Keywords
Hemolytic uremic syndrome; Treatment of HUS; Kidney disease; Polyclonal antibodies; F(ab’)2 fragments
Introduction
Shiga toxin-producing Escherichia coli associated Hemolytic uremic syndrome (STEC-HUS) is a serious foodborne disease worldwide for which there are currently no specific therapeutic options on the market. The disease begins with the ingestion of contaminated food or water. Around 3 days later, symptoms like abdominal pain, fever, diarrhoea and vomiting appear. One to three days later diarrhoea becomes bloody and patients are monitored on an outpatient basis for HUS progression. At this point, the unequivocal and quick diagnostic of the presence of a bacterial infection or Shiga toxin (Stx) in feces is of critical importance for management of the patient [1]. Bloody diarrhoea can be solved spontaneously at day 7 but 5-15% of the patients develop HUS. This disease is characterized by a triad of symptoms: acute kidney damage, microangiopathic Hemolytic anaemia and thrombocytopenia (low platelet count). Until now there is no specific treatment available, only supportive care can be provided and 50% of the patients require a period of dialysis. With the proper cares HUS mortality is around 3-5% but in 30% of the survivors the disease leaves sequels such as chronic renal failure, hypertension, neurological symptoms and proteinuria [2].
Although two major Stx types (Stx1 and Stx2) and several subtypes (variants) have been described, it has been reported that strains that produce Stx2a, Stx2c or Stx2d are more pathogenic than those strains producing variants of Stx1 alone or Stx1 / Stx2 [3]. Given the key role of the Shiga toxin, STEC-HUS is considered a toxemic disorder more than a bacterial disease, suggesting that early intervention with neutralizing antibodies may have a therapeutic benefit. Several monoclonal antibodies (mAbs) that block Stx and prevent its internalization through its specific Gb3 (globotriaosylceramide) receptor have been developed. These mAbs showed promising results in preclinical studies but not conclusive effectiveness in clinical trials were demonstrated [4-6].
Polyclonal antibodies (pAbs) appear to have many advantages for anti-toxin therapies, since they recognize a vast array of epitopes and tend to develop greater avidity than mAbs for their cognate antigens. Furthermore, pAbs are also able to recognize different variants of pathogens, venoms or toxins, diminishing the risk of escape mutations and allowing for rapid development and production of a novel efficient treatment. Use of pAbs is well established for many human pathological disorders; most notably exposure to rabies virus or botulinum toxin and treatment of venomous snake bite and scorpion sting [7,8]. Equine polyclonal antibodies (EpAbs) are easy to manufacture and are being successfully applied to several diseases. While in the past serum sickness and anaphylactic shock, mainly due to the presence of Fc fragments, disfavoured the use of EpAbs, the new generation (third generation serums) of processed and purified EpAbs containing highly purified F(ab’)2 fragments, are well tolerated [9]. This kind of products have been used for decades in the management of clinical emergencies, such as snakebite envenomation, severe poisoning (e.g., tetanus toxin, botulin toxin, digoxin, scorpion bites) [10,11] or severe infectious diseases like Avian influenza [12,13]. Taking into account the magnitude of social and economic problems caused by STEC infections, Inmunova has developed a novel therapy based on Neutralizing Equine Anti Shiga Toxin (NEAST), composed of F(ab’)2 fragments from equine immunoglobulin’s. NEAST is aimed to prevent the onset of HUS in STEC infected patients. Here we present the preclinical analysis of NEAST that validate an on-going clinical development plan.
Materials and Methods
Proteins and immunogens
BLS-Stx2B was purified as previously described [14]. The Stx1B-coding sequence:5´GGAGATATACATATGCATACGCCTGATTGTGTAACTGGAAAGGTGGAGTATACAAAATATAAT GATGACGATACCTTTACAGTTAAAGTGGGTGATAAAGAATTATTTACCAACAGATGGAATCTTC AGTCTCTTCTTCTCAGTGCGCAAATTACGGGGATGACTGTAACCATTAAAACTAATGCCTGTCAT AATGGAGGGGGATTCAGCGAAGTTATTTTTCGTGGTTCTGGTTCTGGTTCTGGTTCTGGTTCT CTTAAGACATCCTTTAAA3’ was obtained from Genscript®. The gene synthesized included the coding sequence for a 10-aa linker (bold type) and was sub cloned in the NsiI and AflII sites (underlined in the sequence) upstream to the BLS gene, in a pET11a vector that was generated previously and contains the BLS sequence [15]. The plasmid pET-BLS-Stx1B was transformed into competent E. coli BL21 (DE3) cells. The inclusion bodies, containing the majority of the BLS-Stx1B protein, were solubilized by overnight incubation in 8 M urea, 50 mm Tris/HCl, 5 mm EDTA pH=8 buffer. The unfolded material was purified by ionic exchange chromatography in a Q-sepharose column using an HPLC apparatus (Dionex Ultimate 3000). Elution was performed using a linear gradient between 0 and 1 M NaCl in buffer consisting of 8 M urea and 50 mm Tris/HCl pH=8.5. Refolding of BLS-Stx1B was conducted by dialysis against PBS. Purified Stx1 and Stx2 toxins were bought to Phoenix Lab., Boston, Massachusetts, USA. Stx variants were obtained from supernatants of STEC strains, from National Reference Laboratory for HUS, Servicio de Fisiopatogenia, Instituto Malbrán-ANLIS.
Immunization of horses and production of F(ab’)2 fragments
This work was done at Instituto Biológico Argentino- BIOL facilities. Four horses were immunized 4 times, 15 days apart, with a mixture of 0.5 mg of BLS-Stx1B and 0.5 mg of BLS-Stx2B emulsified in Freund´s adjuvant. The first inoculation was prepared using equal volumes of the immunogen and Complete Freund Adjuvant. Subsequent inoculations were done using Incomplete Freund Adjuvant. Serum titers were evaluated by ELISA using Stx1 and Stx2 as antigens. The F(ab’)2 fragments were obtained by pepsin digestion of equine immunoglobulin. Blood was obtained using a closed system by jugular venipuncture and the plasma was separated by gravity. In a stainless-steel reactor, the plasma was diluted with 2 parts of an aqueous solution of phenol 2.5 g/l concentration. The digestion begins with the addition of pepsin 1: 10.000 at pH 3.2 and 30°C temperature for 30 minutes. The enzymatic reaction was stopped by raising the pH to 4.3. Ammonium sulphate was added to the digested material up to a final concentration of 12%. The mix was heated for 1 hour to 55°C and pH 5.2-5.3. The non-immunoglobulin proteins were denaturated by the heat and precipitated. Caprylic acid was added and the mixture was incubated at a temperature of 30°C for 30 minutes. This clarification treatment allowed the removal of remaining lipids. Celite® was added as a filtering aid, and non-immunoglobulin proteins were separated by filtering and discarded. The pH of the filtrate was adjusted up to a value between 6.0 and 7.0. Ammonium sulphate was then added up to a final concentration of 24%. The precipitate formed, rich in immunoglobulins, was harvested by filtration. The immunoglobulin-rich precipitate was dissolved in saline solution and diafiltered through a 30 kDA cut off cartridge. After the ammonium sulphate was removed, phenol was incorporated at a concentration of 2.5 g/l as a preservative and the solution was sterilized by filtration. The filter contains quaternary ammonium ligands that allow the retention of peptides not of interest, endotoxins and other possible impurities, whereas the product of interest was not retained. The immunoglobulins concentrate obtained was sterilized by filtration and stored at 2-8°C. The bulk solution was sterilized through a cartridge of 0.2 μm pore and aliquoted in sealed glass vials (lot 3945). The product had a protein concentration of 23.2 mg/ml and contains phenol in quantities below 2.5 mg/ml, used as preservative during the manufacturing process. This lot was used for the assays described below.
Preclinical assays using in vitro and in vivo models
Vero cells neutralization assays:
Assay A: Serial dilutions of the product covering the range from minimum neutralization effect to total neutralization were preincubated with 10CD50 of Stx1 and Stx2 purified toxins 1 h at 37°C. The aim was to estimate using a mathematical adjustment the IC50, defined as the amount of NEAST (in this case expressed in ug/ml) that neutralizes 50% of the maximum toxic effect.
Assay B: Serial dilutions of culture supernatants of strains producing Stx1a, Stx1c, Stx1d, Stx2a, Stx2b, Stx2c, Stx2d, Stx2e,
Stx2f and Stx2g were preincubated 1 h at 37°C with or without 0.25 mg/ml of NEAST. This assay was performed to evaluate the neutralizing capacity of NEAST against Stx variants. In both assays the preparations were transferred to a 96-well culture plate containing 1.5 × 104 Vero cells/well, and incubated for 48 hours at 37°C in 5% CO2. The cells were stained with crystal violet and absorbance was measured at 570 and 655 nm with MTT dye, and the absorbance ratio at 570-655 nm was determined. The absorbance results were evaluated with GraphPad Prism 6.
Evaluation of neutralizing capacity in vivo
These assays were conducted according to the Standard Operating Procedures (SOPs) applied at the Center for Comparative Medicine (CMC), Universidad Nacional del Litoral, CONICET as follows:
Determination of minimal effective dose of NEAST
A total of 72 BALB/c male mice were used to test the neutralizing capacity of NEAST against purified Stx1a and Stx2a toxins with the corresponding control groups. Groups of mice (n=6) were administered intravenously with 0.1 ml of the corresponding product dilution and intraperitoneally with a single dose of 0.5 ml containing 3DL50 of Stx1 (1560 ng) or 5DL50 of Stx2 (20 ng). The control groups received physiological solution in replacement of antitoxin. The animals were monitored and deaths were recorded for 7 days.
Evaluation of the neutralizing capacity in a murine model of sequential dose administration of toxin
A dose corresponding to 5LD50 of Stx2 (20 ng) was administered intraperitoneally to BALB/c groups of mice (n=8), with the following scheme: Group 1) A 20% of the dose (4 ng) at day 0, Group 2) A 20% of the dose on day 0 plus 20% on day 1, Group 3) A 20% of the dose on days 0 and 1, and 30% of the dose (6 ng) on day 2, Group A 20% of the doses at days 0 and 1 followed by 30% at days 2 and 3. A control Group received 100% of the dose at time 0. The deaths of the animals were recorded for 10 days. Subsequently, groups of mice received 5DL50 of Stx2 according to the sequential dose administration scheme, accompanied by the intravenous administration of a single dose of 10 mg/kg of NEAST at different times: Group A at day 0, Group B at day 1, Group C at day 2, Group D at day 3 and Group Eat day 4 (24 hours after the last administration of toxin). Survival of the animals was evaluated for 10 days.
Mouse infection model
For the in vivo efficacy evaluation of NEAST we selected a streptomycin sulphate (Str) and mitomycin C-treated intragastrically inoculated mouse model which is able to reflect the Stx-mediated damage and the pathogenesis of STEC in humans [16]. The STEC strain of Escherichia coli O157:H7 (746) selected for this study was stx2+ (provided by Dr. Gerardo Leotta). This strain was routinely grown in Luria-Bertani broth (LBB; SIGMA-ALDRICH, USA). All stocks were maintained at -80°C in 20% glycerol. Spontaneous streptomycin sulphate-resistant (Strr) mutants of the E. coli O157:H7 strain were obtained by plating overnight cultures of the wild type strain on LB agar plates (LBA; LBB plus 1.5% agar – agar, agar bacteriological, Oxoid, UK) supplemented with 30, 50, 80 and 100 μg/ml Str (Str; Richet, Argentina). Incubation time was 3 to 6 days. Putative E. coli O157:H7 Strr colonies were streaked three times on the same medium (LBA plus 100 μg/ml Str) for purification. Isolated E. coli O157:H7 Strr mutants were used for in vivo infection model. Streptomycin-treated mouse model of STEC infection was performed following a previously reported protocol [16] with slight modifications. E. coli O157:H7 Strr was streaked onto LBA plates with the addition of 100 μg/ml Str and grown overnight at 37°C. Single colonies were picked and cultured in LBB plus 100 μg/ml Str for 24 h at 37°C. A 1:50 dilution was made and the Strr mutants were grown in LBB at 37°C in a shaking incubator (MaxQ HP 4450, Thermo Scientific) overnight (150 rpm × 18 h). Bacterial cells were pelleted by centrifugation (SORVALL RC6+, Thermo Scientific) at 8,000 rpm for 20 minutes. The pellet was resuspended in sterile PBS. E. coli O157:H7 Strr suspension was serially diluted and plated via spread plate method onto LBA plates containing 100 μg/ml Str and the inoculum concentration (CFU/ml) was estimated. Prior to the animal infection, Stx2 production by E. coli O157:H7 Strr was confirmed using a rapid membrane enzyme immunoassay kit (Shiga Toxin Quik Chek; TECHLAB, USA). The BALB/c female and male mice were approximately 18-21 days old and were housed in a individually ventilated caging (IVC) systems (Allentown Inc, USA) and maintained at an average temperature and humidity of 23.0°C and 60%, respectively. Mice were given drinking water containing Str (5 g/l) 3 days before infection. Water bottles were replaced every day with freshly prepared water. Str treatment continued throughout the course of the experiment. On day 3 of the Str administration, mice were starved for 6 h. Forty animals (5 males and 5 female/group) were administered orally by gavage with 1 × 107 CFU/kg of the E. coli O157:H7 Strr suspension diluted in sterile PBS (100 μl/mouse) through a stainless steel catheter with a blunted end (outer diameter, 0.90 mm; JELCO). Simultaneously, they were injected intraperitoneally with mitomycin C (MMC; LKM, Argentina) at a dose of 2.5 mg/kg. Twenty animals received the product at a dose of 10 mg/kg (50 μl/animal) via I.V, at 24 h (Group 1), or at 24, 72 and 120 h (Group 2) after bacterial infection. Ten control mice (Group 3) received bacteria and MMC injections but no product. This Group was considered as control of the appropriate Str-treated mouse model of STEC infection. Mice survival was monitored daily for 14 days post-infection.
Safety and pharmacokinetics profiles in mice
Balb/cJ mice (120 animals/dose) were used. For single administration pharmacokinetics, 2 doses of product were tested: 11.6 and 116 mg/kg, administered intravenously. Each dose was administered to 3 groups of animals: Group A, received the product and serum samples were obtained at different times until 72 h; Group B received a second administration of the same dose at 30 days and Group C received a third administration at 60 days; in both groups, 15 days after the last administration, samples were obtained to evaluate immunogenicity by ELISA. To evaluate the pharmacokinetics of repeated administrations of the product, a dose of 116 mg/kg administered at time 0, 24, and 48 h was used. Samples of serum were obtained at different times and F (ab’)2 presence was quantified by ELISA. The ELISA technique for quantification of NEAST in serum was designed at Inmunova and set-up and validated at the CMC according to FDA guidelines (Guidance for Industry: Bionalytical Method Validation). The method consists of sandwich ELISA, in which the samples are incubated in a plate sensitized with an anti-equine F (ab’)2 specific antibody (LS-C60458), and revealed by the addition of an anti-equine immunoglobulins antibody conjugated with peroxidase (Santa Cruz, SC-2448). Finally, a chromogenic substrate (TMB) is added, which develops an absorbance proportional to the amount of NEAST in the sample. The signal is interpolated in a standard curve evaluated in the same assay and adjusted to a multi-parameter model.
Safety evaluation and pharmacokinetics in rabbits
Females of the New Zealand strain were used (n=6). The animals received a single administration corresponding to 116 mg/Kg. Blood was obtained at different times until 72 h. For repeated dose pharmacokinetics, 6 administrations of 40 mg/kg (every 12 h) were performed and serum samples were obtained at different times up to 264 h. The F(ab’)2 circulating was quantified by ELISA as described above. The systemic response was analyzed by complete blood count and biochemical variables at different times. The animals were observed daily and examined clinically.
Evaluation of repeated dose toxicity in mice and rabbits
Product doses corresponding to 116.00, 38.66 or 7.73 mg / kg were administered to mice 3 times a week for 4 weeks. Young male rabbits of New Zealand strain (n=10) were used. Product doses corresponding to 116 mg / kg were administered to the animals 3 times per week for 4 weeks. In both trials a control Group received physiological solution with the same frequency. The animals were observed daily, and a complete clinical examination was performed. After the last inoculation, a complete necropsy and the study of biochemical and hematological variables were performed.
Determination of cross-reactivity in human tissues
This assay was performed in Lifespan Bioscience, USA. Reactivity of the product was evaluated by immunohistochemistry on a panel of 30 frozen human tissues. In the optimization of the staining, transfected HEK293 cells overexpressing BLS-Stx2 were used as controls. An antiophidic F(ab')2 was included as a negative control. In the final test, the primary antisera (NEAST) was used at a concentration of 4 μg/ml and the secondary anti-equine at a dilution of 1:400, with the ABC-HRP kit (catalog # PK-4000) and the Chromogen substrate DAKO DAB + ( catalog # K3468).
Results
NEAST production
Considering the relevance of Shiga toxin in the sequence of events leading to HUS, one of the biggest challenges for developing a successful therapy is to engineer an immunogen capable to induce high titers of neutralizing antibodies against it. The B subunit of Stx2 (Stx2B) is the most attractive candidate, since it is involved in receptor binding and cell entry. However, Stx2B is a very poor immunogen, mainly because of its low thermodynamics stability [17]. On the other hand, the enzyme lumazine synthase from Brucella spp. (BLS) is a highly immunogenic and stable dimer of pentamers and a scaffold with enormous plasticity on which to display foreign antigens [15,18-22]. A chimeric protein was obtained in which the B subunit of Shiga toxin 2a was displayed on the BLS scaffold. The resulting recombinant protein (BLS-Stx2B) showed a remarkable stability, as demonstrated by Static Light Scattering (SLS) and Circular Dichroism (CD) analyses [14]. In accordance, the chimera shows a remarkable capacity to induce very strong and long lasting humoral immune responses, and was able to induce antibodies with high neutralizing capacity for Stx2 and its variants [14,23,24]. Thus, BLS strongly enhanced Stx2B immunogenicity by stabilizing its native tertiary and quaternary structure [14].
With a focus on obtaining a therapy with broad neutralizing capacity against different strains of Shiga toxins, we also generated another chimeric protein between the B subunit of Stx1a and BLS. In Figure 1, the molecular modeling of BLS-Stx1B obtained with the program PyMOL 1.5 is shown. Each Stx1B pentamer is depicted in a different shade of pink, and the BLS decamer is showed in turquoise tones. The display of Stx1B using BLS also enhanced the immunogenicity of this subunit (results not shown).
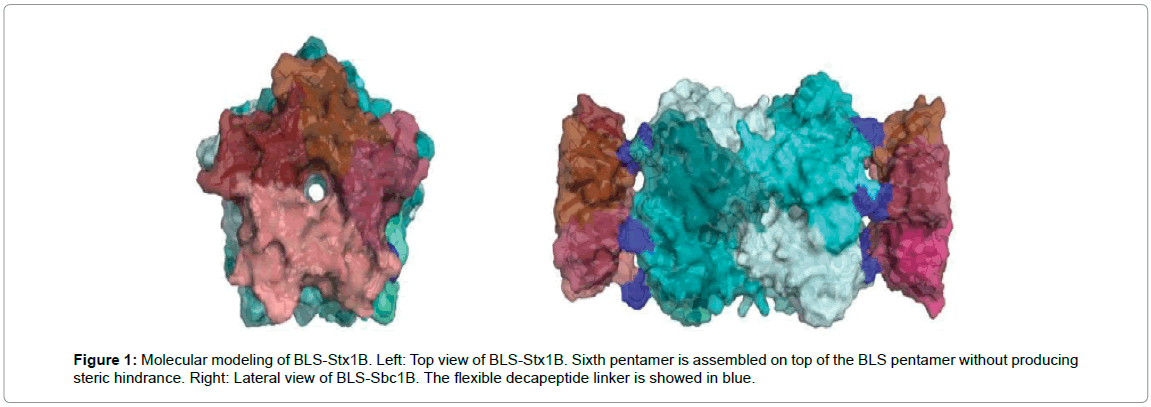
Figure 1: Molecular modeling of BLS-Stx1B. Left: Top view of BLS-Stx1B. Sixth pentamer is assembled on top of the BLS pentamer without producing steric hindrance. Right: Lateral view of BLS-Sbc1B. The flexible decapeptide linker is showed in blue.
We then took advantage of the high immunogenicity of both BLSStxBs proteins to generate EpAbs with very high titers of neutralizing antibodies against both Stx1 and Stx2. Four horses were administered four times, 15 days apart, with a mixture of the BLS-Stx1B and BLS-Stx2B chimeras. This immunization plan allowed us to develop specific antibodies against Stx1 and Stx2 with titers higher than 1x105 as shown in Figure 2A.
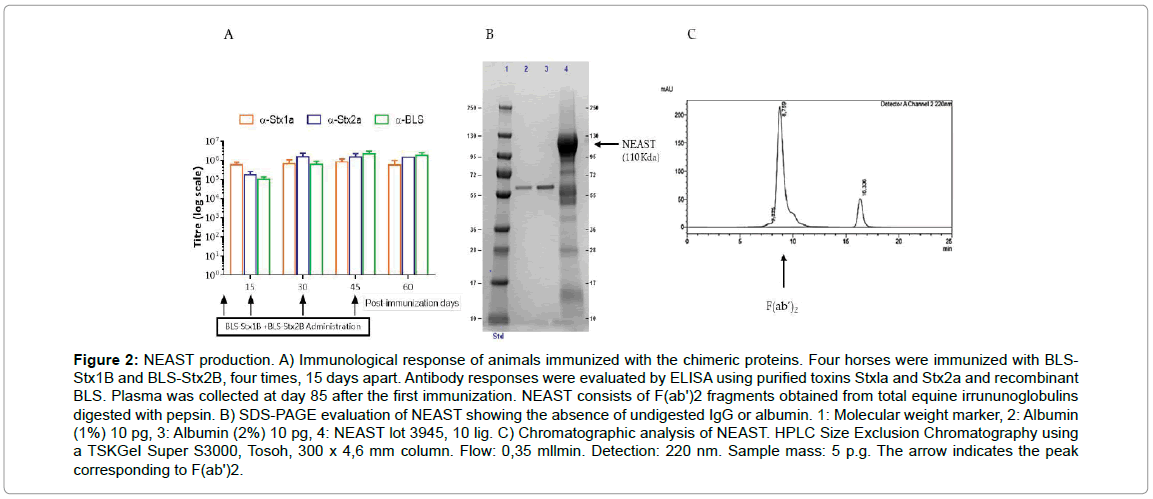
Figure 2: NEAST production. A) Immunological response of animals immunized with the chimeric proteins. Four horses were immunized with BLS-Stx1B and BLS-Stx2B, four times, 15 days apart. Antibody responses were evaluated by ELISA using purified toxins Stxla and Stx2a and recombinant BLS. Plasma was collected at day 85 after the first immunization. NEAST consists of F(ab')2 fragments obtained from total equine irrununoglobulins digested with pepsin. B) SDS-PAGE evaluation of NEAST showing the absence of undigested IgG or albumin. 1: Molecular weight marker, 2: Albumin (1%) 10 pg, 3: Albumin (2%) 10 pg, 4: NEAST lot 3945, 10 lig. C) Chromatographic analysis of NEAST. HPLC Size Exclusion Chromatography using a TSKGeI Super S3000, Tosoh, 300 x 4,6 mm column. Flow: 0,35 mllmin. Detection: 220 nm. Sample mass: 5 p.g. The arrow indicates the peak corresponding to F(ab')2.
Plasma proteins from immunized horses were digested with pepsin at acidic pH, then non-immunoglobulin proteins were precipitated by heating at 55°C and acidic pH in the presence of 12% ammonium sulphate; subsequently, the immunoglobulin fragments were precipitated and concentrated by adding 24% ammonium sulphate at neutral pH (see material and methods for details). Digestion of the immunoglobulins with pepsin generates F(ab’)2 fragments, which are depleted of the constant region, leaving the intact hinge region. These fragments have two F(ab) binding domains connected by a disulfide bridge, and are therefore divalent with a molecular weight of approximately 110 kDa. This fraction has an average pI of 8.5. SDS-PAGE and HPLC Size exclusion chromatography assays of NEAST were conducted in order to evaluate the presence of possible contaminants as albumin or undigested antibodies. In SDS-PAGE analysis (Figure 2B) NEAST shows a majority band around 110 KDa corresponding to F(ab’)2 fragments (lane 4) and the absence of higher molecular weight bands corresponding to undigested IgG antibodies. In addition, albumin is absent or present in less than the accepted amounts according to the product protein content (lanes 2 and 3). The HPLC Chromatogram (Figure 2C) shows the absence of aggregates and only one peak corresponding to F(ab’)2 is observed (black arrow).
Determination of Shiga toxin neutralization capacity of NEAST in vitro
Vero cells are highly sensitive to the toxic effect of Stx, thus we used an analysis of the capacity of NEAST to neutralize this toxicity as a mean to measure its potency. We determined the mass of NEAST able to neutralize 50% of the maximum toxic effect (IC50). NEAST is effective to neutralize 50% of the maximum toxic effect exerted by 10 cytotoxic doses (10CD50) of Stx1a at a concentration of 0.66 μg/ml and 10CD50 of Stx2a at a concentration of 4.55 μg/ml (mean of three independent assays for each toxin). The neutralization capacity of NEAST against other Shiga toxin variants was also determined. Pre-treatment with NEAST (0.25 mg/mL) neutralized the cytotoxic effect of Stx1a, Stx2a and its variants, Stx1c, Stx2c, Stx2d, Stx2e, Stx2f and Stx2g (Figure 3 and Supplemental Figure S1). These results show a wide capacity of NEAST for neutralizing 8 out of 10 Shiga toxin variants, including the more relevant for HUS development.
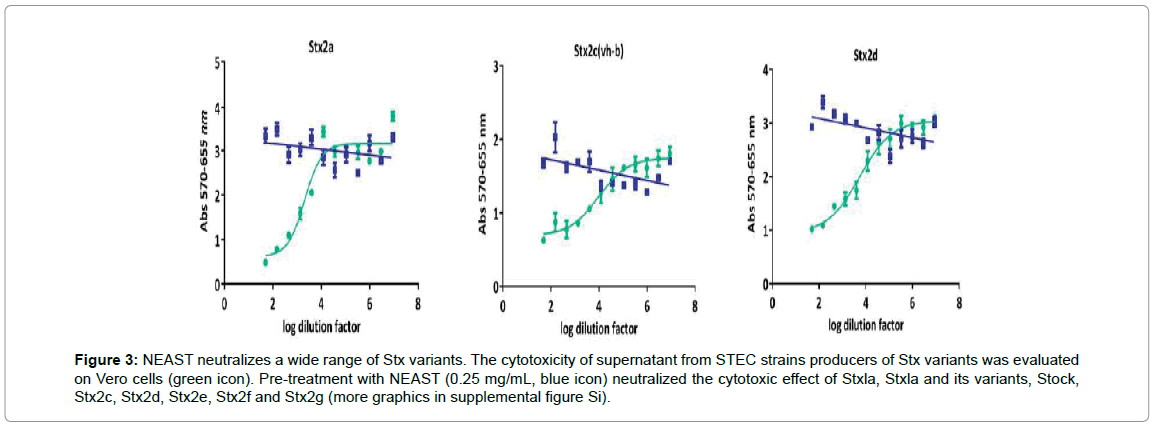
Figure 3: NEAST neutralizes a wide range of Stx variants. The cytotoxicity of supernatant from STEC strains producers of Stx variants was evaluated on Vero cells (green icon). Pre-treatment with NEAST (0.25 mg/mL, blue icon) neutralized the cytotoxic effect of Stxla, Stxla and its variants, Stock, Stx2c, Stx2d, Stx2e, Stx2f and Stx2g (more graphics in supplemental figure Si).
Determination of Shiga toxin neutralization capacity of NEAST in vivo
Neutralization studies in the mice model of death produced by Stx were conducted. We determined the potency and the minimal effective dose by simultaneous injection of Stx1a or Stx2a with serial dilutions of NEAST. Groups of mice received 3 lethal doses (LD50) of Stx1a (equivalent to 1560 ng of toxin) or 5LD50 of Stx2a (20 ng). The minimal dose of NEAST required to protect mice against the lethal effects of Stxs was 1.0 mg/kg for Stx2a and 5.0 mg/kg for Stx1a (Figure 4). The potency, defined as the number of LD50 of Stx neutralized by 1 ml of product, was 488 LD50/ml for Stx1a and 7043 LD50/ml for Stx2a.
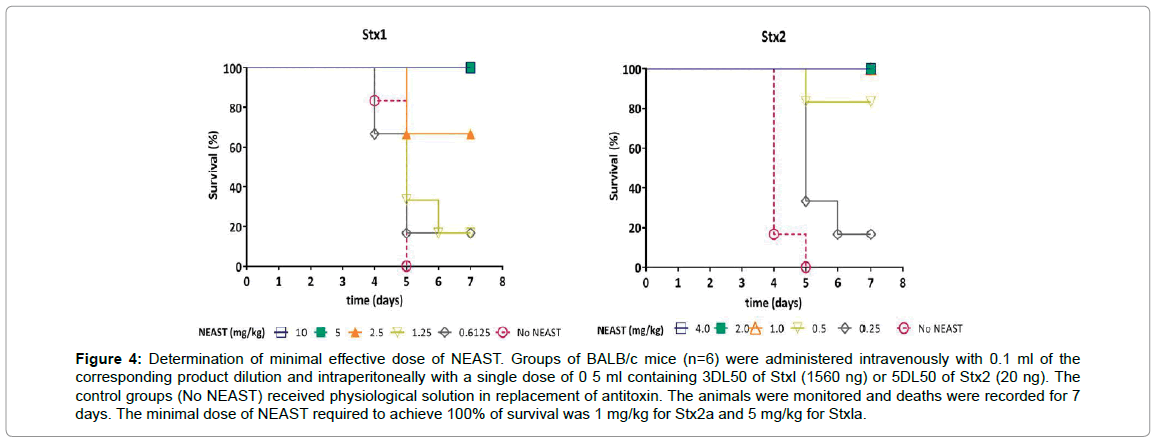
Figure 4: Determination of minimal effective dose of NEAST. Groups of BALB/c mice (n=6) were administered intravenously with 0.1 ml of the corresponding product dilution and intraperitoneally with a single dose of 0 5 ml containing 3DL50 of Stxl (1560 ng) or 5DL50 of Stx2 (20 ng). The control groups (No NEAST) received physiological solution in replacement of antitoxin. The animals were monitored and deaths were recorded for 7 days. The minimal dose of NEAST required to achieve 100% of survival was 1 mg/kg for Stx2a and 5 mg/kg for Stxla.
Determination of a therapeutic window for NEAST administration
In order to simulate the progressive excretion of Stx during a STEC infection, we used a murine model similar to one previously described [25]. In this sequential toxicity model, a total of 5LD50 of Stx2a were administered in an incremental manner as sublethal and sequential doses (see methods section) while NEAST was co-administered alternatively at days 0, 1, 2, 3 or 4. In a first test, the sub-lethality of partial doses of Stx2a was verified (Figure 5A). Subsequently, the effect of intervention with NEAST in the toxin administration scheme was evaluated (Figure 5B). In this case, it can be seen that 100% of the animals survive if NEAST is injected together with the first or the second dose of toxin (Groups A and B). When these groups are compared with groups 1 and 2, it can be seen that mortality goes down from 50% or 100% respectively, to 0%. If Group 3 is compared to Group C, it is observed that in the first Group all mice died before the fifth day, whereas with the application of NEAST 40% of the animals survive. It is important to emphasize that in groups A and B, where NEAST is administered with the first and second doses of Stx2a, no deaths are observed after the administration of the product even though 3 LD50 of Stx2a were injected in the following two days, demonstrating the persistence of the product in the body and its ability to neutralize the toxin. On the other hand, when the product is applied with the fourth dose of toxin (reaching 5LD50) or 24 hours after the 4th dose, the protective effect is no longer observed, possibly because these animals have been exposed to high concentrations of toxin for a long time before the application of the product. Then, it can be concluded that in the murine model used here, there is a therapeutic window in which the progression to death can be stopped by the administration of NEAST because of the strong neutralizing capacity showed even when animals have been exposed to the toxin for 48 hours.
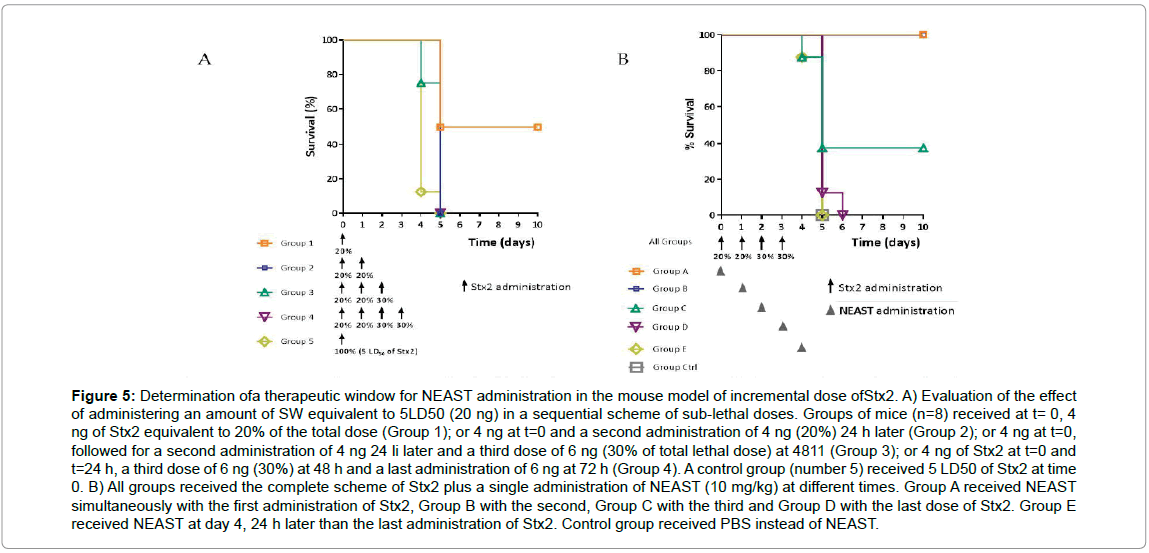
Figure 5: Determination ofa therapeutic window for NEAST administration in the mouse model of incremental dose ofStx2. A) Evaluation of the effect of administering an amount of SW equivalent to 5LD50 (20 ng) in a sequential scheme of sub-lethal doses. Groups of mice (n=8) received at t= 0, 4 ng of Stx2 equivalent to 20% of the total dose (Group 1); or 4 ng at t=0 and a second administration of 4 ng (20%) 24 h later (Group 2); or 4 ng at t=0, followed for a second administration of 4 ng 24 li later and a third dose of 6 ng (30% of total lethal dose) at 4811 (Group 3); or 4 ng of Stx2 at t=0 and t=24 h, a third dose of 6 ng (30%) at 48 h and a last administration of 6 ng at 72 h (Group 4). A control group (number 5) received 5 LD50 of Stx2 at time 0. B) All groups received the complete scheme of Stx2 plus a single administration of NEAST (10 mg/kg) at different times. Group A received NEAST simultaneously with the first administration of Stx2, Group B with the second, Group C with the third and Group D with the last dose of Stx2. Group E received NEAST at day 4, 24 h later than the last administration of Stx2. Control group received PBS instead of NEAST.
Determination of the neutralization efficacy of NEAST against Shiga toxin in an in vivo infection model
We next tested the efficacy of NEAST to in vivo neutralize Stx in a proven infection model that more closely resembles human infection.
For this purpose, we selected a streptomycin sulfate and mitomycin C-treated intragastrically inoculated mouse model which is able to reflect the Stx- mediated damage and the pathogenesis of STEC in humans [16].
As can be seen in Figure 6, most of the animals die about 5 to 7 days after oral infection in the control group, whereas NEAST is able to completely protect from death if administered three times at days 1, 3 and 5 after infection. Remarkably, the mice receiving only one dose of NEAST at 24 hr post-infection showed more than 80% of protection. Overall, these results clearly show that NEAST is highly protective in this more physiological setting.
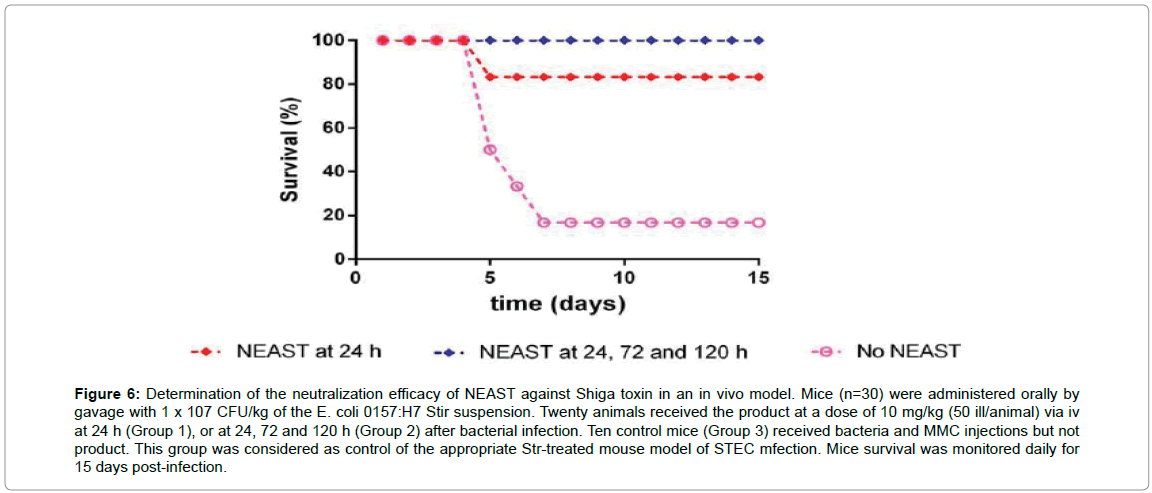
Figure 6: Determination of the neutralization efficacy of NEAST against Shiga toxin in an in vivo model. Mice (n=30) were administered orally by gavage with 1 x 107 CFU/kg of the E. coli 0157:H7 Stir suspension. Twenty animals received the product at a dose of 10 mg/kg (50 ill/animal) via iv at 24 h (Group 1), or at 24, 72 and 120 h (Group 2) after bacterial infection. Ten control mice (Group 3) received bacteria and MMC injections but not product. This group was considered as control of the appropriate Str-treated mouse model of STEC mfection. Mice survival was monitored daily for 15 days post-infection.
Pharmacokinetics and in vivo safety profile of NEAST
To ensure complete bioavailability of the product, systemic exposure to NEAST was evaluated in mice (rodent species) and rabbits (non-rodent species) by intravenous (I.V.) application on a mg/ kg basis. For pharmacokinetics profile analysis, the maximum dose administered (116 mg/kg) corresponds to the 5 ml/kg body weight limit established for the intravenous route, taking into account the concentration of batch 3945. Besides, a dose tenth fold lower (11.6 mg/ kg) close to the planned therapeutic dose, was evaluated. For toxicology studies, intermediate doses were evaluated.
Pharmacokinetics in mice: The pharmacokinetics profile of the product in mice was evaluated after the administration of single or repeated doses. The maximum concentration of NEAST in the serum after the administration of a dose of 116 mg/kg was 1,029.8 μg/ml. When a single administration of 11.6 mg/kg was carried out, a maximum proportional serum product concentration of 102.3 μg/ml was observed (Figure 7, left). In all tests, the maximum concentration of the product was reached in 1 hour. In the case of repeated administration of the 116 mg/kg dose, the result was slightly higher (1,164.5 μg/ml), due to the accumulation of the compound (Figure 7, right). The clearance of the product in the single administration assays was similar for the two doses used (0.22 ml/h), indicating that the elimination capacity was not saturated. The half-life (t1/2) of the product with a single dose was 19.76 h, similar to that obtained in the repeated administrations scheme (19.26 h).
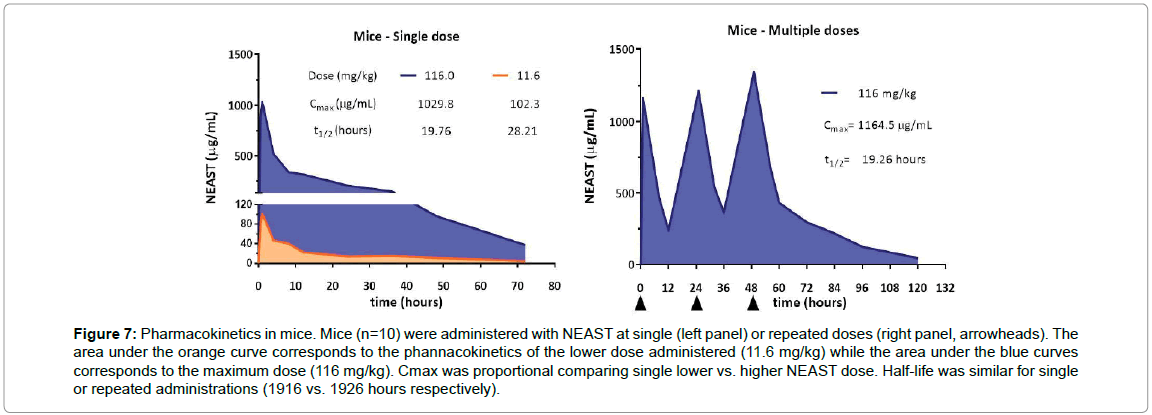
Figure 7: Pharmacokinetics in mice. Mice (n=10) were administered with NEAST at single (left panel) or repeated doses (right panel, arrowheads). The area under the orange curve corresponds to the phannacokinetics of the lower dose administered (11.6 mg/kg) while the area under the blue curves corresponds to the maximum dose (116 mg/kg). Cmax was proportional comparing single lower vs. higher NEAST dose. Half-life was similar for single or repeated administrations (1916 vs. 1926 hours respectively).
Pharmacokinetics in rabbits: The pharmacokinetics profile of NEAST was evaluated after the administration in rabbits of a single dose of 116 mg/kg or repeated doses of 40 mg/kg. The maximum concentration of product in serum, after the administration of a single dose, was 3,264 μg/ml and it was reached at 0.5 h (Figure 8). The half-life (t1/2) of the product after administration of a single dose of 116 mg/ kg was 23.61 hours. However, in the repeated administration assay, t1/2 was found to be 49.27 h. This increase is probably associated with the saturation of the elimination mechanisms and the frequency of administration (12 h). For immunogenicity tests, the antibody titers developed in the animals against the administered F(ab')2 were not significant. In all trials, F(ab')2 was detected up to 72 h after the last administration.
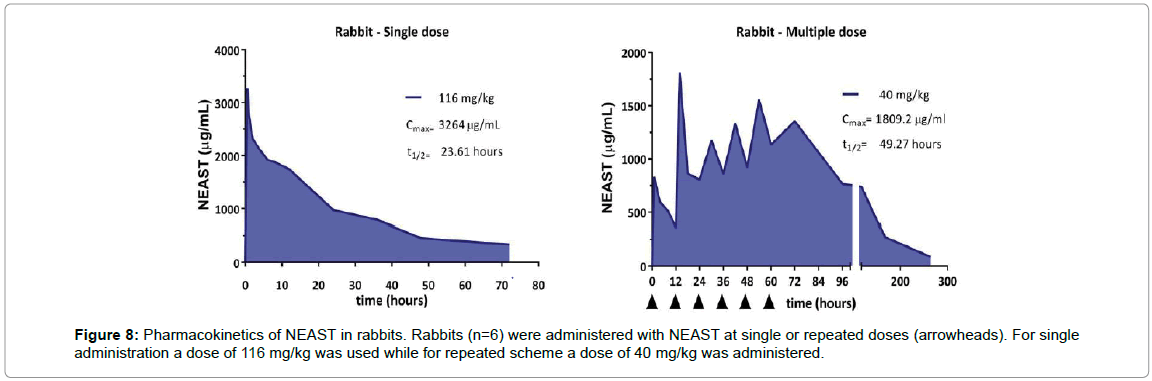
Figure 8: Pharmacokinetics of NEAST in rabbits. Rabbits (n=6) were administered with NEAST at single or repeated doses (arrowheads). For single administration a dose of 116 mg/kg was used while for repeated scheme a dose of 40 mg/kg was administered.
In vivo toxicology of NEAST at single and repeated doses
A series of single and repeated doses and toxicity studies (see material and methods) were conducted in mice and rabbits in order to evaluate the effects of NEAST administration on clinical and biochemical parameters including skin, mucous membranes, motor activity and conduct patterns, haematology and laboratory. Supplementary Table S1 summarizes the findings of the studies. There were no significant clinical effects associated with NEAST doses and frequency administration in both animal models.
Absence of NEAST crossâ€ÂÂÂÂreactivity in human tissues
We performed tissue cross-reactivity (TCR) analysis in order to demonstrate the lack of reactivity of NEAST in immunochemistry tests against a vast array of human healthy tissues. The first step in the TCR analysis is the determination of the working dilution for these tests. NEAST F(ab´)2 antisera (Batch 3495) and polyvalent anti-ophidic F(ab´)2 antisera (batch 3935, purified with the same process as NEAST) were evaluated at 4 or 8 μg/ml with a secondary anti-equine antibody, using HEK293 cells overexpressing Shiga toxin B and as negative control HEK293 cells expressing only an empty vector (Figure 9).
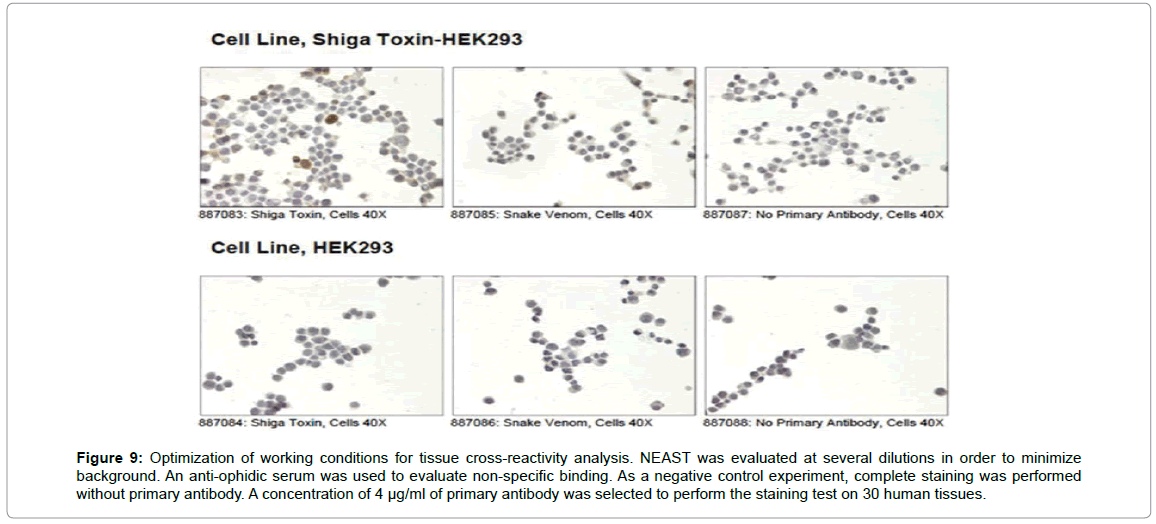
Figure 9: Optimization of working conditions for tissue cross-reactivity analysis. NEAST was evaluated at several dilutions in order to minimize background. An anti-ophidic serum was used to evaluate non-specific binding. As a negative control experiment, complete staining was performed without primary antibody. A concentration of 4 μg/ml of primary antibody was selected to perform the staining test on 30 human tissues.
Once the optimal concentration of primary antibody was determined, a TCR analysis was conducted using 30 frozen tissues from 3 unrelated human donors, as recommended by FDA. As negative control the full immunohistochemistry procedure was conducted in the absence of the primary antibody, to determine the level of the non-specific signal produced by the secondary antibody and the detection system. The immunochemical reactions were evaluated by certified pathologists by establishing a score for the degree of staining. Table 1 summarizes the results obtained. This analysis shows that, at a concentration where NEAST showed strong staining within the positive control cell line, there was no significant staining beyond background against any of the human tissues tested in this study. This result indicates that NEAST lacks undesired reactivity against human healthy tissues. As a general conclusion, NEAST shows a very strong neutralizing capacity against Shiga toxin variants in preclinical models of STEC-HUS. NEAST is also able to neutralize pathologic effects after previous exposure to Shiga Toxin, defining a potential therapeutic window. Besides, NEAST shows an excellent pharmacokinetics and safety profile in animal models. Overall, these results indicate that NEAST is a very good drug candidate for preventing the onset of HUS in STEC-infected patients.
| Tissue |
Shiga toxic Score |
Interpretation |
| Adrenal |
1 |
negative |
| Bone Marrow |
1-2 |
negative |
| Breast |
0 |
negative |
| Brain; cerebellum |
0 |
negative |
| Brain, cerebral cortex |
0 |
negative |
| Brain, pituitary |
0 |
Negative |
| Colon |
2 |
Negative |
| Endothelium |
0 |
Negative |
| Esophagus |
0 |
Negative |
| Fallopian tube |
0 |
Negative |
| Heart, ventricle |
1 |
Negative |
| Kidney |
1-2 |
Negative |
| Liver |
1 |
Negative |
| Lung |
0 |
Negative |
| Lymph Node |
0 |
negative |
| Ovary |
0 |
Negative |
| Pancreas |
0 |
negative |
| Placenta |
0 |
negative |
| Prostate |
0 |
Negative |
| Skin |
0 |
Negative |
| Spinal cord |
0 |
Negative |
| Spleen |
2 |
Negative |
| Stomach |
2 |
Negative |
| Striated muscle |
1 |
Negative |
| Testis |
0 |
Negative |
| Thymus |
2 |
Negative |
| Thyroid |
0 |
Negative |
| Ureter |
0 |
Negative |
Table 1: Evaluation of tissue staining by pathologists. The Lifespan’s scoring system for MC is a modification of the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines for testing for Estrogen and Progesterone Receptors (ER/PR) and Her2/neu protein for breast cancer. Pathology scoring numeric scale: 0=Negative, 1=Blush, 2=Faint, 3=Moderate, 4=Strong.
Discussion
Although several therapeutic strategies have been tested against STEC-HUS, none of the approaches have been completely effective and there are currently no specific or preventive treatments that allow for the control of the level of kidney damage caused by the toxin. The still elevated number of cases of HUS, and the concomitant morbidity and mortality, requires an answer that not only leads to the reinforcement of preventive mechanisms, but also to offer in the short term a treatment that prevents the development of the disease in children infected with STEC.
Monoclonal antibodies (mAbs) are being successfully used for many therapeutic applications as the treatment of cancer or inflammatory conditions. There have also been a few preliminary trials of humanized mAbs for the treatment of infectious diseases, but none of these treatments are currently in use. One of the challenges facing antibody-based Shiga toxin neutralizers is the variety of toxin subtypes present, since each STEC strain can secrete one or more subtypes. In this way, pAbs appear to have many advantages: since they recognize a vast array of epitopes, pAbs tend to develop greater avidity than mAbs for their cognate antigens, and they would also be able to recognize different variants of Shiga toxins.
In the present work, we took advantage of the high immunogenicity of BLS-StxBs chimeras to generate NEAST; potent antisera against Shiga toxins based on F(ab’)2 fragments from equine immunoglobulins. NEAST showed a broad spectrum of neutralizing capacity against several variants of Shiga toxins in the preclinical models evaluated and, remarkably, protected against the deadly infection with Shiga toxin-producing bacteria. These results indicate that NEAST would be useful for the treatment of STEC infections and prevention of development of HUS. Likewise, NEAST showed to be safe in animals that were administered with much higher doses or number of injections than the planned in a human clinical trial or therapeutic setting. These preclinical results are in agreement with the excellent safety profile shown by polyclonal F(ab’)2 fragments from equine immunoglobulins currently in medical use.
The preclinical results obtained validated the conduction of a first in human clinical study of NEAST in order to assess its safety, tolerance and pharmacokinetics profiles (clinicaltrial.gov number NCT03388216). This phase I clinical trial was conducted at Hospital Italiano de Buenos Aires in Argentina, with 14 healthy adult volunteers from 18 to 55 years old, applying a protocol that has been approved by the local Ethics Committee and the local Health Authority (ANMAT). The study was executed in 2 stages, prospective, randomized, and controlled with placebo. In the first stage a dose escalation design was used, starting with the administration of a simple dose of 2 mg/kg, and then increasing it to 4 mg/kg. Subsequently, having confirmed the safety of the product at the doses studied, a repeated dose scheme was administered by administering 3 product doses of 4 mg/kg. The phase I clinical trial has been successfully completed and the results will be published elsewhere.
As an sporadic acute disease, no Shiga toxin-specific therapeutics has advanced beyond Phase II clinical trials in the United States [26] where most outbreaks are less than 100 patients [27,28]. The usual HUS rate during an outbreak difficult the statistics and makes extremely expensive a Phase II clinical trial. In this sense, Argentina turns an appropriate scenario for clinical development, since HUS is an endemic disease with around 500 new cases annually. Inmunova has designed a pivotal adaptive design phase 2/3 clinical trial which has currently begun and is active for recruitment.
Conclusion
This trial will be conducted in 10 to 15 clinical sites in Argentina and the focus will be STEC infected children between one and ten years. The primary objective will be to assess the safety and the efficacy of NEAST as the first line treatment for children with bloody diarrhoea that yield a positive result for Shiga-Toxin Producing Escherichia coli in feces (STEC infection). The aim is to prevent the occurrence of STEC-HUS and to decrease the incidence and severity of STEC-HUS sequelae. Since the main indication of this clinical trial is prevention of HUS, the challenge will be to recruit around 400 children early enough, in their clinical setting of STEC infection, to make an overlapping between the therapeutic and the diagnostic windows. For this purpose, the most sensitive culture independent diagnostic tests (enzyme immuno assays and real time PCR) will be applied to test the presence of Shiga toxin in feces of children having bloody diarrhoea. As part of the Clinical Development Plan for NEAST, the Sponsor has requested scientific advice meetings with regulatory agencies in Argentina, EMA and FDA to review clinical trial design for the pivotal clinical studies. Because NEAST targets a severe unmet medical need, the product was included in the Innovation and Support Program from Ministry of Health in Argentina (SAI003-16). This program aims to accelerate the time to market as well as provide continuing support by the Agency throughout all the development phases of the product. EMA has recently granted an orphan drug designation for NEAST, and we are requesting a similar designation from FDA. Should this clinical trial be successful, NEAST will constitute a new therapeutic option for an orphan disease with large impact worldwide.
24641
References
- Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, et al. (2005) Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 115: e673-80.
- Rosales A, Hofer J, Zimmerhackl LB, Jungraithmayr TC, Riedl MG, et al. (2012) Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis 54: 1413-1421.
- Scheutz F (2014) Taxonomy meets public health: The Case of Shiga Toxin-Producing Escherichia coli. Microbiol Spectr 2.
- Lopez EL, Contrini MM, Glatstein E, Ayala SG, Santoro R, et al. (2010) Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in paediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrob Agents Chemother 54: 239-243.
- Bitzan M, Poole R, Mehran M, Sicard E, Brockus C (2009) Safety and pharmacokinetics of chimeric anti-shiga toxin 1 and anti-shiga toxin 2 monoclonal antibodies in healthy volunteers. Antimicrob Agents Chemother 53: 3081-3087.
- Melton-Celsa AR, Carvalho HM, Thuning-Roberson C, O’Brien AD (2015) Protective efficacy and pharmacokinetics of human/mouse chimeric anti-Stx1 and anti-Stx2 antibodies in mice. Clin Vaccine Immunol.
- Karliner JS, Belaval GS (1965) Incidence of Reactions Following Administration of Antirabies Serum; Study of 526 Cases Jama 193: 359-362.
- De Haro L, Lang J, Bedry R, Guelon D, Harry P (1998) Snake bite by European vipers. A multicenter study of tolerance to Viperfav, a new intravenous antivenom. Ann Fr Anesth Reanim 17: 681-687.
- Boyer L, Degan J, Ruha AM, Mallie J, Mangin E (2013) Safety of intravenous equine F (ab’)2: Insights following clinical trials involving 1534 recipients of scorpion antivenom. Toxicon 76: 386-393.
- Lang J, Attanath P, Quiambao B, Singhasivanon V, Chanthavanich P, et al. (1998) Evaluation of the safety, immunogenicity, and pharmacokinetic profile of a new, highly purified, heat-treated equine rabies immunoglobulin, administered either alone or in association with a purified, Vero-cell rabies vaccine. Acta Trop 70, 317-333.
- Quiambao BP, DyTioco HZ, Dizon RM, Crisostomo ME, Laot TM (2008) Rabies post-exposure prophylaxis in the Philippines: Health status of patients having received purified equine F(ab’)2 fragment rabies immunoglobulin (Favirab). PLoS Negl Trop Dis 2: e243.
- Herbreteau CH, Jacquot F, Rith S, Vacher L, Nguyen L, et al. (2014) Specific polyclonal F(ab’) 2 neutralize a large panel of highly pathogenic avian influenza A viruses (H5N1) and control infection in mice. Immunotherapy 6: 699-708.
- Bal C, Herbreteau CH, Buchy P, Rith S, Zaid M, et al. (2015) Safety, potential efficacy, and pharmacokinetics of specific polyclonal immunoglobulin F(ab’)2 fragments against avian influenza A (H5N1) in healthy volunteers: A single-centre, randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis.
- Mejias MP, Ghersi G, Craig PO, Panek CA, Bentancor LV, et al. (2013) Immunization with a chimera consisting of the B subunit of Shiga toxin type 2 and brucella lumazine synthase confers total protection against Shiga toxins in mice. J Immunol 191: 2403-2411.
- Laplagne D, Zylberman V, Ainciart N, Steward MW, Sciutto E, et al. (2004) Engineering of a polymeric bacterial protein as a scaffold for the multiple display of peptides. Proteins 57: 820-828.
- Melton-Celsa A, O´Brien A (2003) Animal Models for STEC-Mediated Disease. In: Methods in Molecular Medicine, vol.73: E. coli. Shiga toxin methods and protocols. Humana Press Inc., Totowa, N. 349.
- Marcato P, Mulvey G, Read RJ, Vander Helm K, Nation PN (2001) Immunoprophylactic Potential of Cloned Shiga Toxin 2 B Subunit. J. Infect. Dis.183, 435-443.
- Velikovsky CA, Cassataro J, Giambartolomei GH, Goldbaum FA, Estein S (2002) A DNA vaccine encoding lumazine synthase from Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 70, 2507-2511.
- Zylberman V, Craig PO, Klinke S, Braden BC, Cauerhff A (2004) A High order quaternary arrangement confers increased structural stability to Brucella sp. lumazine synthase. J Biol Chem 279: 8093-8101.
- Bellido D, Craig PO, Mozgovoj MV, Gonzalez DD, Wigdorovitz A, et al. (2009) Brucella spp. lumazine synthase as a bovine rotavirus antigen delivery system. Vaccine 27: 136-145.
- Cassataro J, Pasquevich KA, Estein SM, Laplagne DA, Velikovsky CA, et al. (2007) A recombinant subunit vaccine based on the insertion of 27 amino acids from Omp31 to the N-terminus of BLS induced a similar degree of protection against B. ovis than Rev.1 vaccination. Vaccine 25: 4437-4446.
- Velikovsky CA, Goldbaum FA, Cassataro J, Estein S, Bowden RA, et al. (2003) Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect Immun 71: 5750-5755.
- Mejias MP, Cabrera G, Fernandez-Brando RJ, Baschkier A, Ghersi G, et al. (2014) Protection of Mice against Shiga Toxin 2 (Stx2)-Associated Damage by Maternal Immunization with a Brucella Lumazine Synthase-Stx2 B Subunit Chimera. Infect Immun 82: 1491-1499.
- Mejias MP, Hiriart Y, Lauché C, Fernández-Brando RJ, Pardo R, et al. (2016) Development of came lid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS). Nat Publ Gr.
- Mejias MP, Fernandez-Brando RJ, Ramos MV, Abrey-Recalde MJ, Zotta E, et al. (2016) Development of a Mouse Model of Shiga Toxin 2 (STX2) Intoxication for Testing Therapeutic Agents against Hemolytic Uremic Syndrome (HUS). Curr Pharm Des.
- Melton-Celsa AR, O’Brien AD (2014) New therapeutic developments against Shiga Toxin-producing Escherichia coli. Microbiol Spectr 2.
- Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL (2005) Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg Infect Dis 11: 603-609.
- Luna-Gierke RE, Griffin PM, Gould LH, Herman K, Bopp CA, et al. (2014) Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol Infect 142: 2270-2280.















