Keywords
UTI; Antimicrobial resistance; Uropathogens
Introduction
Infections of urinary tract are implicated as one of the severe public health problems that impose high morbidity and mortality as well as severe economic consequences Worldwide [1].It is the most common bacterial infection in clinical practices and accounts one-third of all nosocomial infections [2]. Infections of urinary tract (UTIs) manifest a spectrum of severity that ranging from mild self-limiting infection to life-threatening systemic disease. Globally, 250 million people are affected per year and estimated 300 million people are at risk [2]. The frequency of UTIs depends on many risk factors such as diabetes mellitus, advanced age, urinary tract obstructions, immune-suppression, and neurological disorders [3]. Albeit a wide variety of causative agents were adduced with UTIs, the most common pathogens are E. coli, K. pneumoniae, P. mirabilis, E. faecalis and S. saprophyticus [4]. During the last decades there has been a tremendous increase in the reports pertaining to the antibiotic resistance of UTIs causing bacteria worldwide. It is envisaged that causative agents for UTIs and its susceptibility pattern can diverge according the geographical, social and biological status [5]. Prompt diagnostic and culture facilities are not available in many parts of developing countries. And this may leads to incorrect diagnosis or self-medication whereupon causes the development of antimicrobial resistance among uropathogens [6]. Therefore, the selection of empiric antibiotic therapy for UTIs should be based on the institutional susceptibility profiles of the causative bacteria.
In Ethiopia, several instances of prevalence and drug resistant patterns of UTIs were corroborated [4-8]. Our preliminary study conjectured that, UTI is one of the top most bacterial infections reporting at the Arba Minch hospital, Arba Minch, Ethiopia. Howbeit, the prevalence, etiological profile and antibiotic susceptibility pattern of bacterial uropathogens from the study area is seldom being investigated. In the light of this, the present study was initiated to investigate the prevalence, etiological profile and antibiotic susceptibility pattern of bacterial uropathogens.
Materials and Methods
Details of the study area and participants
A prospective study was conducted in adult patients clinically suspected to have urinary tract infection who consulted at the Internal Medicine Unit of Arba Minch Hospital (AMH) during the period of 8 months (from January 2015 to September 2015). The inclusion criteria for the study participants was all adult patients with the UTI visited at AMH during the above study period. The exclusion criteria for the study participants were 1) Those who were underwent antibiotic treatment during the time of specimen collection or for the last one week, 2) patients who were admitted to Internal Medicine ward for more than 48 hours, 3) who were declined to participate, 4) age less than 15 were excluded from the study.
Prior to the specimen and data collection, the purpose of the study was explained to all respondents and informed consent was obtained from all participants. The study has been approved by the Ethical Review Board of Arba Minch University.
Sampling and isolation of uropathogenic bacteria
The first voided midstream urine samples were collected in sterile wide mouth disposable containers (5 ml) from 129 adult subjects (15-55 years old). Only one sample per patient was sourced. Collected samples were labeled and immediately transported to the laboratory following appropriate safety precautions and SOPs (Standard operating procedures) as described elsewhere [9]. The urine culture and antibiotic sensitivity tests were performed in our Microbiology and Parasitology Laboratory, Department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University. The processing and culture of urine was performed within 1 hr of sampling. Urine samples were inoculated using a calibrated inoculation needle onto various isolation media such as 5% blood agar, MacConkey, and Manitol Salt Agar (MSA) agar (Oxoid Ltd, Bashingstore, Hampire, UK). Subsequently, plates were incubated in both aerobic and anaerobic conditions for 24 to 48 hours at 37°C. Afterwards, plates were inspected for bacterial growth. The samples that exhibiting significant bacterial growth according to Kass count (single species count of >105 organisms/mL) was considered as positive results for UTI [10]. Only samples with significant bacteriuria (≥105 cfu/ml) were taken up for bacteriological analysis.
Identification of uropathogenic bacterial isolates
The pure cultures of respective uropathogens were further subjected to species identification and confirmation. Biochemical, morphological and physiological characteristics of isolated uropathogens was confirmed by adopting standard laboratory methods including Gram staining, colony morphology on different media, growth on selective media, lactose and mannitol fermentation, H2S production, catalase, oxidase, coagulase, indole, and citrate utilization, and urease tests [11]. Corresponding American Type Culture Collection (ATCC) strains were used as reference standard during biochemical identification of isolated uropathogenic bacteria.
Antibiotic sensitivity test
All uropathogenic isolates were subjected to antibiotic sensitivity test. Antibiotic sensitivity profile was determined by the Kirby–Bauer disk diffusion method [12] using oxoid antibiotic discs. Nine commercially available antibiotic disks were used with their respective concentrations: [ciprofloxacin (CIP, 5 μg), tetracycline (TE, 30 μg), chloramphenicol (C, 30 μg), erythromycin (E, 15 μg), penicillin (P, 10 μg), gentamicin (CN, 10 μg), trimethoprim-sulfamethoxazole (TMP-SMX, 1.25+23.75 μg), amoxicillin (AML, 30 μg) and ampicillin (AMP, 10 μg) Oxoid, Hampshire, UK]. In brief, the respective bacterial suspensions of 10-fold dilutions were prepared using 18-h fresh shake culture (Tryptic Soy broth) and was inoculated onto the Muller Hinton agar plates to get a lawn concentration of about 1.5 × 106 CFU/cm2. Six disks were dispensed on the seeded lawn at 60O apart from each other. The diameter of inhibition zone around the disks was recorded after incubation at 37°C for 24 h. The reference standard was used for the inspection of isolate sensitivity/resistance pattern. The results of the antimicrobial susceptibility tests were interpreted by following the National Committee for Clinical Laboratory Standards [13].
Data analysis
The data were analyzed using SPSS for Windows version 20 (Statistical Package for Social Services, Chicago, IL, USA).
Results
Sample size, prevalence and general characteristics of study participants
In the present study, a total of 129 study participants clinically suspected to have UTI consulted at Arba Minch Hospital were selected for participation according to the inclusion criteria during the study period of eight months. The age of the study participants ranged from 15 to 55 years with a mean age of 29.09 (SD=11.4 years). Of the overall samples screened, 37.2% (n=48) of samples were found positive for uropathogenic bacteria and remaining 62.7% (n=81) were negative. The urine samples with colony count ≥105 CFU/mL were considered as positive and those with a colony count <105 CFU/ml were considered as negative. Among the positive study participants (n=48), UTI was more prevalent in females (n=33; 68.75%). From 20 positive samples, in the case of 15-24 years old, 8 samples were from males and 12 samples were from females. In the case 25-34 years old, there was a total of 12 positive samples were collected from 3 males and 9 females. In the case of 35-44 years old, from 7 positive samples 5 were females and 2 were males. In the 45-54 years old, of the 5 positive samples 4 were female and 1 was male. In the >55 years old, of the 4 positive samples, 3 were females. In the present study, no correlation was observed between culture positivity and age of UTI patients. The positive samples showing distinct colonies were further taken up for biochemical characterization and antimicrobial susceptibility screening.
Etiological profile of UTIs
Of the 48 isolates, 33.3% (n=32) represents Gram positive cocci and remaining 66.7% (n=16) represents Gram negative bacilli. The bacterial co- infections (both Gram-negative bacilli and Gram positive cocci) was not found in the present study. Based on the colony morphology, culture and biochemical characteristics, isolates were identified and grouped into seven species including Gram negative bacilli such as E. coli, K. pneumoniae, Proteus mirabilis, Citrobacter sp. and Gram positive cocci such as S. aureus, Coagulase negative Staphylococcus (CNS), and E. faecalis. Among the Gram negative uropathogenic bacilli identified, E. coli was the most predominant species representing 41.6% followed by K. pneumoniae (16.6%). In the case of Gram positive cocci, the most common uropathogen was S. aureus (20.08%). The percentage of different uropathogenic bacterial isolates is depicted in Figure 1.
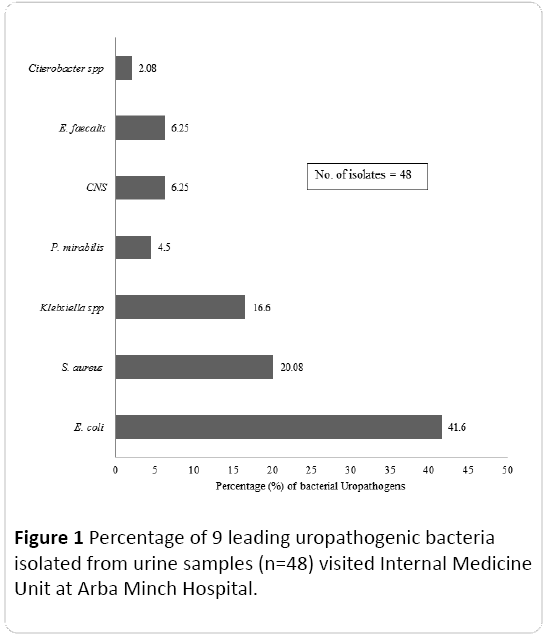
Figure 1: Percentage of 9 leading uropathogenic bacteria isolated from urine samples (n=48) visited Internal Medicine Unit at Arba Minch Hospital.
Antibiogram profile
The antibiotic resistance pattern of all uropathogenic Gram positive cocci and Gram negative bacilli is tabulated in Tables1 and2. In the present study, 81.62% of Gram-negative bacilli were resistant to ampicillin, penicillin and amoxicillin respectively, whereas 68.75% of the bacilli were resistant to erythromycin, indicating low susceptibility to these drugs. Among the most prevalent Gram negative bacilli, 85% of E. coli isolates were resistant against ampicillin, penicillin and amoxicillin.And 70% E. coli isolates exhibited resistant to erythromycin followed by tetracycline (60%). In contrast, low rank of resistance was observed to ciprofloxacin (20%), gentamicin (25%), trimethoprim-sulfamethoxazole (30%) and chloramphenicol (30%).Nearly 62.5% of K. pneumoniae isolates were resistant to erythromycin and trimethoprim-sulfamethoxazole.
| Uropathogenic Gram Negative bacilli |
Number of isolates |
Drug resistant isolates |
| TE |
CIP |
GM |
AML |
E |
TMP-SMX |
AMP |
CAF |
P |
| E. coli |
20 |
12 |
4 |
5 |
17 |
14 |
6 |
17 |
6 |
17 |
| K. pneumoniae |
8 |
0 |
2 |
0 |
4 |
5 |
5 |
4 |
1 |
4 |
| P. mirabilis |
3 |
3 |
2 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
| Citrobacter sp. |
1 |
1 |
0 |
0 |
1 |
0 |
1 |
1 |
1 |
1 |
| |
32 |
16 (50%) |
8 (25%) |
8 (25%) |
25 (78.1%) |
22 (68.7%) |
15 (46.8%) |
25 (78.1%) |
11 (34.5%) |
25 (78.1%) |
TE: Tetracycline, CIP: Ciprofloxacin, GM: Gentamycin, AML: Amoxicillin, E: Erythromycin, TMP-SMX: Trimethoprim-sulfamethoxazole, AMP: Ampicillin, CAF: Chloramphenicol, P: Penicillin
Table 1: Drug resistance pattern of Gram Negative bacilli from urine sample of urinary tract infection patients, Arba Minch Hospital.
| Uropathogenic Gram positive cocci |
Number of isolates |
Drug Resistant Isolates |
| TE |
CIP |
GM |
AML |
E |
TMP-SMX |
AMP |
CAF |
P |
| S. aureus |
10 |
8 |
3 |
6 |
10 |
3 |
10 |
10 |
5 |
10 |
| E. faecalis |
3 |
2 |
1 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
| CNS |
3 |
1 |
1 |
2 |
3 |
1 |
3 |
3 |
1 |
3 |
| |
16 |
11 (68.75%) |
5 (31.25%) |
11(68.75%) |
16 (100%) |
7 (43.75%) |
16 (100%) |
16 (100%) |
9 (56.25%) |
16 (100%) |
TE: Tetracycline, CIP: Ciprofloxacin, GM: Gentamycin, AML: Amoxicillin, E: Erythromycin, TMP-SMX: Trimethoprim-sulfamethoxazole, AMP: Ampicillin, CAF: Chloramphenicol, P: Penicillin
Table 2: Drug resistance pattern of Gram positive cocci from urine sample of urinary tract infection patients, Arba Minch Hospital.
Regarding the Gram positive cocci, 100% of isolates expressed high rank of resistance to amoxicillin, trimethoprim-sulfamethoxazole, ampicillinand penicillin whereas 68.75% of isolates were resistant to tetracycline and gentamicin. Among the resistant Gram positive cocci, 100% of S. aureus displayed resistance to amoxicillin, trimethoprim-sulfamethoxazole, ampicillin and penicillin. Eighty percent of S. aureus exhibited resistant to tetracycline followed by gentamicin (60%) and chloramphenicol (50%).
Discussion
Worldwide, urinary tract infections (UTIs) are recognized as the most prevalent bacterial infections in human and a major public health concern. It is speculated that nationwide or region-based surveillance of uropathogenic bacterial diversity and its resistance patterns may facilitate the medical practitioners to select the exact empirical therapy [14]. Albeit several studies pertained to UTIs are reported from the various regions of Ethiopia, studies with regards to the same from Arba Minch province is scanty. Thence, in the present study, we delineated the prevalence, diversity and antimicrobial resistance of uropathogenic bacteria isolated from the UTI patients of Arba Minch province. From the present study it was found that overall prevalence rate of UTI was 37.2% during the study period of eight months. Our result is consistent with those of two similar kinds of studies done in other regions of Ethiopia [15,16]. In contrast to our findings, the prevalence rate of UTI was different in two previous studies cited from Ethiopia [4,8]. Its envisaged that prevalence of UTI depend on diverse factors such as geographical area, climate, sex, age, social habit, the presence of urodynamic disorders, compliance with treatments for overactive bladder symptoms, and the immunological status [17]. Among the positive study participants, highest rate of prevalence was observed in females. It’s a universally averred that frequency of UTI is especially high among women due to their reproductive physiology [18]. Howbeit, no correlation was observed between culture positivity and age of UTI patients. The prevalence of UTI was more or less uniform in all age groups.
For the effective management and treatment of urinary tract infections, elucidating the diversity of uropathogens as well as drug resistance pattern is pivotal.The percentage and diversity of uropathogenic bacterial isolates observed in the present study is concordance with the previous literatures [4,7]. Of the diverse uropathogens isolated, Gram negative bacilli were the predominant group. It is a known fact that Gram-negative isolates are the most prominent uropathogens compared to Gram-positive isolates. Amongst the Gram positive bacilli, E. coli was the most prevalent isolates in the urine samples. In accordance to our study, similar pattern of prevalence was noted in Ethiopia [15,16,19] and other locales of the World [20,21]. Moreover, globally E. coli is envisaged to cause 70-95% upper and lower UTIs [22]. The second most prevalent isolates amongst the Gram positive bacilli was K. pneumoniae [4]. Among the Gram positive cocci, S. aureus was the most frequently isolated uropathogens. Moges et al. [16] opined high prevalence of S. aureus in the urine samples collected from other region of Ethiopia. The present study has few limitations such as small sample size and mixed population. Therefore, the results are generalizable.
The development of resistances among uropathogens to multiple drugs is a major crisis which restricts drug of choice for the treatment of UTI.In the present study, the pattern of antibiotic resistance of uropathogenic isolates against commonly prescribed empirically antibiotics was explored. Of the Gram negative bacilli, E. coli isolates exhibited high rank of resistance against ampicillin, and tetracycline, annulling its empirical usage.The similar trend of resistant was observed in other studies conducted at Ethiopia and India [15,21]. Howbeit, rate of resistance was lower in another study done at Ethiopia [8]. The high rank of resistance could be due to the overuse/self-prescription of these antibiotics in our study area.Apart from E. coli, other Gram negative bacilli such as K. pneumoniae and P. mirabilis were also exhibited resistance against trimethoprim-sulfamethoxazole, ampicillin and chloramphenicol which well-nigh resembled with other reports [8,15]. In general, Gram negative bacilli were susceptible to the ciprofloxacin and gentamicin. Based on this result, it could be inferred that antibiotics such as ciprofloxacin and gentamicin might be useful for the management of Gram negative uropathogens in the study area. In the case of Gram positive cocci, it was evidenced that S. aureus were more resistance than other two species. It displayed a high rank of resistance against tetracycline, ampicillin, amoxicillin and penicillin which is comparable to another study conducted at Ethiopia [15]. Gram positive cocci showed sensitivity to ciprofloxacin. Thence, overall results of present study could be useful to improve therapeutic tactics of uropathogens in the study area.
Conclusion
This study may be the first on the prevalence, diversity and antimicrobial susceptibility of uropathogenic bacterial isolates sourced from the UTI patients of Arba Minch Province, Ethiopia. Findings of the present study revealed that, overall prevalence rate of UTI was 37.2%. Of the diverse uropathogens isolated, Gram negative bacilli were the predominant group followed by Gram positive cocci. In vitro antimicrobial susceptibility was elucidated only against nine antibiotics. In antimicrobial susceptibility test, majority of the uropathogens demonstrated high rank of resistance to ampicillin, penicillin amoxicillin and erythromycin.From the results of the antimicrobial susceptibility test it’s envisaged that ciprofloxacin is found to be effective against Gram negative and Gram positive uropathogens respectively. Detailed study is being underway on the aspects of mechanism of antibiosis, biofilm forming potential and molecular characterization of uropathogens.
13613
References
- Smith AC, Almond M (2007) Management of urinary tract infections in the elderly. Trends UrolGynaecol Sex Health 12: 31-34.
- Lee SW, Kim YH (2015) Uropathogens based on Antibiotic Susceptibility. Urogenit Tract Infect 10:67-73.
- Redder JD, Leth RA, Moller JK (2016) Analysing risk factors for urinary tract infection based on automated monitoring of hospital-acquired infection. J Hosp Infect 92:397-400.
- Kibret M,Abera B (2014) Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. Asian Pac J Trop Biomed 4: 164-168.
- Rowe TA, Juthani-Mehta M (2013) Urinary tract infection in older adults. Aging Health 9.
- Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, et al. (2012) High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes 5:38.
- Demile, T, Beyene G, Melaku S, Tsegaye W (2012) Urinary bacterial profile and antibiotic susceptibilty pattern among pregnant women in North West Ethiopia. Ethiopian J Health Sci 22: 121-128.
- Beyene G, Tsegaye W (2011) Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in Jimma University specialized hospital, southwest Ethiopia. Ethiop J Health Sci 21:141-146.
- Cheesbrough M (2004) Medical Laboratory Manual for Tropical Countries, II Microbiology. (ELBS), Butterworth: Kent, UK. 23-78.
- Kass EH (1955) Chemotherapeutic and antibiotic drugs in the management of infections of the urinary tract. Amer J Med 18: 764-781.
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology (9thedn). Williams and Wikins co, Baltimore, USA.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493-496.
- National Committee for Clinical Laboratory Standards (NCCLS). (2010) Performance Standards for antimicrobial susceptibility testing. 20th Informational Supplement. M100-S20 (ISBN 1-56238- 716 -2). Clinical and Laboratory Standards Institute. Pennsylvania. USA.
- Lee SJ, Lee DS, Choe HS, Shim BS, Kim CS, et al. (2011) Antimicrobial resistance in community-acquired urinary tract infections: results from the Korean Antimicrobial Resistance Monitoring System. J Infect Chemother 17:440-446.
- Biadglegne F, Abera B (2009) Antimicrobial resistance of bacterial isolates from urinary tract infections at FelgeHiwot Referral Hospital, Ethiopia. Ethiop J Health Dev 23:236-238.
- Moges AF, Genetu A, Mengistu G (2002) Antibiotic sensitivities of common bacterial pathogens in urinary tract infections at Gondar Hospital, Ethiopia. East Afr Med J 79: 140-142.
- Kirill VK, Loparev SA, Ivanovskaya MA, Kosilova LV (2015) The efficacy of different doses of solifenacin in elderly patients after treating a urinary tract infection. Arab J Urol 13: 203-208.
- Mody L, JuthaniMM (2014) Urinary Tract Infections in Older Women A Clinical Review. JAMA 311: 844-854.
- Abejew AA,Denboba AA,Mekonnen AG (2014) Prevalence and antibiotic resistance pattern of urinary tract bacterial infections in Dessie area, North-East Ethiopia. BMC Res Notes 7: 687.
- Kurtaran B, Candevir A, Tasova Y, Kibar F, Inal AS, et al. (2010) Antibiotic resistance in community-acquired urinary tract infections: prevalence and risk factors. Med Sci Monit 16: 46-51.
- Nerurkar A, Solanky P, Naik SS (2012) Bacterial pathogens in urinary tract infection and antibiotic susceptibility pattern. J Pharm Biomed Sci 21: 1-3.
- Behzadi P, Behzadi E, Yazdandod H,Aghapour R, Akbaricheshmeh M, et al. (2010) A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar) 5: 111-115.






