Keywords
3D printing technology; Polyvinyl alcohol; Fused deposition modeling; Drop-on-drop deposition; Poly (?-Caprolactone)
Introduction
Drug delivery can be defined as an approach through which systems, technologies, and formulation can be developed, which helps explicitly in drug transportation within the biological fluid and succeeding desired biological effects [1,2]. With the increased development of science and technologies in the pharmaceutical field, there have been new concepts in the design of drugs, manufacturing technology, processes, and for better understanding which helps to achieve a high quality of dosage form [3]. Within the last few decades, the development of drug product has been under study, and numerous novel dosage forms and the technological process has been developed [4]. Noticeably, in most of the cases, special consideration was given for the physicochemical and biopharmaceutical properties of APIs and regulatory requirements during each stage of product development [5]. Now-a-days different ethnic background, food habits, different circadian cycles, an inter-individual difference of patients propagates creates some enormous challenges for pharmaceutical scientists to deliver uniformity in medicine. Hence, personalization of medicine is in a hike for the last few years [6]. Over the years, scientists are stressing to optimize treatments according to the pharmacogenetics of the populations and individual pharmacokinetic profiles [7]. In the field of customized medicine, Three-dimensional (3D) printing technology is emerging out to be the blockbuster in personalized medicine. Scientists are developing 3D printing technology as a powerful tool for designing novel formulations and disease modelling [8]. Computer-based drug design along with 3D printing technology quickens manufacturing of personalized pharmaceutical drug products [9]. Using inkjet printing; in which a semi-liquid binding solution was mixed with powder bed to produces adhesive particles. The 3D printing pharmaceutical formulations are successfully prepared in recent years [10]. For obtaining the desired shape and size of the formulation, optimization of tools and techniques is a prerequisite. The first 3D printed drug that was approved by the FDA (August 2015) is SPRITAM (Levetiracetam) [11]. In this review, we are trying to highlight recent research and findings of 3D printing technology in the pharmaceutical sector.
Literature Review
What is 3D printing?
3D printing means the process which involves the formation of three-dimensional solid objects from a computerized or digital (ordinal) files [12]. The process of spraying or the laying down of additives continues unless successive layers create an object. The thinly sliced horizontal cross-sections of the eventual object have been seen in every layer.
Terminology related to 3D printing
Drug products: A drug product means a finished dosage form; capsule, tablet, solution, emulsion, suspension, etc. containing active pharmaceutical ingredients. 3D printing can fabricate other regulated products materials, which have well-defined quality purpose and regulation [13].
Three-Dimensional (3D) Printing
On the basis of the USGAO (United States Government Accountability Office), Three-dimensional objects from digital model are produced by 3D printing which uses method such as layer-by-layer process [14] in which underlying digital model is changed during processing. There are many synonyms affixed for 3D printing technology viz. rapid prototyping, solid free from, additive manufacturing and fabrication. [14,15]. The term preferred by the ASME (American Society of Mechanical Engineers) in place of 3D printing is additive manufacturing, for the purpose of pharmaceutical manufacturing process [16]. The process is called additive process but it could create confusion with other additive process like the process of capsule filling, coating or the film lamination process [10].
Brief outline of recent 3D printing technologies
A numerous variation of 3D printing techniques has presented by the RP Industries limited for the last 15 years [17]. Three-dimensional methods are commonly in use like laser-based writing systems, nozzlebased deposition systems, and printing based inkjet systems [18].
Printing-based inkjet systems: Two types of methods were used while performing the inkjet printing system. They are continuous inkjet printing (CIJ) and drop-on-demand (DoD) inkjet printing [19]. During inkjet proceedings, viscosity and fluid viscosity drop formation have to be controlled for proper 3D printing. While 3D printing, print head is equipped with multiple nozzles with approximately around 100 to 1000 in numbers. The disappearance of liquid is very palpable as temperature increase up to around 300°C while the electrical resistance taking place during printing [20]. As a result, bubble expands and conveys the energy essential to eject a droplet. Water is used as a solvent in thermal DoD inkjet systems because the high temperature is generated [21]. A hard coating layer of RP (Rapid Prototyping) building material is made when the printer head shoots out the articulated droplets; this process is known as Drop on Drop deposition [22]. It is also known as a drop on solid deposition when the solid material is made by the help of shoots droplets. Although the drop on drop deposition method is relatively hard as compared to the drop on solid deposition [23], these methods are used for printing an extensive pharmaceutical active ingredient [API]. Drop-on-powder and dropon- bed deposition is also known as the drop on solid deposition as the droplets of the binder is shooting into the powder material [24]
Drop-on-powder deposition: Due to the mixture of powder (bed) and binder (ink) they make a solid structure in a layer-wise manner [24]. They permit the elimination of remaining volatile solvents for the stability of the final product. Powder particle sticks due to the ink binder and causes the solidification. Powder topology and material reactivity by binder are the major two characteristics of powder in the drop on powder deposition [25].
Drop-on-drop deposition: Using a drop-on-drop deposition method, almost around 20 dissimilar geometrical shaped formulations embedded with nanocarriers can be possible which also offers a higher drug loading [26]. Polyethylene glycol [PEG] droplets of Ibuprofen were printed by Elele and coworkers. They then swallowed the material into a porous substrate which is formed by HPMC [27]. The threedimensional dosage form is created by superposition; the freeze-drying method was used to make a cellulosic derivative substrate and thus the Three-dimensional printing was not used to make a final structure [24]. To make a 3D printing PLGA and PLA are used in various methods such as drop-on-drop deposition, inkjet printing technique [24].
Fused-deposition modelling: In this method, the liquid thermoplastic polymer is runout by the help of two rollers across a high-temperature nozzle then moved to solidify on a build plate [28]. There is a free movement of print head inside the x- and y-axes however the platform moves only straight up along the z-axis to make a 3D structure by combining the layers together [29]. Some parameters are to be controlled like speed, height of the layer, temperature of the nozzle or building plates [30].
Laser-based writing systems: This method was the first commercially presented SFF (solid freeform fabrication) techniques and developed in 1986. In the field of bioengineering, they are heavily used and reviewed [24]. To make a model, prototypes, patterns, and production parts using a photochemical process with the help of printing technology in the form of Stereolithography [SLA] [31]. This research came during the 1970s, but the term was coined by Chuck Hull in 1984 when he patented his process, which was granted in 1986. Stereolithography is a material which can be used to make a medical model, and computer hardware and other sides. But they are very costly due to their property.
Nozzle-based deposition systems: Nozzle-based deposition systems allow direct writing, which relies on computer-controlled manufacturing methods that deposit ink direct through a nozzle to create a 3D pattern layer-bilayer with controlled composition and architecture [32]. Such systems may basically be divided into processes based on material melting and processes without material melting. Although numerous methods have been described in bioengineering applications, only a few have been used in the pharmaceuticals field [24]. More attention has been paid to SFF techniques based on pressure-assisted micro syringes (PAM) and hot-melt printing (HMP), in free-melting and melting material processes [24]. The PAM printing method is based on extruding a viscous semi-liquid material from a syringe to create a desired 3D shape. The process can be performed in a continuous flow at room temperature. The dispenser is usually based on a pressured-air piston [24].
Fused-deposition modeling: In fused-deposition modeling (FDM), a molten thermoplastic polymer filament is extruded by two rollers through a high temperature nozzle and thereafter solidifies onto a build plate [33]. The print head can move within the x- and y-axes whereas the platform, which can be thermostatic, can move vertically on the z-axis, creating 3D structures layer-bilayer by fusing the layers together [34]. Typical parameters that must be controlled during an FDM process are the infill density, the speed of the extruder, the height of the layers and the temperature of both the nozzle and the building plate [24].
Advantages
Customization: The biggest advantages of 3D printing is customization, which means any shaped and sized product can be prepared using this technique
Enhanced productivity: Mostly 3D printing is flawless and it is completely automated and computerized. In this technique possibilities of making prototypes or the small-scale version of real object in the stipulated timeline is possible which ultimately improvise the quality and customize the product.
Economical: The initial setup of the 3D printing premises is definitely very costly but due to lesser need of manpower while manufacturing 3D printed products, makes it reasonably inexpensive. Most importantly, the possibilities of human error while manufacturing produces are sensibly negligible.
Warehousing: Since 3D printing justifies customize principles, hence chances of large-scale production can be mitigated and storage cost can be cleaved.
Empowerment: There is a huge possibility to create a large number of jobs in design engineering and allied software engineering.
Healthcare: Now-a-days 3D printing technology is going in the next level; using bioprinting technology the possibilities of preparing artificial human body parts and organs is possible. Scientists are working in a direction where possibilities of making organs with unique characteristics of patient DNA would be possible.
Disadvantages
The demotion of manufacturing jobs: Since labor cost and demand of workforce is limited in 3D printing technology, there is a huge chance to have limited jobs in this sector. Which could ultimately lead to an economic slowdown.
Limited size and shape: Using 3D printing techniques huge structural production is a big challenge.
Utilization of limited raw materials: Very few raw materials are compatibles with 3D printers. Hence still lots of work to be done to include more polymers and materials in the list.
Copyright infringement: The possibilities of counterfeiting products or replica of the original object is more anticipating along with 3D printing technology advancement, which could boost the number of cases in copyright violation.
Production of derogatory products: With 3D printing devices possibilities are there to prepare lethal devises, weapons, guns which could be very much affordable and could boost terrorist and criminal activities.
Various methods utilized in 3D Printing technology
During the journey of 3D printing technology, different techniques were invented and evolved for the last 40 years. The following main methods are listed below:
• Powder Solidification process
• Liquid solidification process
• Extrusion process
Each and every 3D printers work according to a different mode which needs some sufficient material to be solidified for subsequent object fabrication (Figure 1).
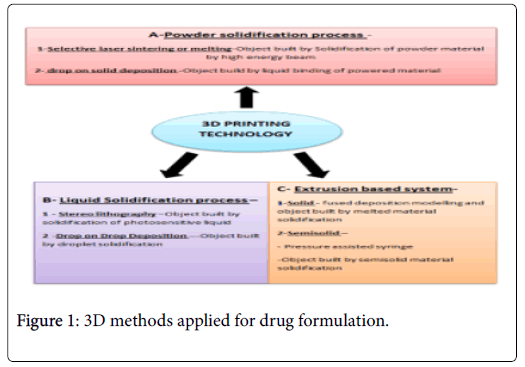
Figure 1: 3D methods applied for drug formulation.
According to printer specification, the 3D design objects with the computer-aided drug design and the optimization of geometry [35]. The generation of layers and software is done by the import of the file, which will be printed [36]. The printed layer light a key influence on the printed object quality and also in printing time [37]. The specific printing method involves the fabrications of the object with the help of following application (like- the process of solidification) of the material layers.
The development of 3D printed objects
In the pharmaceutical and medical applied field, the threedimensional printing method achieves more importance because tailor-made objects have the possibility of rapid preparation which can be used to apply for medicine or personalized therapy [5]. In dosage form, the route of administration is vital, the favourite one being the oral dosage form in which large scale research works are managed. In drug design, the growing interest in 3D printed products can be demonstrated by using different 3D printing techniques. Threedimensional printing techniques is the new method of 3D printing which is used in the development of different pharmaceutical dosage form and it can also be found on the approach available in patent entitled TDPT [38]. Drop on solid deposition is a printing process and its action mode is same to the DIP (desktop inkjet printers) and it is also called the drop on solid or powder bed jetting. The droplet ink sprayed from the print head binds to the layer of free powder particle, act like preventing support material from porous structures or collapsing or overhang [39]. After each step, free powder layer is applied by powder jetting system or roller and the formed object is lowered and further process is carried out [6]. The piezoelectric or thermal print heads deliver bonding agent available commercially in well-equipped condition [40]. The modifying agent, as well as active pharmaceutical ingredient, can be either distributed in powder bed or dispersed or dissolved in ink [41]. This process was used due to its similarity to the classical formulation process and its potential to apply the excipients usually used in the field of pharmaceutical techniques especially in solid (powder or tablet) dosage formulation [42]. The ink properties and powder influence product quality attributes. The flowability of powder and particle size influences mainly the layer height and also cohesion force between the part of printer or powder wettability and particles [43]. In the form of solvent used as ink constituents, modified excipients or APIs tend to change droplet size, viscosity and the process influence the powder binding efficiency [44]. The process parameter in the product development like droplet volume, printing speed, distance from powder bed and can have an impact on the powder bonding especially between layer Z-axis. The various solid systems prepared by Drop-on-solid method such as in combination with rifampicin, or implants with levofloxacin, rifampicin, and isoniazid, shows pulsatile release of APIs. Various developments in the 3D printing area have explored Zip Dose® technology commercialization by Aprecia® pharmaceuticals. Moreover, in 2015 Spritam® was first 3D (three dimensional) printed drug which was approved by the FDA [45]. Other, the release rate depends on the drug content were observed for Kollicoat® based formulation with the pH-independent release whereas Eudragit® formulation show pHdependent, modified release profiles which did not depend on drug loading [46]. The application of another polymer i.e., Hydro propyl methylcellulose or Kollidon® VA 64 and printing parameter optimization influences printlets characteristics and increases the laser scanner speed, results in more porous structure and also decreases the mechanical strength. The fast dissolution characteristics in case of Kollidon® VA64 printlets in 5 min cover 90% and rapid disintegration time 4S -7 [47].
Turning process of solidification
The idea of manufacturing object by solidification of liquid is same to powder solidification technique [48]. The nozzles spray ‘Droplets of ink’ which accumulate on the thin layers surface by in pressure of cooling air or high energy light. Due to the absence of suitable powder bed or polyjet technology, it requires the use of additional material to create support for the process of overhang geometries [25]. In process of printing, the print head moves along X and Y axis, the print platform bed is lowered along Z–axis after the deposition of material on each layer. Multi-material is used in full-colour printing, in this process different technical solution is used based on different techniques [49]. The curing method depends on the spraying material properties which also improve the quality of the printed product. And waxes as material are used in this technique firstly [50]. At present in pharmaceutical technology, 3D printing technology can be found for the formulation of modified released dosage form. [51]. For the prevention of rapid solidification in heating chamber, the hybrid platform was sprayed by molten wax. In another research the researcher prepared matrix tablets containing fenofibrate and beeswax by three-Dimensional products jetting of molten wax and its geometries effect on dissolution profile were revealed. The application of first attempt of Drop-on-Drop Method in drug formulation field revealed which is tested matrix material and is suitable for the fabrication of prolonged-release dosage forms [52]. The wide range polymer needs to be evaluated which is the challenge in this process. The modification in application of hydrophilic polymers or wax matrix composition has a great impact on dissolutions behavior [53]. The solidification method is based on UV light so possibility of Active Pharmaceutical ingredients decomposition and also the stability matter should be taken in consideration for this process [54]. The postprocessing removal of unbound polymer and photo-initiator should be done whereas the toxicity of the components should be checked before applying it in drug formulation [54].
Extrusion based method
In the pharmaceutical technology area, hot-melt extrusion (HME) and extrusion of semisolid materials are well-established processes [55]. This technical method is related to progress possibility of relatively inexpensive equipment and compact size, and increasing popularity of printing method. Two ways of printing method are well known [56].
Extrusion process of semi-molten materials or semi-solid (gels, pastes) at room temperature or elevated temperature
Extrusion process of molten celluloid or thermoplastic rod shape (filament) materials.
In both methods, the materials are extruded from the nozzle and are spread in subsequent layers on the build platform surface [57]. The distance of print head to build plate is created on a defined printing path and it is influenced by the nozzle orifice diameter. The quality of the printed object is affected by two-parameter nozzle orifice diameter and print speed [58]. Another layer is applied when the print head or print plate moves along z-axis at the distance of layer height [59]. Three-dimension printers and the technological solution are based on printing materials. Mostly world-wide extrusion-based method is used in 3D printing technique. In pharmaceutical manufacturing, this functional and versatile method is growing in a very interest rate [25]. The robotically actuated nozzle is used for material extrusion in an extrusion process. Any material can be printed by extrusion method or powder bed. In Figure 2 how HME methods help to prepare 3D printed polymeric Nano capsules were explained. 3D printer and 3D model: Various 3D printers and models are discussed in Table 1.
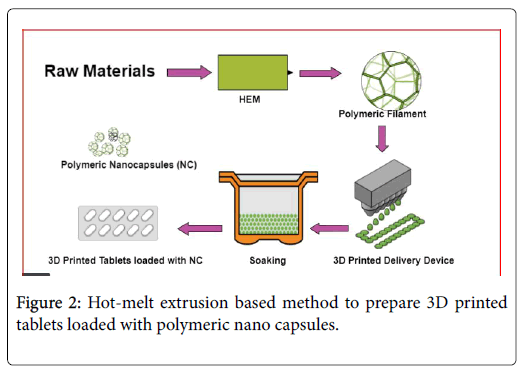
Figure 2: Hot-melt extrusion based method to prepare 3D printed tablets loaded with polymeric nano capsules.
| Printing |
Resolution |
Advantage |
Disadvantage |
Cell viability |
Commercially available bio printer / 3D model |
Reference |
| Techniques |
| Inkjet Bio printing |
100 μm |
High speed linear |
Low precision of droplet size and its placement |
80-85% |
Jet Lab 4 (Micro Fab), |
[155] |
| Piezoelectric/Thermal Inkjet Electrodynamics |
10-20 μm |
Fabrication availability |
Low viscosity bio ink needed slower volumetric speed. |
~ 95% |
Alpha / omega (3 Dynamic system) Bio Assembly Bot (Advanced life sciences) Aether 1 (Aether) |
| Acoustic Droplet Ejection (ADE) |
37- 150 μm |
High precision and controlled directionality |
Low viscosityBioink needed |
40-90% based on the force of extrusion |
Allevi 1,2,6 ( Allevi) Inkredible+, BIOX (Cellink) Bioplotter (Envision TEC) |
| Micro-Extrusion Bio printing |
15- 400 μm |
Extrudes high viscosity Bioink and prints with higher cell density |
Distortion of call structure and chances of reduction in cell viability |
40-90% based on the force of extrusion |
BioX (cell Link) 3D- Bio plotter (Envision TEC) 3D Discovery (regen HU) Bio Scaffolder 3.1 (GeSim) |
| Laser Based Bio –printing- |
10-100 μm |
High resolution precision, ability to use medium to high viscosity bio link and print high cell density |
Very Low Volumetric speed, time consuming high cost |
95% |
Poietis (France),Regenova (Cyfusebiomedical),Regemat 3DV1 (Regemat3D) |
[156] |
| BioLP/AFA.LIFT/MAPLE-DW |
100 nm |
| Laser guided Direct Writing |
10 μm |
| Stereo lithography (SLA) |
∼1 mm |
Highly accurate and quick fabrication |
UV light exposure is potentially harmful for cells under long exposure, post-processing, limited number of compatible biomaterials |
~ 90% |
|
[157] |
| Direct 3D Cell Printing (3DP) |
260-500 μm |
Scaffold-free printing method and no physical or chemical crosslinking required |
Structures are initially weak and difficult to handle mechanically,require time to mature and become self-standing tissue and specific to limited cell types |
~ 95% |
Regemat 3D V1 (Regemat, Spain) |
[158] |
| Selective Laser Sintering (SLS) |
20–200 μm |
High mechanical strength scaffolds fabricated |
Cell-free fabrication method, specific to few materials that can be sintered, slow degradation and resorption of scaffolds |
-- |
-- |
[159] |
Table 1: 3D printer and 3D model commercially based, printing technique, resolution, advantage and disadvantage.
Current challenges in drug development
The overall challenges for pharmaceutical industries are paramount regarding successful infusion of new drug molecules or medicaments into the market [60]. The rate of approval of drugs is very limited; hence it becomes necessary for scientists to identify suitable drugs promptly using preclinical or clinical studies during early phase of drug development process. In low cost, there are multiple formulations to support flexible bioavailability with easy administration [61]. But drug solubility and stability are a big concern for materializing new drug into a novel formulation.
Examples of current 3D printing technologies in pharmaceutical drug delivery
Depositing material layer by layer, 3DP can fabricate objects of almost any shape and size on demand [62]. Structures are made from a digital 3D file created using computer-aided design (CAD) software [63]. Enabling the bespoke and personalized objects to be readily manufactured. As such, 3DP has beneficial applications in many industries, engineering to personalized devices in medicine.
Development of 3D printing formulations
3D printing (3DP) predicted to be a highly advanced technology within the pharmaceutical area. 3D printing is the manufacturing process of making three dimensional solid objects from a digital file [57]. This technology has been applied to pharmaceuticals to manufacture medical devices and print lets, which is a term that we have coined to refer to 3D-printed solid oral dosage forms [64]. Thus far, a range of formulations has been produced, including those containing multiple active pharmaceutical ingredients (APIs), with different release characteristics. This technology enables precise doses to be deposited based on the initial ‘ ink ’ concentration and the physical dimensions of the formulation. in 2016, the first 3D-printed tablet approved by the US Food and Drug Administration (FDA) was commercialized for the treatment of epilepsy (Spritam1 by Aprecia Pharmaceuticals) [65]. Formulations are prepared in a layer-by-layer, which are ready for ‘dispensing’. This method of production in the printing platform is used [66]. This novel method of formulation production could be beneficial in several clinical applications, from streamlining the drug development process through to creating personalized medicines [67]. Examples of the types of formulations that have been produced using each technology are provided in table. In year of 2015 first 3D printed tablet manufactured by Aprecia Pharmaceuticals was approved by the FDA. Two years later, GlaxoSmithKline completed a study where inkjet 3D printing and ultraviolet (UV) curing were used to create tablets that treat Parkinson’s disease [68]. In Table 2 various 3D printed technology and various excipients used in oral dosage form was explained.
| 3D printing technology |
Formulation |
API |
Excipients |
References |
| FDM |
Caplets |
Paracetamol or caffeine |
Polyvinyl alcohol (PVA) |
[160] |
| |
Caplets |
Budesonide |
PVA |
[161] |
| |
Tablets |
4-aminosalicylic acid (ASA) or 5- ASA |
PVA |
[162] |
| |
Tablets |
Hydrochlorothiazide |
PVA and mannitol: inner, polylactic acid (PLA): outer |
[163] |
| |
Tablets |
Theophylline |
Eudragit RL or RS or E or hydroxyl propyl cellulose (HPC) SSL, varying concentrations of triethyl citrate (TEC) or triacetin |
[164] |
| |
Tablets |
Prednisolone |
PVA |
[138] |
| |
Oral films |
Aripiprazole |
PVA |
[165] |
| Binder jet printing |
Tabular device |
Methylene blue and alizarin yellow (dyes) |
Polycaprolactone (PCL) and polyethylene oxide (PEO) |
[166] |
| |
Tablets |
Paracetamol and alizarin yellow (dye) |
Colloidal silicon dioxide (SiO2), mannitol, polyvinylpyrrolidone (PVP) K30, and lactose |
[167] |
| |
Cubic tabular devices |
Pseudoephedrine |
Kollidon SR, hydroxypropylmethylcellulose (HPMC) |
[72] |
| |
Tablets |
Chlorphenamine maleate and fluorescein |
Avicel PH301, Eudragit E-100, RLPO in ethanol or acetone, or PVP and Tween 20 in deionized water |
[168] |
| |
Orodispersible tablets |
Levetiracetam |
Microcrystalline cellulose (MCC), glycerine, Tween 80, povidone, sucralose |
[72] |
| SSE |
Bi-layered tablets (polypill) |
Guaifenesin |
Polyacrylic acid (PAA), MCC and sodium starch glycolate |
[2] |
| |
Multiactive tablets (polypill) |
Nifedipine, glipizide, and captopril |
HPMC |
[72] |
| |
Multiactive tablets (polypill) |
Hydrochlorothiazide, aspirin, pravastatin, atenolol and ramipril |
Polyethylene glycol (PEG) 600, D-mannitol, cellulose acetate |
[169] |
| SLS |
Tablets |
Paracetamol |
Kollicoat IR or Eudragit L, can during gold sheen |
[54] |
| |
Drug delivery device |
Progesterone |
PCL |
[170] |
| SLA |
Tablets |
Paracetamol or 4-ASA |
Polyethylene glycol diacrylate (PEGDA), PEG 300 and diphenyl (2,4,6-trimethyl benzoyl)phosphine oxide |
[2] |
| |
Hydrogels |
Ibuprofen |
PEGDA, PEG 300, diphenyl (2,4,6- trimethyl benzoyl)phosphine oxide or riboflavin and triethanolamine |
[171] |
| |
Facial mask |
Salicylic acid |
PEGDA, PEG 300, diphenyl (2,4,6- trimethyl benzoyl)phosphine oxide |
[92] |
| Desktop 3D printer |
Tablet |
Guaifenesin |
--- |
[172] |
| A laboratory –scale 3D machine |
Capsule |
Pseudoephedrine |
--- |
[173] |
| Fused deposition modeling (FDM) |
Tablet |
5-Aminosalicylic acid (5-ASA mesalazine) and 4-aminosalicylic acid (4-ASA) |
--- |
[162] |
| 3DP extrusion-based printing |
Tablet |
Captopril with Nifedipine and Glipizide |
--- |
[72] |
| 3DP technology |
Implant |
Acetaminophen |
--- |
[73] |
| Inkjet 3DP |
Nanosuspension |
Levofloxacin |
--- |
[173] |
| 3DP machine |
Multi-drug implant |
Rifampicin and Isoniazid |
--- |
[168] |
| Inkjet 3DP |
Nanoparticles |
Folic acid |
--- |
[173] |
Table 2: Examples of oral dosage forms using different 3DP technologies.
Early-phase drug development
The pharmaceutical companies faced various problems with the development of the successful new drug reaching the markets [69]. The rate of drug failure is enough during the early-phase development of the drug. Therefore, it is essential for companies to quickly identify suitable drugs at the low cost and as sharply as possible during the drug development process; mostly preclinical studies or clinical trials [70]. There are various formulations to support early-phase drug development, displays high dose flexibility and high bioavailability, are easy to administer and support fast case study progress at low cost [68]. Furthermore, current formulation strategies come along with different associated challenges, from issues around drug solubility and stability.
Application of 3D printing technology in the drug delivery system
Inkjet printing: In this technique, for the personalized medication, inject printers are mainly used with different combinations of active ingredients and excipients (ink), which is precisely sprayed in small droplets, via drug on-demand or continuous jet method in varying sizes, layer by layer into a non-powder substrate [71]. The idea of inkjet printing originated from the uses of computer-operated inkjet printing which makes a digital image by ink droplets on to the paper [72]. The two types of inkjet dispensing printers have been investigated Thermal & piezoelectric inkjet printers for the pharmaceutical application. This technique is a powder-based 3D printing that uses a foundation or powder for the sprayed ink where it is solidified into a solid dosage form. Most of the inkjet drug printer offers a significant advantage of accurate control of dose combinations and pattern release [73].
Direct-write: It uses a computer-controlled translational stage that moves a pattern-generating device to achieve, layer-by-layer, 3D microstructure [74].
Zip dose: It provides a personalized dose in addition to the delivery of a high drug-load with high disintegration and dissolution levels by manufacturing highly porous material [75].
Thermal Inkjet (TIJ) printing: TIJ system consists of a micro-resistor that heats a thin film of ink fluid (located in the ink reservoir) forming a vapor bubble that nucleates and expands to push the ink drop out of a nozzle. TIJ affords the opportunity of dispensing extemporaneous preparation/solution of the drug onto 3D scaffolds (drug carriers/ films) [76].
Fused deposition modeling (FDM): Another name of FDM is Fused Filament Fabrication which first came into the market by 1991 [77]. It is one of the most common low-cost techniques in 3D printing. Usually, this technology has been established for non-pharmaceutical purposes with a wide range of low-cost printers available [78]. Recently, FDM 3D printing has been found in the drug research and development where thermoplastic polymers such as PVA have been used as drug carriers [72]. This process can be applied to multiple dosage forms that are applied in polymers as part of the framework such as implants, zero-order release tablets, multi-layered tablets, and fast-dissolving devices. In the process, the polymer is melted and extruded through a movable heated nozzle. The layer by layer ejection of the polymer is repeated along the various stage, followed by solidification to create a shape previously defined by the computeraided design models [79].
The polymeric approach in 3D printing technology for the controlled drug delivery system:
Bio fabrication using polymers in 3D printing dosage form is a new technique which could alter the release rate of drug and could provide paramount stability for incorporated active pharmaceutical ingredient [64]. Polymers of natural and synthetic origin are vividly in use to decorate 3D printing dosage form [80]. Various natural polymers such as chitosan, alginate, and gelatine have been in use to develop 3D printed dosage form but these polymers often dictate crosslinkers which could be cytotoxic [81]. Hence, synthetic polymers such as Hydroxypropyl methylcellulose (HPMC),Poly (lactic-co-glycolic acid) (PLGA), Polyvinyl alcohol (PVA), Poly (methacrylates) (Eudragit), Polylactic acid (PLA), Polyvinylpyrrolidone (PVP), Poly (ethylene glycol) diacrylate (PEGDA), Polyurethane (PU), Poly (?-caprolactone) [PCL], Ethylcellulose (EC), Poly (methyl methacrylate) [PMMA], Poly (N- (2-hydroxypropyl) meth acrylamide-mono/diacylamine-mono/ dilactate)-polyethylene glycol triblock copolymer [M15P10] has gained attention to circumvent all associated problems caused by natural polymers [82]. Some important polymers used in 3D printing formulations and their significance are illustrated bellow:
Polyvinyl Alcohol (PVA): Certain polymers like PVA has thermoplastic and higher water-soluble properties which improvise its mechanical strength and reabsorption qualities [83]. The best part of selecting PVA as 3D printing material is its gas transmission temperature; which is almost around 84-86°C [84]. Nevertheless, PVA starts degrading at a higher temperature of around 340-450°C, which makes it is more ambient for 3D printing [85]. As far as the toxicity is concerned, PVA has reported minor toxic in mice model; as LD50 value was found to be 14.7 g/kg. Nowadays many types of research is going on to incorporate PVA in 3D printing formulations [86]. One of such research is in ethanolic solution 2% w/v fluorescein loaded PVA filament. This PVA filament was fabricated in tablets using Marker Blot Replicator 2 × 3D printer. The software which was used in fabrication operation was Marker Ware [87]. Noticeably, ethanol enhances the drug encapsulation proprieties of PVA. For thermolabile drugs, PVA has limitation because of its higher melting point. Now a day’ s scientists are using PVA and PLA combination to fabricate 3D printed formulations. Using 3D printing technique it is now possible to incorporate two incompatible drugs together in a single dosage form like, rifampicin and isoniazid [88]. The significance of FDM 3D printers were checked by altering different molecules in PVA filaments.
Polyurethane (PU): Basically, polyurethane is a biocompatible thermoplastic polymer which could mimic like a human tissue due to the presence of soft oligodiol and diisocyanate [89]. Ionization of PU helps to disperse in water. The PU has several advantages like versatile used in biomedical wound dressing, surgical dressings & drug carriers [90]. As per recent studies theophylline and metformin 3D printed formulation was prepared using PU using fused deposition modeling (FDM) technique [91]. In this technique the temperature was affixed at 100-180°C for the preparation. Noticeably, formulation surface was rough and almost around 60% drug loading was observed in the filament. In 3D printing technique, PU helps to produce more porosity in the body surface of the tablets, hence indirectly PU helps in to improve sustain release property of the formulation [92]. In another study, PU and Polycaprolactone (PCL) synthesis together to produce sol-gel transition characteristic in human skin contact but remain less viscous at room temperature. In upcoming years PU could be seen in injectable hydrogel and injectable preparation [93].
Polylactide (PLA) and Poly (lactide-co-glycolide) [PLGA]: The best part of PLA is its less toxicity and easy fabrication quality [94]. Even though PLA is hydrophobic and partial crystalline in nature but it can easily be hydrolyzed and removed from the normal body clearance [95]. PLA is very stigmatic to produce laevorotatory (L) and dextrorotatory (D) configuration which ultimately results in stereo complexes of L-PLA & D-PLA. The combination of PLA and Polyethylene oxide (PEO) is now-a-days featuring in various 3D printed formulation, because PLA produces good biodegradability and hydrophilicity, where else, PEO produces good biocompatibility [96]. Recently, multi-head -dispersion system (MHDS); a melting head technique for 3D printing, is in use to produce PLGA-PCL coated 5- FU sustained release dosage form for colorectal cancer treatment [97]. In the MHDS technique, the melted blend was extruded from the storage tank at 140°C and maintaining pressure at 600kPa [98].
Poly (?-caprolactone) [PCL]: The remarkable advantages of USFDA approved PCL was its outstanding biocompatibility, fewer toxicity, elasticity, semi-crystalline behavior with limited water absorbability [99]. Jenny Hollander et al. [100] developed indomethacin loaded 3D printed T-shaped architype intrauterine system (IUS) using fused deposition modeling (FDM™) technique. In this research the principal polymer was used for the filament was poly (ε-caprolactone). In another research, Alvaro Goyanes et al. [101] developed salicylic acid loaded 3D printed formulations for the treatment of acne using fused deposition modeling (FDM) and stereolithography (SLA). In this research, Flex EcoPLA™ (FPLA) and polycaprolactone (PCL) filaments was loaded with 2% w/v salicylic acid using hot-melt extrusion (HME) method [92]. Drug loading was found be little high (1.2% w/w) in PCL-salicylic acid 3D printed patches as compare to the FPLA-salicylic acid 3D printed patches (0.4% w/w) respectively. In recent era of 3D printing technology porous scaffolds have gained many attentions aseptically in the field of bone tissue engineering [102]. The modified silica-based stabilized nanoparticles were embedded in scaffolds which comprise of PLA and PCL [103].
Hydroxypropyl Methylcellulose (HPMC): For the better development of impanated printable drug-polymer filament, HPMC has extensive use [104]. Using hot melt-extrusion and 3D printing technology, Jiaxiang Zhang et al. [105] developed 3D structured tablets for constructed for zero-order release of active pharmaceutical ingredient. The specific mathematical models responsible for drug release from the 3D printed tablets were studied. For this research acetaminophen was considered as a model drug, where else HPMC E5 and polyethylene glycol, polyvinyl acetate and polyvinyl caprolactamebased graft copolymer (Soluplus®) were taken to formulate nine 3D printed tablets with different inner and outer core-shell thickness [54]. The results of this research indicating successful fabrication of 3D structured tablets using hot-melt extrusion (HME) and 3D printing technology for zero-order drug release. In similar trend, Hossam Kadry et al. [106] developed 3D printed Diltiazem and HPMC impregnated multi-purpose filaments. The authors did study the crystalline properties of the Diltiazem, cytotoxicity of the multipurpose filaments, drug release profile, drug loading etc. The Selective laser sintering (SLS) an effective three-dimensional printing (3DP) technology has wide-angle use in fabrication of metal and plastic materials [107]. In a recent study, Fabrizio Fina et al. [108] Demonstrated the possibilities of using SLS to fabricate novel solid dosage form for accelerated drug release with thinking to produce a qualitative orally disintegrating formulation. For this novel preparation, HPMCE-5 & vinylpyrrolidone-vinyl acetate copolymer (Kollidon® VA 64)) was used as a principal polymer [109]. These polymers were mixed separately with 5% acetaminophen and 3% Candurin® Gold Sheen colorant to proceeds SLS printing which ultimately results in 3DP tablets. SLS printings help to alter the release patterns of the printlets [110].
Eudragit (Methyl prop-2-enoate;2-methylprop-2-enoic acid): The Poly (meth)acrylates commonly known as Eudragit is basically an ester of arylene and methacrylic acid [111]. The Eudragit is available in L, S, FS & E, etc. grades with enabling alkaline or acidic groups for pH depending release of drug [112]. Eudragit RS & RL polymers with alkaline and neutral group help in pH-dependent drug release and control the release of drug [113]. As per Muzna Sadia et al. [114] work, a universal filament on pharmaceutical usage was being prepared using methacrylic polymer (Eudragit EPO) and TCP (tribasic calcium phosphate). Using hot-melt extrusion (HME) and fused deposition modeling (FDM) 3D printing techniques 5-ASA, captopril, theophylline, and prednisolone drugs were incorporated in patienttailored immediate-release tablets. This unique implementation of an idea can provide low-cost production and customize medicine. In another research Muqdad Alhijjaj et al. [115] stressed out the production of personalized medicine using FDM 3D printing technique. In their research felodipine was successfully fabricated using FDM 3D printing technique using tween 80, PEG, PEO, Eudragit E PO [116]. The obtained polymer blends exhibit splendid printability and well-suited with commercially available FDM 3D printers [117].
Polyvinyl Pyrrolidone (PVP): PVP is well equipped with properties like biocompatibility, excellent stability, non-significant toxicity; wide range of solubility in different organic and non-organic solvents and most importantly, affinity to complex with both hydrophilic a hydrophobic substance [118]. These qualities make is best suitable polymer for drug delivery system. In gastroprotective dosage form, PVP has enormous use. As per Wei Long Ng et al. [119] drop-ondemand 3D bioprinters are having huge significance in biological applications. The entire printing bio-ink (0-3% w/v) was made by polyvinylpyrrolidone (PVP) macromolecules [120]. In this type of bioink at initial stage permeability checking is preamble. The Z value was investigated as viability of the printed cell depends on Z value. PVP helps to mitigate the cell addition and sedimentation during bioprinting process [120]. As per optimization results 2-2.5% w/v of PVP postulated most consistent cell output over 30 minutes [121]. The Wei Long Ng et al. tried to convey scientific conclusion that PVP could improvise cell homogeneity and cell viability during drop-on-demand 3D bioprinting process [122]. In similar type of research Tochukwu et al. [123] did prepare immediate-release tablets via lower temperature FDM printing technique by considering polyvinylpyrrolidone (PVP) as principal polymer. The 3D printed caplet shape 10% theophylline loaded filaments were prepared using hot-melt extrusion (HEM) method. The morphology of the tablet was assessed using scanning electron microscopy (SEM) [124]. Other parameters were checked using x-ray powder diffraction, HPLC method. The conclusion of this research indicating that, due to the presence of PVP as an integral polymer at lower temperature (100°C) the integrity and crystalline property of theophylline remain intact and fabricated tablets possessed excellent mechanical properties with excellent immediate in vitro release qualities [125].
Polyethylene Glycol Diacrylate (PEGDA): Polyethylene glycol Diacrylate is basically a low toxic hydrophilic ethylene oxide unit with a polymerizable ending [126]. PEGDA acts like a crosslinking monomer which helps to prepare 3D printed hydrogels. PEGDA has water-soluble acrylate groups which ultimately help in photopolymerization which ultimately benefits in preparing hydrogels [127]. It is also evidence that high concentrated pH-sensitive PEGDA helps in delaying drug release [128]. Nowadays PEG and PEGDA combination are in use to prepare 3DP hydrogels via SLA printed method [129]. Sebastian Joas et al. [130] developed a 3D printed hydrogel for tissue engineering usage using poly (ethylene glycol) Diacrylate (PEG-DA), 2- (acryloyloxy) ethyl trimethylammonium chloride & 3-sulfopropyl acrylate. It was found that presence of 22.5% w/w Poloxamer 407 (P407) in hydrogel improvised yield stress up to 100Pa. Nowadays in the preparation of stable superparamagnetic iron oxide nanoparticles, Polyethylene glycol Diacrylate is in common use.
Other polymers in use in 3D printing technology: Since 3D printing technology is an experimental technology for pharmaceutical products, hence many other polymers like ethyl cellulose (EC) and ethyl vinyl acetate (EVA) are in genuine use. In recent years, for the preparation of acetaminophen 3D printed novel multilayer drug delivery technology, the varying concentration of ethyl cellulose have in used [131]. Due to 3D printing technology, the acetaminophen multilayer formulation shows simultaneous surface erosion which ultimately results in desired drug release system [132]. In similar fashion, using FDM technology Ethyl vinyl acetate has been in use to prepare T-Shaped intrauterine system (IUS) and subcutaneous (SR) rods [133]. A drug like indomethacin can be used in 5% and 15% in 3D printed filament preparation. Mostly, the 3D printed prototype was found to be amorphous in nature [134]. Certain polymers like Kollidon VA64 or amalgamation with Kollidon 12PF help to exterminate drug degradation problem in FDM printing technology [135].
Major obstacles of polymeric 3D printed technology
Since the incorporation of 3D printing technology in pharmaceutical products is nowadays trending in scientific communities, many numbers of polymers are in use to cross-check the best possibilities of 3D printed optimized products [136]. However, regulatory approval from USFDA remains a great concern to commercialize 3D printed products for human consumption [78]. Another major drawback is most of the time 3D printed formulation filaments were prepared in high temperature which may lead to instability for certain thermolabile drugs incorporating in 3D printed films. But this problem can be sorted out by incorporating nanoformulations of the drug in melted polymer [137]. Which ultimately leads to Thermo protection of the drug? Another significant drawback is lower availabilities of fused deposition modeling (FDM); which could help in solid oral preparation [138]. The suitability & compatibility of polymers with FDM is a big concern in 3D printing technology. Since 3D printed formulations are mostly personalized in nature, hence it become very difficult to standardized the formulations.
3D printing in pharmaceuticals
As per United States Government Accountability Office (GAO),3D printing generates 3D objectives from digital models, and these objectives are to produce by layer by layer process.3D printing is achieving increasing attention in pharmaceutical formulation as they produce different dosage from in different shapes, sizes & release characteristics.3D printing technology overcomes some challenges in conventional pharmaceutical preparation [139]. Traditional pharmaceutical preparation involving milling, mixing, granulation, compression which result, drug loading, drug release, drug stability & dosage form stability.3D printing is needed in pharmaceuticals for personalized dosing such as needs variable dose, needs targeted therapy, need orphan drug, needs to adjust dose based on diagnostic response.3D printing medicines are enter into the pharmaceutical market as they are potential to achieve personalized treatments of each patient [78]. Personalized treatment is design by considering patient age, weight, comorbidities, pharmacogenetic and pharmacokinetic characteristics. Ex: Imagine there is an older patient who will have prescribed polypill per day but he forgot to take, it is solved by taking a single pill, if suddenly the patient will have serious problem and don’t have time to go to the doctor or any specialized he has the facility to produce his required medicament by 3D printer [140]. The variability is worldwide problem when treating patients from different medical backgrounds with diverse customs & necessities [141]. This variability has been accepted as part of the therapeutic process all over the year, but now a day ’ s new technologies allow for the optimization of treatments according to population subgroups, based on pharmacogenetics and pharmacokinetic profile [142]. Pharmacokinetic characteristics such as weight & age are important to dose adjustment to achieve the desired therapeutic effect [143]. Polymedicated patients have the risk of side effect, which is minimized by intake of a single pill containing all the drugs required for the patient [141]. This single pill is producing by 3D printing technology. In this way 3D printing enters into the drug therapy [144]. We have reached an era in pharmaceutical field whereby “one size does not fit all”. Science medication must be individual patient to patient. FDA approved 3D printing drug product in August 2015 [145]. The Food and Drug Administration agency (FDA) granted the approval of Spritam®, the first 3D printed tablet for the treatment of epileptic seizures [24]. Table 3 explained about various oral dosage forms produced by 3D printing technology.
| 3D printing technology |
Formulation |
Study aim |
API |
Excipient |
Reference |
| Binder jet printing |
Tabular device |
Fabrication of novel drug delivery Devices. |
Methylene blue and alizarin yellow |
Polycaprolactone (PCL) and polyethylene oxide (PEO) |
[174] |
| Tablets |
Fabrication of fast-dissolving drug delivery device |
Paracetamol and alizarin yellow |
|
| Cubic tabular devices |
Development of near zero-order release dosage forms |
Pseudoephedrine |
Colloidal silicon dioxide (SiO2), mannitol, polyvinylpyrrolidone (PVP) K30, and lactose |
| FDM |
Caplets |
Evaluate microstructure and drug release |
Paracetamol or caffeine |
Polyvinyl alcohol (PVA) |
[33] |
| Caplets |
characteristics of PVA based Caplets |
Budesonide |
Polyvinyl alcohol (PVA) |
| tablets |
Fabrication of controlled-release tablets Fabrication of modified-release |
4-amino salicylic acid (ASA) or 5- ASA |
PVA |
Table 3: Examples of oral dosage forms produced by 3D printing technology.
Application of 3D printed drug
Commercially available 3D printed drugs: Spritam® is marked by Aprecia Pharmaceuticals using the ZipDose technique based on powder bed fusion. Spritam® made by the layer-by-layer production system. The pharmacological efficacy of Spritam® was found to be equivalent to conventional tablets. The great improvement is the solubilization time of Spritam was significantly reduced due to its porous and soluble matrix composition [146].
Personalized topical treatment devices: Nose-shaped masks, loaded with salicylic acid, used for anti-acne treatments, have been developed in a short and efficient manner [92]. The face of the patient was scanned and the taken image was projected to the autocad program, through which the nose section was selected. FDM and SLA, to determine which one was more favorable in terms of engineering, the morphological characteristics of the object, drug release, and the stability during printing. SLA was the most accurate technology for mask manufacture [147].
3D Printing for cancer treatment: Chemotherapy has widely applied in cancer treatment but chemotherapy can cause side effect [148]. Chemotherapeutic drugs have poor solubility in aqueous media; thus, they are administering through a different route. Currently, the construction of patches loaded with 5-fluorouracil, poly (lactic-coglycolic) acid, and PCL have been efficaciously printed and implanted directly into pancreatic cancer [149].
3D printed polypill: The concept polypill described that a combination of several drugs in a single personalized tablet. It provides advantages over poly medicated patient such as elder person [150]. Different polypills using 3D extrusion printing have been successfully created. As an example, captopril, nifedipine, and glipizide, to treat hypertension and type 2 diabetes. In Table 3 examples of recent oral dosage forms produced by 3D printing technology was reported.
Future prospectives: The future of pharmaceutical is represented by 3D printed drug manufacturing technology. 3D printing plays an important role in the field of personalized medicine [151]. It is used in customizing nutritional products, organ, and drugs. Industries along with all society prefer 3D printing as a method for manufacturing medicine & healthcare product [152]. 3D printing helps in the manufacturing of medications with continued research. Drug manufacturing and distribution is a costly process in the pharmaceutical industry. 3D printing tablet production is done within the clinic, local pharmacies or even in the patient home [153]. Personalized medicines for which 3D printing technologies could find huge interest, is based on the biomolecules, which is more sensitive (e.g. solvent, temp, agitation) than familiar chemical entities. Personalized medicine will be a new option in the pharmaceutical field [102]. It will reach new levels of possibility & pharmacist will be trained for this particular application of 3D printing. Most common medication become available in this way, patient will be able to reduce their medication load to one polypill per day, which will produce patient compliance.3D printing technologies may change pharmacy practice by allowing individualized medication and tailored specifically to each patient, although technical and regulatory hurdles remain [47]. Several manufacturing techniques come under 3D printing that is associated to construct freeform geometrics by layer-by-layer. However, freeform fabrication methods are generally assisted with 3D printing [154]. In the area of biomanufacturing, these processes are mostly in use. The main agenda of discussion was to discuss the various methods possibly applicable in pharmaceutics. These are progressive and fascinating techniques that can be used in 3D printing for customized drug delivery systems. 3D printing approach was used to prepare the tablet firstly by Aprecia Pharmaceuticals in 2015 and accepted with FDA [114]. 3D printing technique with ultraviolet (UV) curing was and is used by GlaxoSmithKline to formulate tablet for treatment of Parkinson’s disease [44]. They have the capability to change the pharmaceutical industry.
Conclusion
In 3D printed drug delivery system FDA has approved many polymers such as PLA, PVA, PLGA, PLC etc which has wide range of applications. For the preclinical evaluation now a day’s 3D printed livers and kidneys are being manufactured. The recent paradigm shift in fabrication techniques like printing-based inject system, leaserbased writing and nozzle -based deposition systems in 3D printing drug delivery system created lots of buzz in pharmaceutical sector. The challenges of limited drug loading are also trying to sort out using TheriForm TM process. The applicability of 3D printed drug delivery system is still in early stage as far as the pharmaceutical sector is concerned. But due to physician and patient acceptance in personalize medicine we could see a tremendous acceleration in 3D printing technology in upcoming days. Now a day’s, scientific research stressed to developed bioprinter organ complex which could be the possible future for organ transplantation. Using 3D printing technology, it is possible to achieve recreation of organ and transplantation within 20 years framework. The 3D printing technique also gone to the next level as now-a-days it is possible to recreate organs inside of the human body while body is under full operation. The significance of 3D Printing technology in pharmaceutical sector is growing inevitably. The technology is showing huge potential while compiling personalized dosage forms. The era of 3D printing would transform to smart formulations which could mitigate preclinical and FIH trials. Recently approved 3D printed tablet called Spritam has created a benchmark for the pharmaceutical companies. Many researches is already under way as a matter of fact many scientific journals can be seen in recent times to discuss 3D printed technology or highlighting recent findings of 3D printing technology. Despite its substantial progress drug development using 3D printing technology remain its infancy as many stringent regulatory requirements has to be follow before prevalent inclusion of 3D printing technology in pharmaceuticals sector.
24759
References
- Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater. Rice EW, editor. Washington, DC: American Public Health Association
- Bajracharya SR, Shrestha BR (2011). The status of glaciers in the Hindu Kush-Himalayan region. International Centre for Integrated Mountain Development (ICIMOD).
- Bolch T, Pieczonka T, Benn DI (2011) Multi-decadal mass loss of glaciers in the Everest area (Nepal Himalaya) derived from stereo imagery. The Cryosphere 5: 349-358
- Yde JC, Anderson NJ, Post E, Saros JE, Telling J (2018) Environmental change and impacts in the Kangerlussuaq area, West Greenland. Arct Antarct Alp Res 50: S100001
- Bhatt MP, Masuzawa TO, Yamamoto M, Sakai A, Fujita K (2000) Seasonal changes in dissolved chemical composition and flux of meltwater draining from Lirung Glacier in the Nepal Himalayas. IAHS Publication 2: 277-288
- Rajkarnikar G (2011) Water resources of Nepal in the context of climate change. Government of Nepal, Water and Energy Commission Secretariat
- Change IC (2007) The physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change p. 996
- Moore RD, Fleming SW, Menounos B, Wheate R, Fountain A, et al. (2009) Glacier change in western North America: influences on hydrology, geomorphic hazards and water quality. Hydrol Process 23: 42-61
- Singh VB, Ramanathan AL, Pottakkal JG, Kumar M (2015) Hydrogeochemistry of meltwater of the Chaturangi glacier, Garhwal Himalaya, India. Proceedings of the National Academy of Sciences, India Section A: Physical Sciences, 85: 187-195
- Xu H, Hou Z, An Z, Liu X, Dong J (2010) Major ion chemistry of waters in Lake Qinghai catchments, NE Qinghai-Tibet plateau, China. Quatern Int 212: 35-43
- Singh VB, Ramanathan AL, Pottakkal JG, Sharma P, Linda A, et al. (2012) Chemical characterisation of meltwater draining from Gangotri glacier, Garhwal Himalaya, India. J Earth Syst Sci 121: 625-636
- Ives JD, Shrestha RB, Mool PK (2010) Formation of glacial lakes in the Hindu Kush-Himalayas and GLOF risk assessment Kathmandu: ICIMOD
- Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Eos Trans AGU 25: 914-928
- Shrestha AB, Wake CP, Mayewski PA, Dibb JE (1999) Maximum temperature trends in the Himalaya and its vicinity: an analysis based on temperature records from Nepal for the period 1971–1994. J Clim 12: 2775-2786
- Feng F, Li Z, Jin S, Dong Z, Wang F (2012) Hydrochemical characteristics and solute dynamics of meltwater runoff of Urumqi Glacier No. 1, eastern Tianshan, northwest China. J Mt Sci-Engl 9: 472-482
- Tuladhar A, Kayastha RB, Gurung S, Shrestha A (2015) Hydro-chemical characterization of glacial melt waters draining from Langtang Valley, Nepal. Journal of Water Resource and Protection 7: 605
- Dougall JA (2007) Downstream effects of glaciers on stream water quality (Doctoral dissertation, Portland State University).
- Modenutti BE, Balseiro EG, Queimalinos CP (2000) Ciliate community structure in two South Andean lakes: the effect of lake water on Ophrydium naumanni distribution. Aquat Microb Ecol 21: 299-307
- Koch JC, McKnight DM, Baeseman JL (2010) Effect of unsteady flow on nitrate loss in an oligotrophic, glacial meltwater stream. J Geophys Res Biogeo 115
- Singh VB, Ramanathan AL, Sharma P, Pottakkal JG (2015) Dissolved ion chemistry and suspended sediment characteristics of meltwater draining from Chhota Shigri Glacier, western Himalaya, India. Arab J Geosci 8: 281-293
- Li X, Ding Y, Han T, Kang S, Yu Z, et al. (2019) Seasonal controls of meltwater runoff chemistry and chemical weathering at Urumqi Glacier No. 1 in central Asia. Hydrol Process
- Tsering T, Wahed MSA, Iftekhar S, Sillanpää M (2019) Major ion chemistry of the Teesta River in Sikkim Himalaya, India: Chemical weathering and assessment of water quality. J Hydrol 24: 100612
- Poulton SW, Raiswell R (2005) Chemical and physical characteristics of iron oxides in riverine and glacial meltwater sediments. Chem Geol 218: 203-221








