Shyamapada Mandal*
Laboratory of Microbiology and Experimental Medicine, Department of Zoology, University of Gour Banga, Malda, West Bengal, India
*Corresponding Author:
Prof. Shyamapada Mandal
Laboratory of Microbiology and Experimental Medicine
Department of Zoology, University of Gour Banga
Malda, West Bengal
India
Tel: +91-9831279239
E-mail: samtropmed@gmail.com
Received date: May 15, 2018; Accepted date: June 19, 2018; Published date: June 21, 2018
Citation: Mandal S (2018) Preparedness for Global Dengue Resurgence: An Urgent Need. Transl Biomed. Vol.9 No.2:150
Commentary
Dengue virus (DENV: Genus Flavivirus; family Flaviviridae), which is vectored by female Aedes aegypti (in endemic regions principally in urban areas) as well as female Aedes albopictus (mostly in rural areas) mosquitoes, cause dengue in humans (with the infection of its all four serotypes [1-3]: DENV1, DENV2, DENV3 and DENV4) that arrays from self-limiting mild feverish illness: DF (dengue fever), to severe life-threatening forms, DHF (dengue hemorrhagic fever) and DSS (dengue shock syndrome) leading to death (Figure 1). The disease has been recognized as a major public health problem in developing tropics and subtropics, but is now spreading rapidly globally with worldwide transportation of infective vectors of DENVs [4,5], due to:
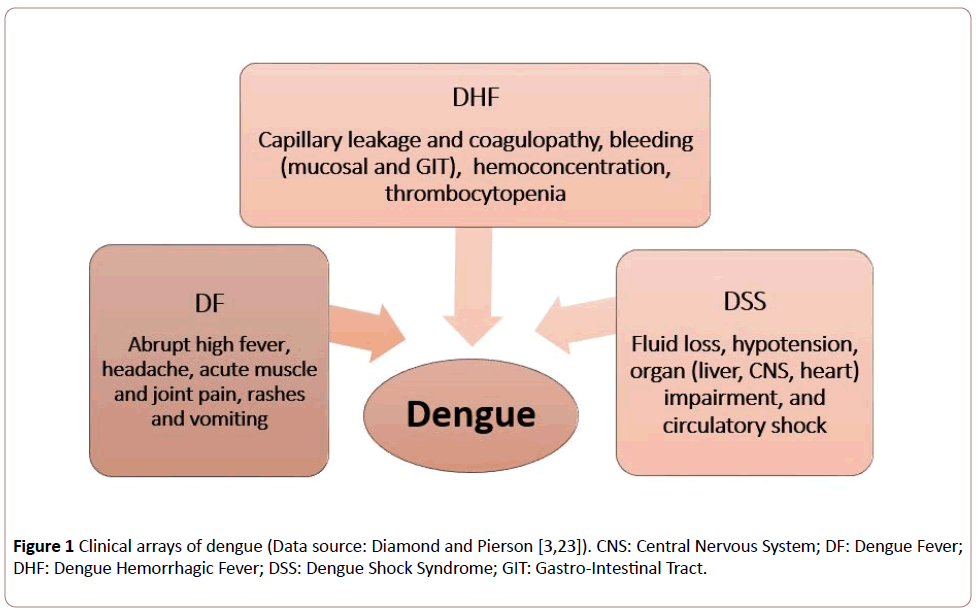
Figure 1: Clinical arrays of dengue (Data source: Diamond and Pierson [3,23]). CNS: Central Nervous System; DF: Dengue Fever; DHF: Dengue Hemorrhagic Fever; DSS: Dengue Shock Syndrome; GIT: Gastro-Intestinal Tract.
(a) climatic (and environmental: temperature, rainfall and humidity) factors as well as human behaviors (urbanization and globalization) and
(b) overall lack of vector control measures and absence of anti-dengue antiviral as well as absence or limitation in usage of licensed dengue (only dengvaxia: CYD-TDV) vaccine [6-9].
DHF was first reported in Calcutta (Kolkata), West Bengal in 1963 and 1964 [10,11], and due to the co-circulation of all four DENV (DENV1, DENV2, DENV3 and DENV4) serotypes this region has been designated as hyper-endemic [12]. Several dengue episodes hit Kolkata from time to time [13,14], while reports of dengue hits have currently been available from previously unaffected rural of West Bengal too [13,15,16].
Dengue cases have been detected among populations from different districts of West Bengal, including Kolkata sharing the majority (74.32%) of the cases, during 2010-2012, due to multiple DENV serotypes: DENV1, DENV3, and DENV4 [17]. Currently, reports are available on the emergence of a new antigenically distinct DENV serotype (DENV5) from Malaysian origin [18] and taking this in account Kolkata and many other parts of India, possessing all suitable niches for the virus as well as the vectors, may provide a favourable home for DENV5 too.
The extrinsic incubation period (EIP: Time span for viral incubation between the ingestion of viremic blood from the human, by a mosquito vector, and detection of DENVs in the mosquito salivary glands for transmission to a new susceptible host) might explain the transmission dynamics of DENVs, and hence the dengue-preparedness for proper management [2]. It has been shown experimentally that at temperature <18°C, DENVs are not detected in the vector’s (A. albopictus) salivary glands, while at 21°C, DENV antigens are detectable, and therefore, reports are aptly available on the decrease of EIP from 9 days (at 26°C) to 5 days (at 30°C), and 8 days to 15 days, respectively at 30.8°C and 23.4°C, suggesting the gradual increase of DEN incidences with increased temperature, peaking at ~ 32°C prior to declining at higher temperatures [2,6,7]. Watts et al. [19] demonstrated in Ae. aegypti model with DENV-2 infection the shortening of EIP from 12 days to 7 days with the increase of temperature from 30°C to 32-34°C. In dengue outbreak as well as sporadic situation, DENV infection occurs during monsoon and post-monsoon seasons in Wes Bengal, India [17], when temperature prevails within the range that facilitates the decrease of the EIP, elevating the chances of DENV transmission.
Vector control (following the principle of integrated vector management), active case surveillance (on the basis of complete health information) and vaccination programmes are required to be considered at the local and global levels, especially in dengue endemic areas, taking into account of the ‘dengue hotspots models’ [8], in order to prevent dengue outbreaks and epidemics [20], wherein awareness of personal protection from mosquito bites and good environmental health might aid the process of prevention of DENV infection and transmission. However, discovery of new serotype, such as DENV5, and the potentiality of the spread of multiple serotypes of DENVs along with their vectors might encumber effective dengue-vaccine discovery thereby causing resurrection of dengue outbreaks/epidemics, even in new geographical regions [21], and therefore, advocating the epidemiological studies with climatic distinctiveness must be the prime requirement for DENV infection control [22,23].
22861
References
- Gubler DJ (2011) Dengue, urbanization and globalization: The unholy trinity of the 21st century. Trop Med Health 39: S3-S11.
- Mutheneni SR (2017) Dengue burden in India: Recent trends and importance of climatic parameters. Emerg Microbes Infect 6: e70.
- Diamond MS, Pierson TC (2015) Molecular insight into dengue virus pathogenesis and its implications for disease control. Cell 162: 488-492.
- Abozeid S (2018) Re-emergence of aedes aegypti in Egypt. Lancet Infect Dis 18: 142-43.
- WHO (2012) Global strategy for dengue prevention and control. WHO Press 2: 1.
- Xiao FZ (2014) The effect of temperature on the extrinsic incubation period and infection rate of dengue virus serotype 2 infection in Aedes albopictus. Arch Virol 159: 3053-3057.
- Colon-Gonzalez FJ (2013) The effects of weather and climate change on dengue. PLoS Negl Trop Dis 7: e2503.
- Sun W (2017) Spatial-temporal distribution of dengue and climate characteristics for two clusters in Sri Lanka from 2012 to 2016. Sci Rep 7: 1-12.
- Aikat BK (1964) Haemorrhagic fever in Calcutta area. Indian J Med Res 152: 660-675.
- Sarkar JK (1972) Sporadic cases of haemorrhage and/or shock during dengue epidemics. Trans R Soc Trop Med Hyg 66: 875–877.
- Sarkar A, Taraphdar D, Chatterjee S (2012) Molecular typing of dengue virus circulating in Kolkata, India in 2010. J Trop Med 4: 1-5.
- Hati AK (2009) Dengue serosurveillance in Kolkata, facing an epidemic in West Bengal, India. J Vector Borne Dis 46: 197-204.
- Debnath F, Ponnaiah M, Acharya P (2017) Dengue fever in a municipality of West Bengal, India, 2015: An outbreak investigation. Indian J Public Health 61: 239-42.
- Biswas DK, Bhunia R, Basu M (2014) Dengue fever in a rural area of West Bengal, India, 2012: An outbreak investigation. WHO South East Asia J Public Health 3: 46‑50.
- Dutta SE (2017) Dengue: An exaggeration or a nemesis? An hospital based study in the Northern part of West Bengal, India. Int J Appl Microbiol Biotechnol Res 5: 82-87.
- Bandyopadhyay B, Bhattacharyya I, Adhikary S, Konar J, Dawar N, et al. (2013) A comprehensive study on the 2012 Dengue fever outbreak in Kolkata, India. ISRN Virology: 1-5
- Mustafa MS, Rasotgi V, Jain S, Gupta V (2015) Discovery of fifth serotype of dengue virus (DENV): A new public health dilemma in dengue control. Med J Armed Forces India 71: 67‚¬Ëœ70.
- Watts D, Whitmire R, Burke D, Nisalak A, Harrison B (1987) Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg 36: 143-152.
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, et al. (2010) Dengue: A continuing global threat. Nat Rev Microbiol 8: S7-S16.
- Borkakoty B, Das M, Sarma K, Jakharia A, Das PK, et al. (2018) Molecular characterisation and phylogenetic analysis of dengue outbreak in Pasighat, Arunachal Pradesh, Northeast India. Indian J Med Microbiol 36: 37-42.
- Vincenti-Gonzalez MF, Tami A, Lizarazo EF, Grillet ME (2018) ENSO-driven climate variability promotes periodic major outbreaks of dengue in Venezuela. Sci Rep 8: 1-11.
- Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB (2016) Dengue infection. Nat Rev Dis Primers 2: 1-25.






