Keywords
BSC Class II drug; Solid dispersions; Lutrol F127; Gelucire 44/14; Dissolution Studies.
Introduction
Chemically, Repaglinide is S (+)-2-ethoxy-4- [N- (1- (2-piperidinophenyl)- 3-methyl- 1-butyl)-amino carbonyl methyl] benzoic acid, which belongs to a new class of hypoglycaemic benzoic acid derivatives, a fast and short-acting meglitinide analog, is widely used for the treatment of diabetes. It has a very low bioavailability (50%) and poor absorption in the upper intestinal tract. Poor solubility in gastrointestinal fluids gives rise variations in its dissolution rate and incomplete bioavailability. An enhancement of the dissolution rates of waterinsoluble drugs remains one of the most challenging tasks of drug development, because the enhanced dissolution rates can increase drug oral bioavailability. Although repaglinide is rapidly absorbed after oral administration, it is critical to improve the dissolution rate of repaglinide to enhance the bioavailability due to its low solubility [1].
SDF (solid dispersion formulation), which was developed by Chiou and Riegelman (1971) [2], is the formulation that possibly enhances the dissolution rate, solubility and oral absorption of a poorly watersoluble drug. Swarbrick (1990) [3], Shargel (1993) [4] and Craig (2001) [5] discussed the increase in drug dissolution rate from SDF and they concluded that the dissolution rate was increased by the following factors: (a) the reduction of the drug particle size to molecular level, (b) the solubilising effect on the drug by the water-soluble carrier, and (c) the enhancement of the wettability and dispersibility of the drug by the carrier material. According to the definition of SDF, both a given drug and carrier should be completely dissolved with organic solvents (solvent method) or fused by the heating (melting method) in order to prepare SDF. With regard to carriers for SDF, many carriers such as polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), hydroxyl propyl methyl cellulose (HPMC), hydroxyl propyl cellulose, hydroxyl propyl methyl cellulose phthalate, gelucires, eudragits and chitosans have been reported to improve the solubility and bioavailability of poorly water-soluble drugs (Okimoto et al., 1997 [6]; Portero et al., 1998 [7]; Jung et al., 1999 [8]; Kohri et al., 1999 [9]; Trapani et al., 1999 [10]; Damian et al., 2002 [11]; Tantishaiyakul et al., 1999 [12]; Yamada et al., 2000 [13] ; Cilurzo et al., 2002 [14]; Kushida et al., 2002 [15]; Nakamichi et al., 2002 [16]), but among them PEG 6000, Gelucire 44/14, Lutrol F127 are considered the most suitable carriers for SDF (Kohri et al., 1999 [9]; Kushida et al., 2002 [15] ).
The mechanisms for the enhancement of the dissolution rate of solid dispersions have been proposed by several investigators. Drugs molecularly dispersed in polymeric carriers may achieve the highest levels of particle size reduction and surface area enhancement, which result in improved dissolution rates. Furthermore, no energy is required to break up the crystal lattice of a drug during dissolution process, and drug solubility and wettability may be increased by surrounding hydrophilic carriers. The methods used to prepare solid dispersions include the melting method, the solvent method and the solvent melting method.
The physicochemical properties of repaglinide in solid dispersions were characterized by differential scanning calorimetry and powder X-ray diffraction, and the effects of various hydrophilic solid dispersion carriers on its dissolution properties were investigated.
An attempt was made in this study to enhance the aqueous solubility and dissolution rate of repaglinide by preparation of SDs using hydrophilic polymers like Lutrol F127, PEG 6000 and Gelucire 44/14 in various concentrations by solvent, melting and melting solvent methods, which improves the bioavailability of repaglinide and their physicochemical properties, were investigated. Therefore, repaglinide would be good candidate for the application of solid dispersions.
Materials and Methods
Materials
Repaglinide was generously supplied as a gift sample by Biocon, (Bangalore, India). Lutrol F127 was purchased from Signet Pharmaceuticals, (Bangalore, India). Gelucire 44/14 was received as a gift sample from ColorCon Pharmaceuticals, (Bangalore, India). PEG 6000 and Ethanol were purchased from SD Fine Chemicals, (Mumbai, India). All other chemicals were of analytical reagent grade and were used as received.
Preformulation Parameters
Determination of Solubility of Repaglinide
Drug solubility studies were performed in triplicate by adding excess amounts of repaglinide to water and buffer solutions having different pH (pH 1.2, 3.0, 4.5, 7, 9.0) and solutions containing flasks were kept on a rotary shaker for 24 h. After 24 h, solutions were analyzed using UV spectrophotometer at 283 nm, which was the absorption maxima determined earlier and drug concentrations were calculated [17].
Compatibility of Excipients
Fourier transform infrared (FT-IR) spectroscopy was employed to characterize the possible interactions between the drug and the carriers in the solid state on Perkin Elmer Spectrum GX (organic Chemistry Unit, IISc, Bangalore) by the conventional KBr pellet method. The spectra were scanned over a frequency range 4000–400 cm-1 [18,19].
Phase Solubility Studies
Solubility measurements were performed in triplicate using the method reported by Higuchi and Connors. An excess amount of repaglinide was added to the aqueous solutions of each carrier in 50 mM citric acid/100 mM sodium phosphate dibasic buffer with a pH of 4.5 containing increasing concentrations of the individual carrier (i.e., 0.1%, 0.25%, 0.5%, 0.75% and 1% w/v). The flasks were sealed and shaken at 37 ºC for 48 hrs in a thermostatically controlled water bath and the samples were filtered. The filtrate was suitably diluted and analyzed spectrophotometrically (Shimadzu 1601, Japan) at 283 nm [19].
Preparation of physical mixtures
Physical mixtures were prepared by grinding repaglinide and individual polymeric carriers in a mortar (the ratio of repaglinide to polymer used was 1:1, 1:3, 1:5 and 1:7) (Table -1) [20, 21].
| Formulation Code |
Carrier |
Drug to Carrier Ratio |
Method of Preparation |
%Drug Content |
%Drug Release |
| GM11 |
Gelucire44/14 |
1:1 |
Melting |
95.52 ± 0.1154 |
70.21 ± 0.1477 |
| GM13 |
Gelucire44/14 |
1:3 |
Melting |
97.03 ± 0.1015 |
74.37 ± 0.1468 |
| GM15 |
Gelucire44/14 |
1:5 |
Melting |
97.25 ± 0.1042 |
77.75 ± 0.1480 |
| GM17 |
Gelucire44/14 |
1:7 |
Melting |
97.90 ± 0.1126 |
83.75 ± 0.1438 |
| GS11 |
Gelucire44/14 |
1:1 |
Solvent |
96.25 ± 0.1130 |
72.53 ± 0.1509 |
| GS13 |
Gelucire44/14 |
1:3 |
Solvent |
97.58 ± 0.1028 |
77.63 ± 0.1374 |
| GS15 |
Gelucire44/14 |
1:5 |
Solvent |
98.29 ± 0.1143 |
82.06 ± 0.1303 |
| GS17 |
Gelucire44/14 |
1:7 |
Solvent |
96.36 ± 0.1032 |
86.24 ± 0.1515 |
| GMS11 |
Gelucire44/14 |
1:1 |
Melting-solvent |
96.83 ± 0.1046 |
75.89 ± 0.1394 |
| GMS13 |
Gelucire44/14 |
1:3 |
Melting-solvent |
96.95 ± 0.1092 |
81.29 ± 0.1264 |
| GMS15 |
Gelucire44/14 |
1:5 |
Melting-solvent |
97.89 ± 0.1025 |
85.32 ± 0.1446 |
| GMS17 |
Gelucire44/14 |
1:7 |
Melting-solvent |
98.25 ± 0.1124 |
86.66 ± 0.1418 |
| LM11 |
Lutrol F127 |
1:1 |
Melting |
96.50 ± 0.1154 |
80.71 ± 0.1256 |
| LM13 |
Lutrol F127 |
1:3 |
Melting |
97.25 ± 0.1058 |
84.57 ± 0.1495 |
| LM15 |
Lutrol F127 |
1:5 |
Melting |
98.50 ± 0.1047 |
87.48 ± 0.1653 |
| LM17 |
Lutrol F127 |
1:7 |
Melting |
99.00 ± 0.0938 |
95.27 ± 0.1267 |
| LS11 |
Lutrol F127 |
1:1 |
Solvent |
96.25 ± 0.1084 |
82.40 ± 0.1188 |
| LS13 |
Lutrol F127 |
1:3 |
Solvent |
97.50 ± 0.0915 |
87.91 ± 0.1464 |
| LS15 |
Lutrol F127 |
1:5 |
Solvent |
98.44 ± 0.0836 |
90.89 ± 0.1359 |
| LS17 |
Lutrol F127 |
1:7 |
Solvent |
97.15 ± 0.1084 |
97.95 ± 0.1305 |
| LMS11 |
Lutrol F127 |
1:1 |
Melting-solvent |
96.85 ± 0.1067 |
84.45± 0.1495 |
| LMS13 |
Lutrol F127 |
1:3 |
Melting-solvent |
97.50 ± 0.0990 |
92.33 ± 0.1810 |
| LMS15 |
Lutrol F127 |
1:5 |
Melting-solvent |
98.90 ± 0.1117 |
93.55 ± 0.1287 |
| LMS17 |
Lutrol F127 |
1:7 |
Melting-solvent |
98.75 ± 0.1068 |
99.82 ± 0.1280 |
| PM11 |
PEG6000 |
1:1 |
Melting |
96.86 ± 0.0993 |
76.14 ± 0.1433 |
| PM13 |
PEG6000 |
1:3 |
Melting |
97.36 ± 0.1038 |
81.23 ± 0.1438 |
| PM15 |
PEG6000 |
1:5 |
Melting |
98.25 ± 0.1076 |
85.16 ± 0.1424 |
| PM17 |
PEG6000 |
1:7 |
Melting |
98.80 ± 0.1045 |
91.14 ± 0.1408 |
| PS11 |
PEG6000 |
1:1 |
Solvent |
97.78 ± 0.0929 |
79.39 ± 0.1523 |
| PS13 |
PEG6000 |
1:3 |
Solvent |
97.44 ± 0.1058 |
84.27 ± 0.1267 |
| PS15 |
PEG6000 |
1:5 |
Solvent |
98.36 ± 0.1021 |
87.93 ± 0.1407 |
| PS17 |
PEG6000 |
1:7 |
Solvent |
97.15 ± 0.1132 |
93.29 ± 0.1392 |
| PMS11 |
PEG6000 |
1:1 |
Melting-solvent |
96.69 ± 0.1107 |
82.13 ± 0.1325 |
| PMS13 |
PEG6000 |
1:3 |
Melting-solvent |
97.85 ± 0.0958 |
88.56 ± 0.1390 |
| PMS15 |
PEG6000 |
1:5 |
Melting-solvent |
98.89 ± 0.1018 |
90.33 ± 0.1207 |
| PMS17 |
PEG6000 |
1:7 |
Melting-solvent |
98.00 ± 0.1014 |
95.29 ± 0.1486 |
| GPM11 |
Gelucire44/14 |
1:1 |
----- |
98.20 ± 0.1120 |
60.81 ± 0.1323 |
| GPM13 |
Gelucire44/14 |
1:3 |
----- |
98.25 ± 0.1040 |
64.26 ± 0.1228 |
| GPM15 |
Gelucire44/14 |
1:5 |
----- |
98.45 ± 0.0964 |
67.17 ± 0.1371 |
| GPM17 |
Gelucire44/14 |
1:7 |
----- |
99.00 ± 0.0936 |
69.87 ± 0.1305 |
| LPM11 |
Lutrol F127 |
1:1 |
----- |
97.33 ± 0.1005 |
74.63 ± 0.1475 |
| LPM13 |
Lutrol F127 |
1:3 |
----- |
98.00 ± 0.1138 |
76.60 ± 0.1262 |
| LPM15 |
Lutrol F127 |
1:5 |
----- |
98.55 ± 0.1025 |
77.50 ± 0.1361 |
| LPM17 |
Lutrol F127 |
1:7 |
----- |
98.51 ± 0.1148 |
81.19 ± 0.1403 |
| PPM11 |
PEG6000 |
1:1 |
----- |
98.30 ± 0.0935 |
69.65 ± 0.1185 |
| PPM13 |
PEG6000 |
1:3 |
----- |
97.70 ± 0.1086 |
73.15 ± 0.1344 |
| PPM15 |
PEG6000 |
1:5 |
----- |
98.45 ± 0.1053 |
74.72 ± 0.1284 |
| PPM17 |
PEG6000 |
1:7 |
----- |
98.89 ± 0.0987 |
77.71 ± 0.1244 |
Table 1: Preparation of SDs and Physical Mixtures of Repaglinide with Different Carriers; Comparison of Drug Content and Drug Release Data.
Preparation of Solid Dispersions
Solid dispersions at different drug-to-polymer ratios were prepared by melting, solvent and melting– solvent methods (Table - 1) [18, 22-24].
The preparation of dispersions of repaglinide with various water-soluble carriers viz. PEG 6000, Gelucire 44/14, Lutrol F127 by the melting method was carried out by melting each carrier over a thermostatically controlled magnetic stirrer at its respective melting point and drug was incorporated into the molten carrier mass. The blend was heated at the corresponding temperature for 5 min, followed by flash-cooling on an ice bath.
According to the solvent method, different amounts of repaglinide and carriers were accurately weighed and separately dissolved in a solvent ethanol and the solutions were mixed with constant stirring. Solvent was removed under vacuum at 40 ºC using a rotary evaporator. The solid dispersions were obtained were ground in a mortar.
For preparing solid dispersions by the melting– solvent method, repaglinide was dissolved in a solvent and this solution was added to the melted carrier (60 ºC). The excess of solvent was eliminated under vacuum at 40 ºC using a rotary evaporator. All the resultant dispersions were then dried in a dessiccator at room temperature. The solid dispersions prepared by the melting, solvent and melting-solvent method contained 1:1, 1:3, 1:5 and 1:7 drug/polymer ratios.
Drug Content
Solid dispersions and physical mixtures equivalent to 2 mg drug were taken and dissolved in minimum quantity of methanol and volume was made up to 50 ml. From this solution, 5 ml was taken and again diluted with methanol up to 50 ml. The solution was assayed for drug content using spectrophotometry method by measuring the absorbance at 283 nm [25].
In Vitro Release studies
In vitro release studies of repaglinide, physical mixtures and SDs (equivalent to 2 mg repaglinide filled in hard gelatin capsule) were performed using USP XXII tablet dissolution test apparatus at the basket rotation speed of 75 rpm in 900 ml 50 mM citric acid/100 mM sodium phosphate dibasic buffer with a pH of 4.5 at 37 ºC. At the specified time, 5 ml of samples were withdrawn, filtered and assayed for repaglinide using specrophotometry method by measuring the absorbance at 283 nm. The volume was replaced by the dissolution medium after each sampling.
Dissolution profiles of the formulations were analyzed by plotting drug release versus time [17, 26].
Powder X-Ray Diffraction Studies (PXRD)
The powder X-ray diffraction study was carried out at Solid State Structural Chemistry Unit, IISc, Bangalore, to characterize state of repaglinide. A Philips X’Pert PW 3040/60 (Almelo, Netherlands) was used as X-ray generator for Cu Ká radiation (ë = 1.54178 a.u.). Data was collected in the continuous scan mode using step size of 0.010 2è. The scanned range was 2-500[22, 27] .
Differential Scanning Calorimetry (DSC)
The differential scanning calorimetry of repaglinide and solid dispersion of repaglinide/Lutrol F127 was done by using Shimadzu DSC-60 Calorimeter, Tokyo, Japan [28, 29].
Stability Studies
The selected formulations were packed in ambercolored bottles, which were tightly plugged with cotton and capped with aluminium. They were then stored at 25ºC / 60% RH, 30ºC / 65% RH, & 40ºC / 75% RH for 6 months and evaluated for their drug content and dissolution study.
Results and Discussion
Preformulation Parameters
Solid dispersion technique for many poorly watersoluble drugs with hydrophilic carriers provides an efficient method to improve drugs dissolution rate. The ability of solid dispersions to afford drug release as fine, dispersed particles has resulted in improvements in dissolution rates of poorly watersoluble drugs, which have also been reflected in increase in oral bioavailability.
Solubility of repaglinide in water was found to be 0.0340 mg/ml indicating it is practically insoluble in water. Repaglinide exhibits two pKa values of 4.19 and 5.78, and being a weakly acidic compound, the drug is ionized at higher pH values, owing to its higher aqueous solubility at higher pH values.
In order to test for possible intermolecular interaction between repaglinide and the excipients of the dispersion matrix, FTIR was carried out. The FTIR spectra analysis of repaglinide alone showed that, the principal peaks were observed at wave numbers- 651.71, 761.66, 1221.46, 1565.30, 1631.27 and 1689.25 confirming the purity of drug as per established standards. In the IR spectra of the physical mixture of repaglinide with excipients- Lutrol F 127; PEG 6000 and Gelucire 44/14, the major peaks of repaglinide were observed at same wave numbers. However, some additional peaks were observed with physical mixtures, which could be due to the presence of polymers.
Phase-solubility diagrams (Fig. 1) show that the concentration of repaglinide in pH 4.5 buffer increases as a function of increase in the concentration of Lutrol F127, PEG 6000 and Gelucire44/14. All the curves obtained in the present studies are AL type because of linear increase in solubility with the value of R2 closed to unity. Hydrophilic carries are known to interact with drug molecules mainly by electrostatic forces and occasionally by other type of forces like hydrogen bonds. The solubility enhancement of repaglinide with various carries was found to be in the order of Lutrol F127> Gelucire 44/14> PEG 6000. At 1% w/v concentration of the polymer, Lutrol F127 showed 19.15-fold augmentation in the solubility of pure drug, attributable to be the micellar solubilization of drug. The drug content ranged between 95.52 ± 0.1154% and 99.0 ± 0.938% (Table-1). The results indicate that the processes employed to prepare solid dispersions in this study were capable of producing formulation with uniform drug content.
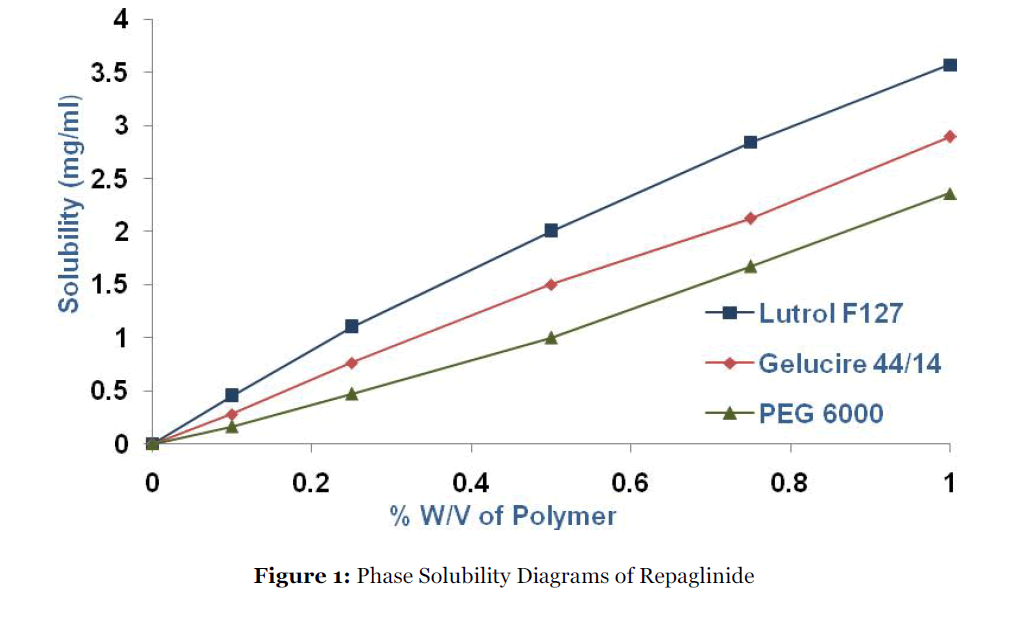
Figure 1: Phase Solubility Diagrams of Repaglinide
Dissolution study
Dissolution rates of repaglinide pure drug, physical mixtures, solid dispersion formulations and repaglinide-marketed tablets were evaluated according to the USP monograph for repaglinide tablets. In comparison to crystalline drug and drug /polymers physical mixtures, products of the solid dispersions prepared by melting, solvent and melting solvent methods generally showed a significant increase in dissolution rate up to 1:7 ratio of drug: polymer (Table - 1). Among three different methods used, the order of dissolution enhancement was found to be melting-solvent > solvent > melting method, where as in case of hydrophilic carries, the order of dissolution enhancement was found to be Lutrol F 127 > PEG 6000 > Gelucire 44/14. Within the first 5 mins, 56%, 50% and 36% of the repaglinide in LMS17, PMS17 and GMS17 was dissolved in the dissolution media, compared with ≈ 24%, 22%, 17% and 40% for LPM17, PPM17, GPM17 and repaglinide marketed tablets, respectively. The bulk drug showed only ≈ 10% drug release after 5 mins, because of the poor wettability, and/or agglomeration of particles. Complete (100%) dissolution of LMS17 was observed after 60 mins, while repaglinide tablets, PMS17, GMS17, LPM17, PPM17 and GPM17 showed only 92%, 95%, 86%, 81%, 77% and 69% release after 60 mins, respectively (Fig. 2).
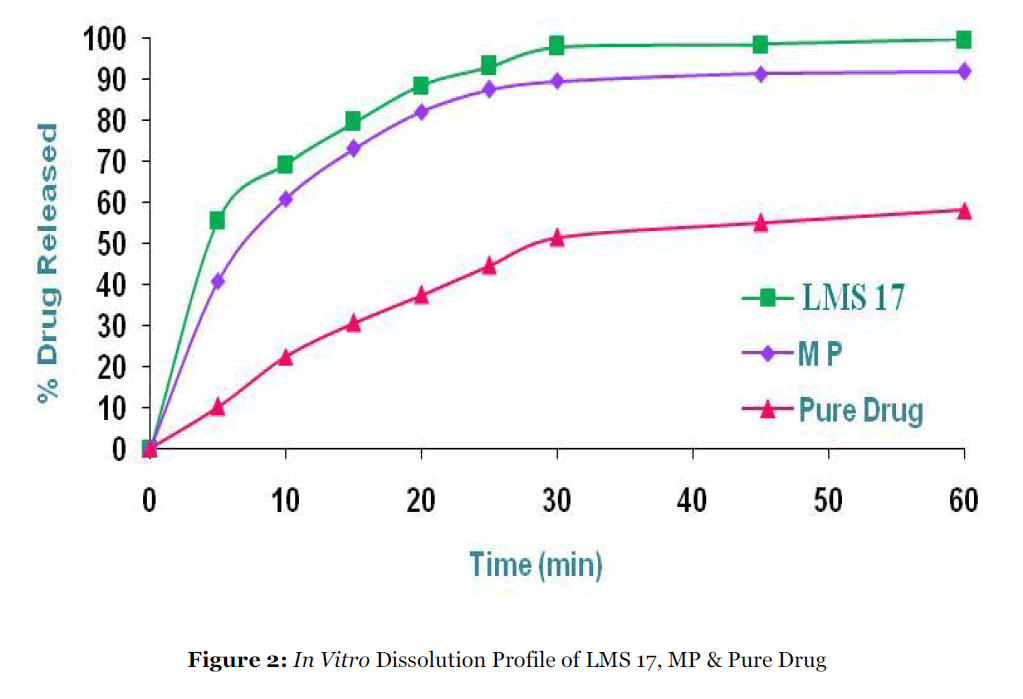
Figure 2:In Vitro Dissolution Profile of LMS 17, MP & Pure Drug.
All formulations tested including repaglinide tablets, showed an increase in dissolution over pure drug repaglinide, which showed only ≈ 58% release after 60 mins.
The order of dissolution enhancement with binary system was found to be Lutrol F127 > PEG 6000 > Gelucire 44/14. This order was not in exact consonance with that obtained during phasesolubility studies, where PEG 6000 showed lower solubility enhancement than Gelucire 44/14. Relatively higher dissolution enhancement in such cases could be credited to more intimate drug carrier interaction during formulation of solid dispersions. Increased dissolution of repaglinide from the dispersion could be ascribed due to Probable reduction in its particle size and Wetting of the hydrophobic particles and augmentation of its solubility by the carriers.
The repaglinide- Lutrol F 127 physical mixtures also showed improvement in drug dissolution profile, but were significantly lower than the corresponding solid dispersions. The Lutrol F127 systems resulted in marked improvement in repaglinide dissolution, assignable to micellar solubilization. Enhancement in drug dissolution performance with PEG 6000 has been attributed to the formation of interstitial solid solutions. The increase in dissolution of drug with the Gelucire 44/14 system, as a result of decreasing interfacial tension between the drug particles and water by microemulsification, was relatively less efficient than Lutrol F127 and PEG 6000.
Solid state study
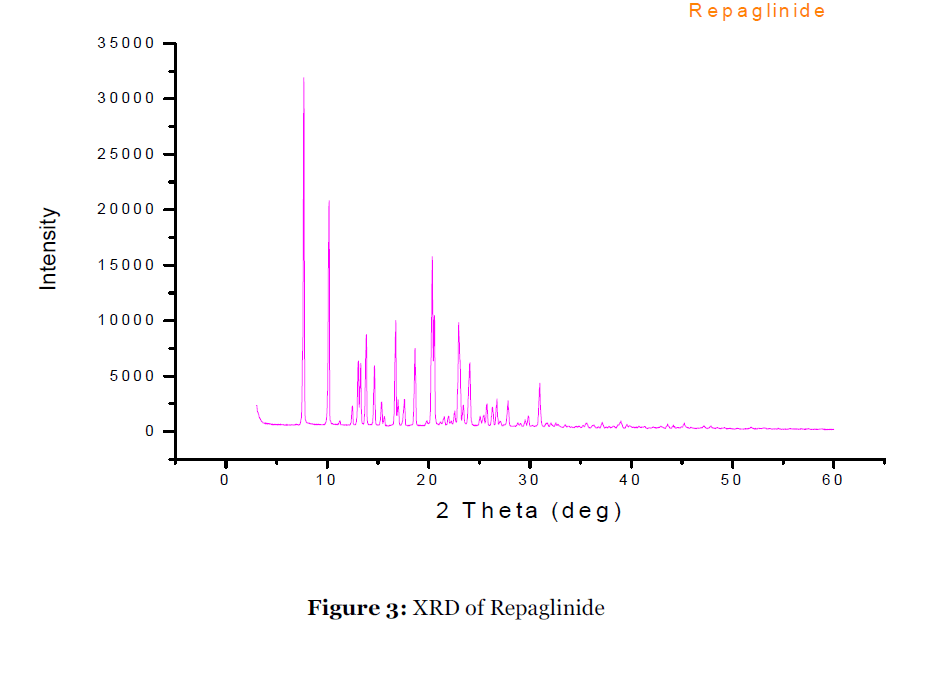
Figure 3:XRD of Repaglinide.
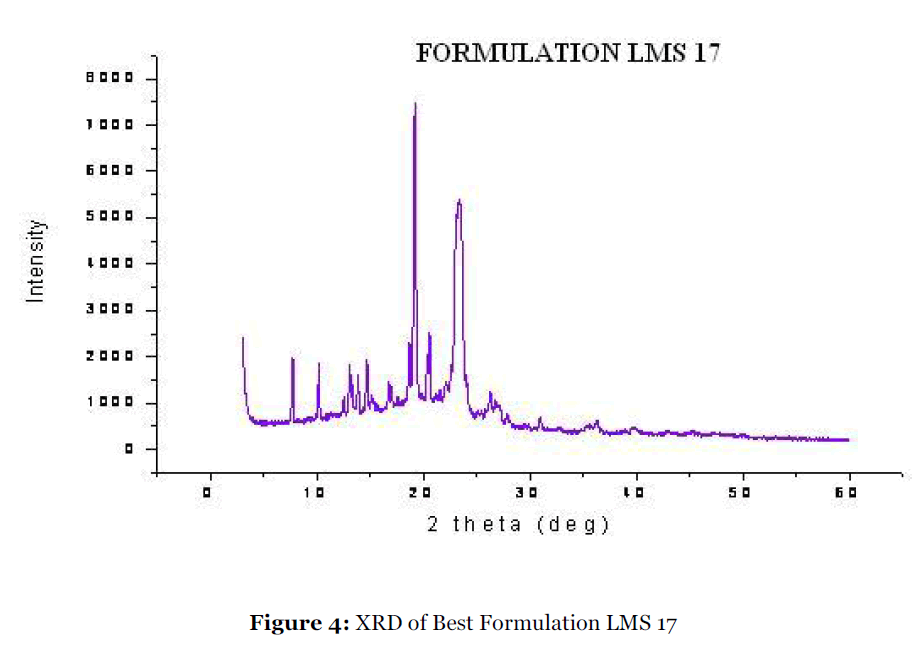
Figure 4:XRD of Best Formulation LMS 17.
To investigate the mechanistic of dissolution rate enhancement of most promising Lutrol F127 systems, the XRD study was carried out for Lutrol F127 solid dispersion. Fig. 3 & 4 show the diffraction spectra of pure repaglinide and the solid dispersion respectively. The diffraction spectrum of the solid dispersion vis-à-vis pure drug indicates the changes produced in the drug crystal structure. The sharp diffraction peaks associated with pure repaglinide are characteristic of its crystalline form. The absence of diffraction peaks in solid dispersion confirms that the repaglinide is present in amorphous form. No new peaks were observed, suggesting the absence of interaction between the drug and the polymer in the formulation. These data are indicative of the transformation of repaglinide from the crystalline to the amorphous state by formation of solid dispersion with Lutrol F127. The formation of an amorphous state proves that the drug was dispersed in a molecular state with the Lutrol F127.
DSC analysis was carried out to confirm the results obtained by X – ray diffraction. DSC provides a systemic way to verify the final state of the overall solid molecular dispersion. The DSC thermograms of repaglinide and of its solid dispersion are shown in Fig. 5 and Fig. 6 respectively. The sharp melting point peak of pure repaglinide appeared at 140.54 ºC, whereas no such peak was observed in solid dispersions prepared with Lutrol F127, suggesting that repaglinide was molecularly dispersed and was in an amorphous form. It was of interest to note that thermal profile of solid dispersions exhibited a single endothermic peak at about 59.64 ºC, corresponding to the fusion of the Lutrol F127: no peak indicating the melting of the drug was present. These results are in accord with previous XRD results, confirming that repaglinide was transformed from a crystal to amorphous form upon dispersion by the melting solvent method.
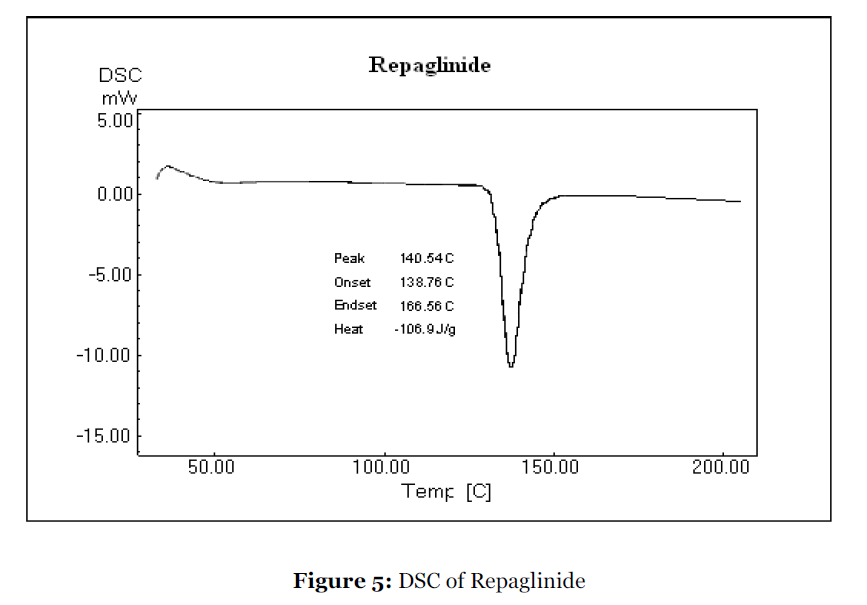
Figure 5:DSC of Repaglinide.
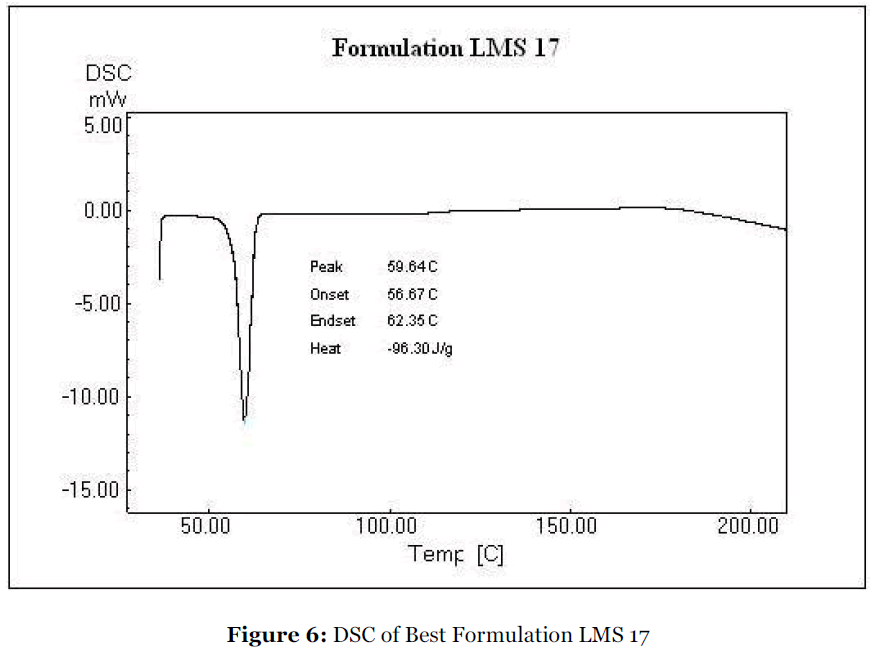
Figure 6:DSC of Best Formulation LMS 17.
Accelerated stability studies of best solid dispersion formulation LMS17, showed no significant change in % drug content and dissolution profile in the applied condition of temperature and humidity (25 ºC / 60% RH, 30°C / 65% RH, & 40 ºC / 75% RH) for a period of 6 months.
Conclusion
Various water-soluble carriers viz.Lutrol F127, PEG 6000, Gelucire 44/14 and different techniques viz. melting, solvent, melting solvent; were investigated in the current study to formulate solid dispersions of repaglinide, which enhanced the solubility and dissolution characteristics of the drug in varying degrees. pH solubility study revealed that the solubility of repaglinide increased as a function of increasing pH. Phase-solubility studies revealed AL type of curves for each carrier, indicating linear increase in the drug solubility with carrier concentration. All the solid dispersions showed dissolution improvement vis-à-vis pure drug to varying degrees, with Lutrol F127 as the most promising carrier. Solid-state characterization studies revealed that the drug crystallinity played pivotal role in governing the solubility characteristics of the drug. As with the repaglinide/Lutrol F127 sample, XRD analysis of the product confirmed a characteristic amorphous pattern, FTIR spectra did not show any evidence for a chemical interaction between the components and DSC showed the absence of melt endotherms, ostensibly accounting for enhancement in dissolution rate. Therefore, it was concluded that melting solvent method of repaglinide with Lutrol F127 (1:7) resulted in amorphous products. The stability studies indicate the Lutrol F127 systems were stable for period of study (90 days).
5539
References
- T Purvis; ME Mattucci; MT Crisp; KP Johnston; RO Williams. AAPS PharmSciTech 2007,8.
- J Swarbrick. Encyclopedia of Pharmaceutical Technology, Marcel Dekker, New York, Vol. 3, 1990
- L Shargel. Applied Biopharmaceutics and Pharmacokinetics, Appleton & Lange, Norwalk, CT, 2nd ed, 1993.
- K Okimoto; M Miyake; R Ibuki; M Yasumura; N Ohnishi; T Nakai. Int. J. Pharm. 1997,159, 85ñ93.
- A Portero; C Remunan-Lopez; JL Vila-Jato. Int. J. Phar 1998, 175, 75ñ84.
- JY Jung; SDF Yoo; SH Lee; KH Kin; DS Yoon; KH Lee. Int. J. Pharm 1999, 187, 209ñ218.
- N Kohri; Y Yamayoshi; H Xin; K Iseki; N Sato; S Todo; K Miyazaki. J. Pharm. Pharmacol 1999, 51, 159ñ164.
- G Trapani; M Franco; A Latrofa; MR Pantaleo; MR Provenzano; E Sanna, E Maciocco; G Liso. Int. J. Pharm. 1999, 184, 121ñ130.
- F Damian; N Blaton; R Kinget; G Van den Mooter.Int. J. Pharm. 2002, 244, 87ñ98.
- V Tantishaiyakul; N Kaewnopparat; S Ingkatawornwong. Int. J. Pharm.1999, 181, 143ñ 151.
- T Yamada; N Saito; M Anraku; T Imai; M Otagiri.Pharm. Dev. Technol. 2000, 5, 443ñ454.
- F Cilurzo; P Minghetti; A Casiraghi; L Montanari.Int. J. Pharm. 2002, 242, 313ñ317.
- K Nakamichi; T Nakano; H Yasuura; S Izumi; Y Kawashima. Int. J. Pharm. 2002 241, 203ñ211.
- T Purvis; ME Mattucci; MT Crisp; KP Johnston; RO Williams. AAPS Pharm Sci Tech. 2007, 8 (3), E1 ñ E9.
- N Ahuja; OP Katare; B Singh. (2007) Eur. J. Pharm. Biopharm.2007, 65, 26ñ38.
- LP Ruan; BY Yu; GM Fu; D Zhu. J. Pharm. Biomed. Analysis. 2005, 38, 457ñ464.
- M Guyot; F Fawaz; J Bildet; F Bonini; AM Lagueny.Int. J. Pharm. 1995,123, 53-63.
- SR Vippagunta; KA Maul; S Tallavajhala; DJW Grant. Int. J. Pharm. 2002, 236, 111ñ123.
- GV Mooter; P Augustijns; N Blaton; R Kinget. Int. J. Pharm.1998,164, 67ñ80.
- EJ Kim; MK Chun; JS Jang; IH Lee; KR Lee; HK Choi. Eur. J. Pharm. Biopharm. 2006, 64, 200ñ 205.
- MC Martinez-Oharriz; C Martin; MM Goni; C Rodriguez-Espinosa; MC Tros-Ilarduya; A Zornoza. Eur. J. Pharm. Sci. 1999, 127-132.
- D Yang; R Kulkarni; RJ Behme; PN Kotiyan. Int. J. Pharm. 2007,329, 72ñ80.
- MM Patel; DM PateL. Fast dissolving valdecoxib tablets containing solid dispersion of valdecoxib. Int. J. Pharm. Sci. 2006; 222-226.
- E Karavas; E Georgarakis; MP Sigalas; K Avgoustakis; D Bikiaris. Eur. J. Pharm. Biopharm. 2007, 66, 334ñ347.
- M Franco; G Trapani; A Latrofa; C Tullio; MR Provenzano; M Serra; M Muggironi; G Biggio; G Liso. Int. J. Pharm. 2001, 225, 63ñ73.
- B Suhagia; HA Patel; SA Shah; I Rathod; VK Parmar. Acta Pharm. 2006, 56, 285ñ298.












