystatin C)
levels between the ALS group and the control group (Fig. 1).
• The correlation analysis of creatinine, uric acid, creatine kinase,
total cholesterol, triglycerides, homocysteine, and cystatin C
levels with ALSFRS-R and DPR, respectively. And the linear
regression analysis on indicators that had obvious correlation
with ALSFRS-R and DPR (Tab. 1 and Fig. 2).
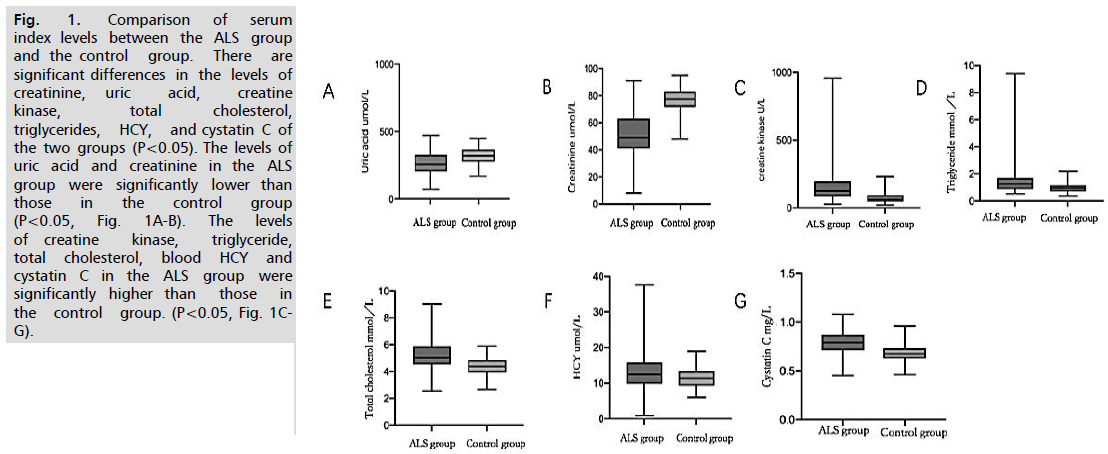
Fig. 1.Comparison of serum index levels between the ALS group and the control group. There are significant differences in the levels of creatinine, uric acid, creatine kinase, total cholesterol, triglycerides, HCY, and cystatin C of the two groups (P<0.05). The levels of uric acid and creatinine in the ALS group were significantly lower than those in the control group (P<0.05, Fig. 1A-B). The levels of creatine kinase, triglyceride, total cholesterol, blood HCY and cystatin C in the ALS group were significantly higher than those in the control group. (P<0.05, Fig. 1C-G).
| |
ALSFRS-S |
DPR |
| |
r |
P |
r |
P |
| Creatinine |
0.567** |
0 |
-0.408** |
0 |
| Creatine kinase |
-0.061 |
0.542 |
-0.097 |
0.332 |
| Uric acid |
0.07 |
0.482 |
-0.102 |
0.305 |
| Triglyceride |
0.075 |
0.452 |
-0.201* |
0.042 |
| Total cholesterol |
0.012 |
0.904 |
-0.210* |
0.033 |
| HCY |
0.007 |
0.947 |
0.088 |
0.378 |
| Cystatin C |
0.04 |
0.689 |
-0.052 |
0.604 |
** At the 0.01 level (two-tailed), the correlation is significant;
*At the 0.05 level (two-tailed), the correlation is significant.
Tab. 1. Correlation analysis between ALSFRS, DPR and serological markers.
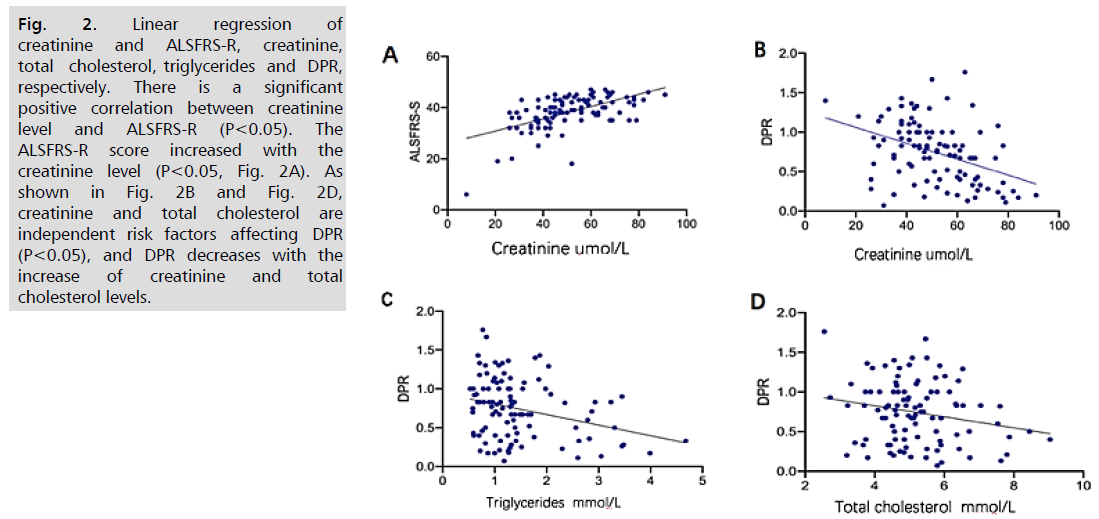
Fig. 2. Linear regression of creatinine and ALSFRS-R, creatinine, total cholesterol, triglycerides and DPR, respectively. There is a significant positive correlation between creatinine level and ALSFRS-R (P<0.05). The
ALSFRS-R score increased with the creatinine level (P<0.05, Fig. 2A). As shown in Fig. 2B and Fig. 2D, creatinine and total cholesterol are independent risk factors affecting DPR (P<0.05), and DPR decreases with the
increase of creatinine and total cholesterol levels.
As shown in Tab. 1, ALSFRS-R has a very significant correlation
with creatinine level (P<0.01), and the correlation coefficient is
0.567 (positive correlation); DPR has a very obvious
correlation with creatinine level (P<0.01), and the correlation is
-0.408 (negative correlation); There is a significant correlation
between DPR and triglyceride levels/total cholesterol levels
(P<0.05), and the cor-relation coefficients are -0.201 and -0.210,
respectively, which are negatively correlated. The residual indexes
had no correlation with ALSFRS-R and DPR.
Discussion
ALS is a disease characterized by irreversible neuronal necrosis.
The overall prognosis of the disease is poor. At present, there is
no method for early diagnosis and effective treatment. Previous
animal experiments have confirmed that biomarkers may be
used as an indicator for early diagnosis and prognosis of ALS.
In this study, we explored the difference between the serum
indicators of ALS patients and the control group, hoping to find
indicators that may prompt the diagnosis and prognosis of ALS, so
as to provide a basis for further experiments.
Creatinine can assess the functional status and muscle strength
of the patients, predict the disease progression rate and prompt
prognosis: The blood creatinine is converted from creatine. The
creatinine level is a direct reflection of the creatine pool, which
reflects the mitochondrial function of muscles [4]. A Japanese
study showed that patients with ALS had lower serum creatinine
levels compared with healthy controls. In addition, the ALS
function rating scale and the rate of decrease in Forced Vital Capacity
(FVC) are negatively correlated with serum creatinine levels [5].
ALSFRS-R and FVC are effective indicators for evaluating limb
function and respiratory function in patients with ALS,
respectively. The results of Van showed that there is a strong correlation between muscle strength and serum creatinine level. In
this study, we observed a smaller variation of serum creatinine
decline rate in patients than that of ALSFRS-R, suggesting that the
rate of creatinine decline is a more stable predictor of ALS disease
progression than ALSFRS-R, and it is more effective in patients
with limb symptoms. The results of a large study in Hua xi
Hospital showed that the serum creatinine of ALS patients was
significantly lower than that of the healthy control group after
adjusting for BMI and age, and those patients with low serum
creatinine levels were more likely to develop severe motor disorders
and low BMI values [6]. But they thought that there was no
significant difference in serum creatinine level among ALS patients
with different disease sites, and there is no relationship between the
serum creatinine level and the survival time of ALS patients [7]. Chio,
et al. suggested that lower creatinine levels were closely associated
with poorer clinical function at diagnosis (clinical function is
evaluated by ALSFRS-R score and FVC). His study showed that
serum creatinine is related to survival, and the predictive sensitivity of
serum creatinine to mortality is similar to FVC, ALSFRS-R score
and age. Patin, et al. suggested that the creatinine level of patients
with limb disease was lower than that of patients with medulla
oblongata disease. The difference still exists after excluding
confounding factors such as gender and weight [8,9]. All the above
studies indicated that blood creatinine may serve as an accurate
measure of muscle quality, and can evaluate limb function, disease
progression rate and prognosis. In this study, the creatinine levels of
the ALS group were significantly lower than that of the healthy
control group. The results of multi-variate analysis showed that:
creatinine level is an independent risk factor for ALSFRS-R and
DPR. Higher creatinine level indicates better limb function and
slower disease progression within a certain range. Serum creatinine is a
cheap and easy to obtain biomarker with good reproducibility, so it
can be used extensively and repeatedly in clinical practice to evaluate
the limb function and prognosis of patients with ALS.
Higher blood lipids indicate better limb function and slower disease
progression: Studies have shown that ALS patients have a higher
probability of dyslipidemia compared with the general population.
Two studies from the United Kingdom and Germany found that
the prevalence of hypercholesterolemia and hypertriglyceridemia in
ALS patients was significantly increased [10,11]. Some researchers
believe that hyperlipidemia is a protective factor for ALS. After muscle
denervation, the body's efforts to regenerate nerves lead to an increase
in energy demand, and the increased demand is supplemented by
lipids, which can also explain the decrease of blood lipid levels in
patients with advanced disease progression. And the total body fat
decreased. In this study, the blood total cholesterol and triglyceride
levels of the ALS group were significantly higher than those of the
control group. However, only the total cholesterol level was
significantly correlated with the disease progression rate after
correlation analysis. As the total cholesterol level increased, the disease
progression rate decreased. The presence of dyslipidemia in ALS
patients is increasingly recognized. The exact cause of hyperlipidemia
in ALS is still uncertain: It may be a compensatory mechanism,
lipids are the preferred energy source for skeletal muscle,
and the regeneration of denervated muscles may lead to increased
energy demand in ALS. It is currently believed that higher blood
lipids may be related to better limb function and slower progression.
In ALS patients, lipid-lowering drugs should be used carefully,
especially statins, which may aggravate muscle damage.
Uric acid reduced the damage to neurons caused by oxidative stress: Uric Acid (UA) is the main end product of human purine metabolism.
As a natural antioxidant, it can remove superoxide and reduce neuronal
death caused by oxidative stress. The study of Bakshi, et al.
showed that urate significantly reduced the death of motor neuron
cells induced by hydrogen peroxide in an astrocyte-dependent
manner [12]. A study in Japan showed that the serum UA level
of ALS patients was significantly higher than that of the healthy
control group, and that the serum UA level was positively correlated
with BMI, ALSFRS-R score and creatinine level, and negatively
correlated with DPR [13]. Paganoni, et al. reported that when
creatinine and BMI were controlled, serum uric acid level was an
important predictor of ALS survival rate and dysfunction [14]. A
prospective study observed that people with higher serum uric acid
levels than their age and sex-matched control group have a slightly
lower risk of ALS in the future [15]. The meta-analysis results of
Zhang, et al. showed that the serum UA level of ALS patients was
significantly lower than that of the control group, and the serum
UA level of ALS patients was negatively correlated with the risk
of death. Nicholson, et al. increased the blood UA level by
intravenous infusion of inosine in 25 ALS patients [16,17]. The
results showed that the biomarkers of oxidative were significantly
reduced, and ALSFRS-R was improved after treatment. Although
the results of various studies are partially contradictory, most of
the current experimental results believe that uric acid as an
antioxidant can reduce the damage of oxidative stress to neurons.
So it is a protective factor for ALS. In this study, the level of uric
acid in the ALS group was significantly lower than that in the
control group, but there was no correlation with ALSFRS-R and
DPR in the multivariate correlation analysis. Further clinical trials
are needed to determine whether uric acid can reduce neuronal
loss, improve limb function and extend life span of ALS patients.
Creatine kinase, homocysteine, and cystatin C are future
research directions: Creatine Kinase (CK) is an enzyme that
catalyzes the reversible conversion of creatine and provides
energy for muscle contraction. Linkhart, et al. believes that the
degeneration of in-nervated motor neurons leads to increased
muscle cell membrane permeability and CK release. CK levels may
be related to the severity of lower motor neuron loss and muscle
atrophy in patients with ALS. Tai, et al. found that the serum CK
level was associated with the persistent low F wave of the median
nerve in the EMG, which confirmed that the CK level in ALS
patients was related to the severity of lower motor nerve loss. In
this study, 31 of 103 (30%) ALS patients had moderately elevated
serum CK (<1000 U/L), The CK level of the ALS group was
significantly higher than that of the control group. However, no
correlation was observed between the CK level and the patient's
physical function score and disease progression rate. It may be
related to the small sample size and the lack of long-term follow-up
of patients. At present, the mechanism of the increase in CK levels
in ALS patients is not very clear. Larger sample studies and more
animal experiments are needed to further clarify [18,19].
Homocysteine (HCY) is a sulfhydryl containing amino acid
produced by the demethylation of methionine. A Japanese small
sample double-blind clinical trial of 24 ALS patients found that
short-term (4 weeks) high-dose (0.5 mg/d) administration of
vitamin B12 effectively improved the compound motor action
potential on the electromyogram of ALS patients, suggesting that
the loss of spinal cord motor neurons is improved [20]. In our
study, the serum HCY level of the ALS group was significantly
higher than that of the control group, and there was no significant
correlation between ALSFSR and DPR and HCY levels in the
correlation analysis. A cohort study on ALS patients is needed to
get a more precise conclusion. However, we believe that HCY may
be one of the factors that cause neuronal damage in ALS. Even if ALS cannot be cured, the treatment of reducing HCY still has a
positive meaning and it is an important part of the treatment of
ALS.
Cystatin C is an endogenous cysteine protease inhibitor, which
plays an important role in the repair of the nervous system after
injury and disease. The neuroprotective activity of CysC includes
inducing autophagy in nerve cells, promoting the degradation of
misfolded or unfolded proteins, and preventing the accumulation
of abnormal mutant proteins [21]. A longitudinal study analysis
showed that the level of CysC in the cerebrospinal fluid of fastprogresses
was lower, while those with slower progress showed an
increasing trend of CysC levels, and higher cerebrospinal fluid
CysC levels suggested slower progress [22]. In this study, the blood
CysC level of the ALS group was significantly higher than that
of the control group, and the correlation analysis had nothing to
do with the functional score and disease progression rate. Further
researches were needed to determine whether there is a correlation
between cerebrospinal fluid and CysC in the blood and whether
increasing the level of CysC in the blood can improve the function,
delay the progression, and improve the prognosis of ALS patients.
Conclusion
In summary, this study performed a statistical comparison of
creatinine, uric acid, creatine kinase, total cholesterol,
triglycerides, HCY, cystatin C and other indicators in the blood of
ALS patients, and found that the serum uric acid and creatinine
levels of ALS patients is lower than healthy people, and the levels of
creatine kinase, triglycerides, total cholesterol, HCY and CysC
are higher than healthy people. Patients with ALS have obvious
metabolic abnormalities. Higher levels of creatinine, blood lipids
and uric acid usually indicate better physical function, slower
disease progression, and better prognosis. However, this study has
some shortcomings. The ALS team has not yet conducted any
human intervention and randomized controlled studies. Our next
study is to conduct a randomized controlled study of every indicator that seems to be meaningful in this trial. As for the indicators that
have not been obtained in this experiment, animal experiments
need to be further validated to discover their role in the diagnosis,
treatment and prognosis of ALS.
Amyotrophic Lateral Sclerosis (ALS) is a devastating condition
with an estimated mortality of 30,000 patients a year worldwide.
The median reported survival time since onset ranges from 24 to
48 months. Therefore, early diagnosis and treatment of ALS are
important. Serum markers may be a promising method to predict
the occurrence, diagnosis, and progress of ALS. The application of
serum markers to the development of ALS drugs will greatly improve
our ability to correctly test drugs in clinical trials and
ultimately find a treatment for ALS. At present, the field of ALS
serum markers is still an active research field with great hope.
Acknowledgements
We would like to thank all patients in this study and
Neurological Sciences for providing this excellent platform for
researchers to communicate and learn.
Author’s Contribution
DLK designed the experiment plan and directed the thesis writing.
XWC, MW, YYL, WZ were in charge of the whole experiment
process and thesis writing. JRL participated in the design of the
experiment. We would like to thank the patient and his families for
their participation in this study.
Conflict of Interest
The authors report no conflicts of interest in this work.
Ethical Approval
None.
References
- Brooks BR, Miller RG, Swash M et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2000;1(5):293-9.
Google Scholar, Crossref, Indexed at
- Cedarbaum JM, Stambler N, Malta E et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1-2):13-21.
Google Scholar, Crossref, Indexed at
- Huang R, Guo X, Chen X et al. The serum lipid profiles of amyotrophic lateral sclerosis patients: a study from south-west China and a meta-analysis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2015;16(5-6):359-65.
Google Scholar, Crossref, Indexed at
- Viollet L, Gailey S, Thornton DJ et al. Utility of cystatin C to monitor renal function in Duchenne muscular dystrophy. Muscle Nerve. 2009;40(3):438-42.
Google Scholar, Crossref, Indexed at
- Ikeda K, Hirayama T, Takazawa T et al. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. 2012;51(12):1501-8.
Google Scholar, Crossref, Indexed at
- van Eijk RP, Eijkemans MJ, Ferguson TA et al. Monitoring disease progression with plasma creatinine in amyotrophic lateral sclerosis clinical trials. J Neurol Neurosurg Psychiatry. 2018;89(2):156-61.
Google Scholar, Crossref, Indexed at
- Chen X, Guo X, Huang R et al. An exploratory study of serum creatinine levels in patients with amyotrophic lateral sclerosis. Neurol Sci. 2014;35:1591-7.
Google Scholar, Crossref, Indexed at
- Chiò A, Calvo A, Bovio G et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA neurol. 2014;71(9):1134-42.
Google Scholar, Crossref, Indexed at
- Patin F, Corcia P, Madji Hounoum B et al. Biological follow‐up in amyotrophic lateral sclerosis: decrease in creatinine levels and increase in ferritin levels predict poor prognosis. Eur J Neurol 2015;22(10):1385-90.
Google Scholar, Crossref, Indexed at
- Rafiq MK, Lee E, Bradburn M et al. Effect of lipid profile on prognosis in the patients with amyotrophic lateral sclerosis: insights from the olesoxime clinical trial. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(7-8):478-84.
Google Scholar, Crossref, Indexed at
- Dorst J, Kühnlein P, Hendrich C et al. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol. 2011;258:613-7.
Google Scholar, Crossref, Indexed at
- Bakshi R, Xu Y, Mueller KA et al. Urate mitigates oxidative stress and motor neuron toxicity of astrocytes derived from ALS-linked SOD1G93A mutant mice. Mol Cell Neurosci. 2018;92:12-6.
Google Scholar, Crossref, Indexed at
- O’Reilly ÉJ, Liu D, Johns DR et al. Serum urate at trial entry and ALS progression in EMPOWER. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(1-2):120-5.
Google Scholar, Crossref, Indexed at
- Paganoni S, Nicholson K, Chan J et al. Urate levels predict survival in amyotrophic lateral sclerosis: analysis of the expanded Pooled Resource Open‐Access ALS clinical trials database. Muscle nerve. 2018;57(3):430-4.
Google Scholar, Crossref, Indexed at
- O’Reilly ÉJ, Bjornevik K, Schwarzschild MA et al. Pre-diagnostic plasma urate and the risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2018;19(3-4):194-200.]
Google Scholar, Crossref, Indexed at
- Zhang F, Zhang Q, Ke Y et al. Serum uric acid levels in patients with amyotrophic lateral sclerosis: a meta-analysis. Scientific reports. 2018 Jan 18;8(1):1100.
Google Scholar, Crossref, Indexed at
- Nicholson K, Chan J, Macklin EA et al. Pilot trial of inosine to elevate urate levels in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2018;5(12):1522-33.
Google Scholar, Crossref, Indexed at
- Linkhart TA, Wilson BW. Appearance of acetylcholinesterase and creatine kinase in plasma of normal chickens after denervation. J Neurol Sci. 1975;26(2):193-201.
Google Scholar, Crossref, Indexed at
- Tai H, Cui L, Liu M et al. Creatine kinase level and its relationship with quantitative electromyographic characteristics in amyotrophic lateral sclerosis. Clin Neurophysio. 2018;129(5):926-30.
Google Scholar, Crossref, Indexed at
- Ikeda K, Iwasaki Y, Kaji R. Neuroprotective effect of ultra-high dose methylcobalamin in wobbler mouse model of amyotrophic lateral sclerosis. J Neurol Sci. 2015;354(1-2):70-4.
Google Scholar, Crossref, Indexed at
- Watanabe S, Hayakawa T, Wakasugi K et al. Cystatin C protects neuronal cells against mutant copper-zinc superoxide dismutase-mediated toxicity. Cell Death Dis. 2014;5(10):e1497-.
Google Scholar, Crossref, Indexed at
- Wilson ME, Boumaza I, Lacomis D et al. Cystatin C: a candidate biomarker for amyotrophic lateral sclerosis. PloS one. 2010;5(12):e15133.
Google Scholar, Crossref, Indexed at







