Keywords
Methotrexate, Cactus cladodes, Oxidative stress, Malondialdehyde level (MDA), Proteins carbonyls, Catalase activity, Peroxidase
Introduction
Methotrexate, a folic acid antagonist, is a cytotoxic chemotherapeutic agent used for leukemia and other malignancies as well as in the treatment of various inflammatory diseases such as psoriasis [1], dermatomyositis and rheumatoid arthritis [2,3]. However its effectiveness is often limited by severe side effects and toxic conditions, such as nausea, vomiting, diarrhea, statutes, gastrointestinal ulcerations and mucositis [4]. Since the cytotoxic effect of MTX is not selected for cancer cells, it affects normal tissues having a high proliferation rate, including gastrointestinal, renal [5] and hepatic toxicity [6]. Its antimitotic effect is known for giving rise to malabsorption syndrome [7], wherein the decrease in the absorption surface area occurs due to histological changes such as shortened microvillus, resulting in a lesser absorption of nutrients [8]. MTX also decreases the brush border membrane protein and lipid contents of the small intestine [9]. The chemical and morphological changes in the small intestine may possibly be triggered by crypt cells damage [10]. In fact, MTX inhibits dihydrofolate reductase, that causes the depletion of the dihydrofolate pool and affects the synthesis of thymidilate, suppressing DNA synthesis [11]. Besides, it inhibits the cytosolic NAD(P)-dependant dehydrogenises [12] and the NADP malic enzyme, thus decreasing NADPH availability in cells [13]. MTX-induced toxicity appears to result from the interaction of many factors: length of treatment, type of disease and the presence of apoptotic factors [14]. Thus, It is therefore important for patients undergoing cancer chemotherapy to reduce MTX side effects by minimizing the mucosal damage. The beneficial effects of various antioxidants, such as vitamin A [15], caffeic acid phenethyl ester [16] taurine [17], aged garlic extract [18] and β-glucan [19] were tested.
Recently, special interest has been focused on the cactus prickly pear belonging to Opuntia species which is viewed as a relevant health promoting food. Cactus (Opuntia spp.), a member of the Cactaceae family, is an important forage crop for livestock in many arid and semi-arid regions of the world. It is widely distributed in Mexico and in all American hemispheres as well as in Africa and in the Mediterranean basin [20]. Recently, special interest has been focused on the cactus prickly pear belonging to Opuntia species. However, Opuntia ficus-indica (L.) Mill. is the cactus species with the highest economic importance worldwide. Indeed prickly pear fruits are recommended for their beneficial and therapeutic properties [20]. In México there are more than 10 500 ha for the production of young cladodes (nopalitos) consumed as vegetables. Data in the literature data report that cladodes and fruits of this plant are also used in folk medicine as an emollient, moisturizing, hypocholersteromic and hypoglycemic agent and in gastric mucosa diseases [21]. Indeed, according to several studies, both cactus fruit and cladode are rich in important nutrients such as minerals, vitamins as well as further antioxidants [22-24]. Furthermore, several studies have reported its efficiency in the treatment of several diseases: cactus extract exhibit anti-tumoral [25], anti-viral [26], anti-inflammatory [27,28], and anti-oxidant effects [19,29], in addition to anti-hyperlipidemic properties [30] and an analgesic action [31]. According to several studies, cactus pear (Opuntia ssp.) yields high values of important nutrients and exhibits antioxidant functions, however hardly considered, most probably due to the scattered information available. These data have made cactus pear fruits and cladodes perfect candidates for cytoprotective investigations.
As previously mentioned, arising attention is giving to the study of natural products which may counteract the detrimental effects of multiple food contaminants and prevent multiple human diseases. In this line, in our laboratory, we have been interested to the study of the protective potential afforded by natural compounds.
The objective of the present study is to find out whether oxidative damage could be relevant for MTX-induced toxicity in vivo using Wistar rats, and to investigate the preventive potential of cactus cladodes. For this purpose, the effect of MTX, on the induction of oxidative stresses whether alone or jointly with cactus ethanol fraction of cactus cladode, was investigated at several levels in the small intestine. Thus, we have monitored malondialdehyde (MDA) level, protein carbonyls generation and Peroxidase and Catalase activities.
Materials and Methods
Chemicals
Methotrexate, Triobarbituric acid (TBA); Trichloroacetic acid (TCA) and Butul hydoxyl toluene (BHT) were obtained from Sigma Chemical Co. (St. Louis, Mo), 2,4-dinitro-phenylhydrazine (2,4- DNPH) and guanidine were purchased from Prolabo (France).
Preparation of the extracts of cactus cladodes
Young cactus cladodes of Opuntia ficus-indica (2-3 weeks of age) collected from the local area were washed with water, chopped into small pieces and dried. Air-dried cladodes were ground to fine powder and successively extracted with solvents of increasing polarity (hexane, dichloromethane, ethyl acetate, ethanol and then methanol). Thus, 20 g of cladodes powder were placed in hexane (200 ml) for 18 h under frequent agitation at ambient pressure and temperature. The mixture was filtered using Whatman paper (GF/A, 110 mm) and the experience was repeated twice. The solvent was evaporated using a rotary evaporator under vacuum at 35 °C. Then, the firstly extracted powder was extracted again with dichloromethane under the same conditions as with hexane. The same procedure was applied for the following solvents: ethyl acetate, ethanol and methanol.
Animals treatment
Adult and healthy male albinos rats (200–250 g) provided from an animal breeding centre (SEXAL St. Doulchard, France following the agreement of the Ethics Committee named National committee of Medical Ethics CNEM, BP 74 - Pasteur Institute, Tunis 1002 TUNISIA) were used. The animals were kept one week for acclimatization under constant conditions of temperature and a 12 hours light/dark cycle. Animals had free access to standard granulated chow and drinking water. Following a single dose of methotrexate (20 mg/kg) given intraperitoneally, either vehicle (saline) or ethanol fraction of cactus cladode (400 mg/kg) was administered intraperitoneally. Treatment was continued daily for 10 consecutive days. In control rats, following a single dose of saline injection, either saline or ethanol fraction of cactus cladode was administered for 10 days. Each group consisted of 6 rats. After treatment, animals were sacrificed by decapitation. Blood and small intestine were dissected out.
Preparation of small intestine extract
The small intestine was homogenized with a Potter (glass-Teflon) in the presence of 10 mM Tris-HCl, pH 7.4 at 4°C and centrifuged at 4000 rpm for 30 min at 4°C. The supernatant was collected for analysis and the protein concentration was determined in the intestinal extract using Protein BioRad assay [32].
Evaluation of lipid peroxidation status
Lipid peroxidation was determined indirectly by measuring the production of MDA in the intestinal extracts following the method of Buege and Aust [33]. Briefly, 200 μl of intestinal extracts were mixed with 150μl of TBS (Tris 50 mM and NaCl 150 mM, pH 7.4) and 250 μl TCA-BHT (20% TCA and BHT 1%). The mixture was vigorously vortexed and centrifuged at 1500 rpm for 10 min. A volume of 400 μl of the supernatant was added to HCl 0.6 N and 320 μl Tris-TBA (Tris 26 mM and TBA 120 mM); the content was mixed and incubated for 10min at 80 °C. Absorbance was measured at 535 nm. The optic density corresponding to the complex formed with the TBA-MDA was proportional to MDA concentration and lipid peroxide. The concentration of μmol of MDA/mg of proteins is calculated from the absorbance at 530 nm using the MDA molar extinction coefficient 1.56 .105 M-1cm-1.
Protein Carbonyl Assay
Protein carbonyls content was determined as described by Mercier et al. [34] in intestinal homogenates by measuring the carbonyl groups reactivity with 2,4-dinitrophenylhydrazine (2,4- DNPH). Thus, 200 μl of small intestine supernatant were placed in two glass tubes. A volume 800 μl of 10 mM DNPH in 2.5 M HCl was added. Tubes were left in the dark for 1 h incubation at room temperature. Samples were vortexed every 15 min. Then 1 ml of 20% TCA was added to samples, and the tubes were left in an ice bucker for 10 min and centrifuged for 5 min in a tabletop centrifuge to collect the protein precipitates, and the supernatants were discarded. Another wash was then performed using 1 ml of 10% TCA, and protein pellets were broken mechanically with the aid of a glass rod. Finally, the pellets were washed with 1ml of ethanolethyl acetate (v/v) to remove the free DNPH. The final precipitates were dissolved in 500 μl of guanidine hydrochloride 6 M and left for 10 min at 37 °C with general vortex mixing. Any insoluble materials were removed by additional centrifugation. Protein carbonyls concentration was determined from absorbance at 370 nm, applying the molar extinction coefficient of 22.0 mM-1cm-1. A range of nmoles of carbonyls per ml is usually obtained for most proteins and is related to the protein content in the pellets.
Catalase activity determination
Catalase activity was measured in the intestinal extract at 240 nm, 25 °C according to Clairbone [35]. Briefly, 20 μl of the extract was added in the quartz cuve containing 780 μl phosphate buffer and 200 μl of H2O2 0.5 M. Catalase activity was calculated using the molar extinction coefficient (0.04 mM-1cm-1). The results were expressed as μmol of H2O2/min/mg of proteins.
Peroxidase activity determination
Peroxidase activity was measured at 25 °C using guaiacol as hydrogen donor [36]. The reaction mixture contained 9 Mm guaiacol, 19 nM H2O2 in 50 mM phosphate buffer pH 7 and 50 μl of enzyme extract in 1 ml final volume. The reaction was initiated by the addition of H2O2 and monitored by measuring the increase in absorbance at 470 nm. Peroxidase activity was expressed in nmol of guaiacol oxidized per min with a molecular extinction coefficient of 26.2 mM-1 for calculation.
Histopathological analysis
The animals were killed by decapitation after urethane anaesthesia. Three tissue samples of the small intestine were cut off at a distance of 5 cm from the proximal end of the small intestine of each animal, fixed with 10% neutral formalin, embedded in paraffin and cut with a microtome set at a thickness of 5-6 μm. The tissue sections were stained with haematoxylin and eosin for histopathological analysis and examined with a light microscope.
Statistical analysis
Each experiment was conducted in triplicate separately. Values were presented as means ± Standard Deviation. Differences were considered significant at P<0.05.
Results
Lipid peroxidation induction
Results of MTX effect alone and jointly with ethanol fraction of cactus cladode on lipid peroxidation induction in intestinal extract as determined by MDA level are shown in Figure 1. A volume of 20 mg/kg b.w of MTX induced a significant increase in MDA formation as compared to control groups. Interestingly, when animals were treated with cladodes extract (400 mg/kg b.w.), 10 days prior to MTX treatment, a sharp decrease in MDA level was noticed in tissues. MDA level was found to be similar to that of control groups (ethanol fraction of cactus cladode and saline group).
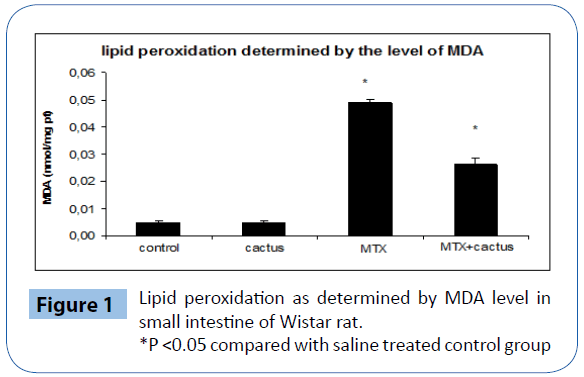
Figure 1: Lipid peroxidation as determined by MDA level in small intestine of Wistar rat.
*P <0.05 compared with saline treated control group
Protein carbonyl assay
Protein carbonyls formation, indicating of severe protein oxidation, was assayed in the small intestine tissue homogenate and results are illustrated in Figure 2. MTX induced protein carbonyls formation in the small intestine compared to control groups. Ethanol fraction of cactus cladode remarkably decreased protein carbonyls formation induced by a fixed dose of MTX (20 mg/Kg b.w.).
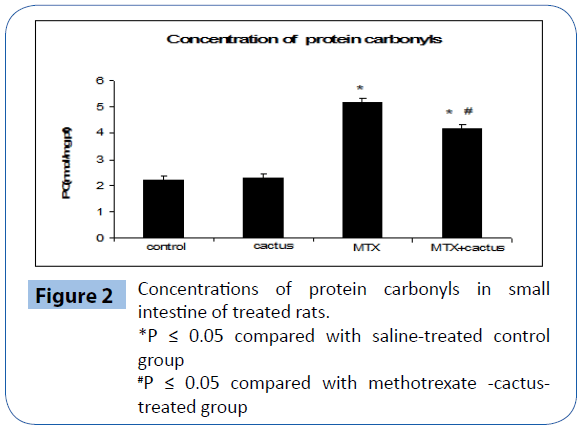
Figure 2: Concentrations of protein carbonyls in small intestine of treated rats.
*P ≤ 0.05 compared with saline-treated control group
#P ≤ 0.05 compared with methotrexate -cactustreated group
Catalase activity
Figure 3 illustrates the effect of MTX and cladodes extract on Catalase activity. MTX induced a marked increase in Catalase activity in the intestinal extract. When animals were given cactus ethanol fraction of cactus cladode, 8 days prior to MTX treatment a striking decrease of this activity in tissue was noticed.
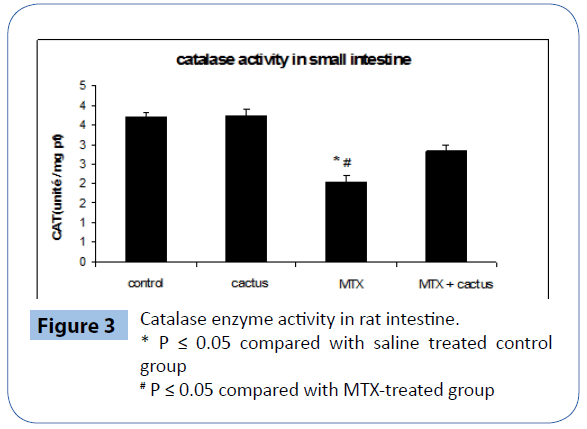
Figure 3: Catalase enzyme activity in rat intestine.
* P ≤ 0.05 compared with saline treated control group
# P ≤ 0.05 compared with MTX-treated group
Peroxidase activity determination
MTX treatment significantly decreased GPX level in the intestinal tissue. However, the supplementation of ethanol fraction of cactus cladode to MTX-treated rats significantly decreased this antioxidant level in the tissue (Figure 4).
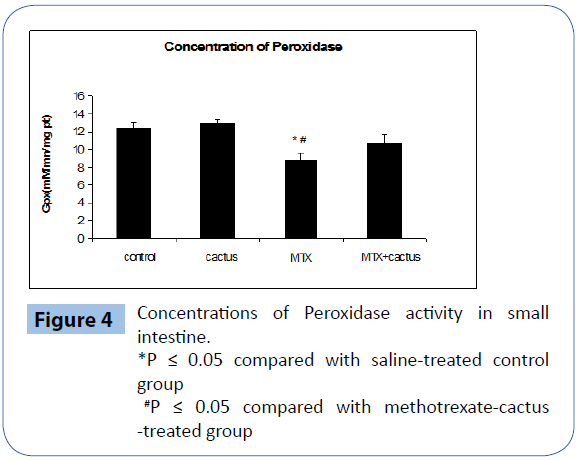
Figure 4: Concentrations of Peroxidase activity in small intestine.
*P ≤ 0.05 compared with saline-treated control group
#P ≤ 0.05 compared with methotrexate-cactus -treated group
Weight loss
The weight of the animals in groups was compared; there was no significant change in the cactus-treated and control groups. When the body weight in the other group was compared, it was lower in the MTX-treated group than the cactus group (P<0.05) (Figure 5).
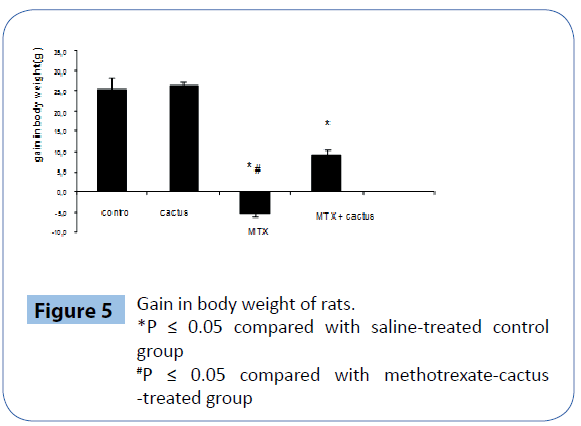
Figure 5: Gain in body weight of rats.
*P ≤ 0.05 compared with saline-treated control group
#P ≤ 0.05 compared with methotrexate-cactus -treated group
Histological studies
MTX treatment induced serious damage to the intestine. The results revealed that MTX-induced small intestinal injury was characterised by villous shortening, variable fusion rates and epithelial atrophy. Clear improvement in the intestine was noticed when the rats were treated with MTX and the ethanol fraction of cactus cladode. The histopathological findings of our study revealed that ethanol fraction ameliorated mucosal destruction and preserved of the intestinal epithelium morphology (Figure 6A, B ,C and D).
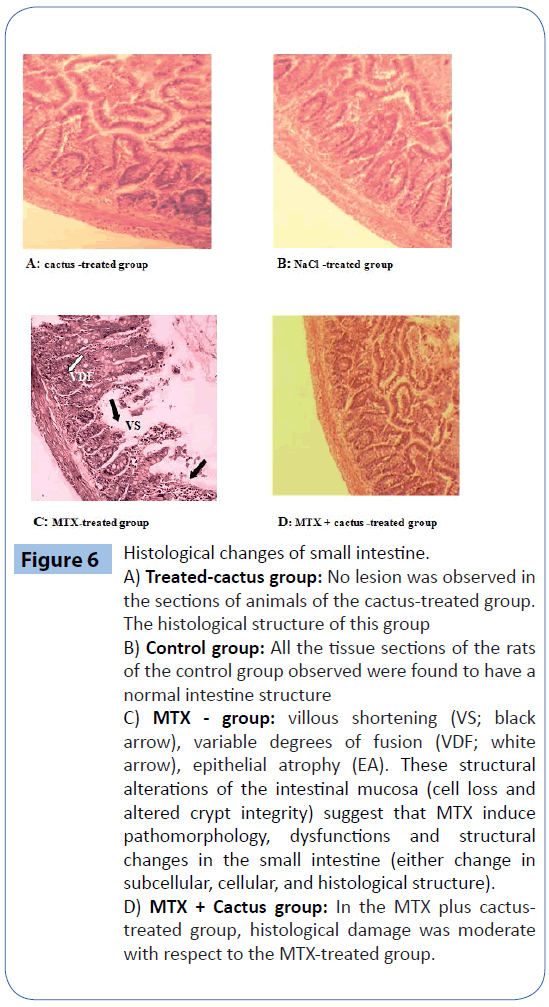
Figure 6: Histological changes of small intestine.
A) Treated-cactus group: No lesion was observed in the sections of animals of the cactus-treated group. The histological structure of this group
B) Control group: All the tissue sections of the rats of the control group observed were found to have a normal intestine structure
C) MTX - group: villous shortening (VS; black arrow), variable degrees of fusion (VDF; white arrow), epithelial atrophy (EA). These structural alterations of the intestinal mucosa (cell loss and altered crypt integrity) suggest that MTX induce pathomorphology, dysfunctions and structural changes in the small intestine (either change in subcellular, cellular, and histological structure).
D) MTX + Cactus group: In the MTX plus cactustreated group, histological damage was moderate with respect to the MTX-treated group.
Discussion
Methotrexate, a widely used drug in antimetabolite cancer therapy or in various forms of arthritis, may have serious unpredictable side effects causing significant clinical problems. Cyclical high doses of methotrexate, used for leukemia [37] or severe psoriasis have been associated with organ toxicity including hepatic fibrosis, cirrhosis and renal failure [38].
Recent advances in medicine have demonstrated that oxygen radicals and hydrogen Peroxidase have been associated with MTX side effects [14]. Free radicals trigger cell damage through binding to cellular macromolecules, particularly membrane lipids and polyunsaturated fatty acids in the endoplasmic reticulum [39]. So, lipid peroxidation may be an important cause of destruction and damage to cell membranes and can contribute to the development of MTX-mediated tissue damage. To further assess MTX induced oxidative damage to Wistar rats, protein carbonyls generation was monitored. Protein carbonyls formation is currently the most widely used marker of severe oxidative stress and a number of assays have been recently developed [40]. Our results clearly showed that MTX induced a marked increase in protein carbonyls generation in small intestine extracts which was totally canceled with ethanol fraction of cactus cladode in both organs (Figure 3). Catalase and Peroxidase activities were also studied in this paper. In our study, we have shown that MTX treatment significantly reduced antioxidant enzyme capacity. Decreased catalse activities were probably due to the decrease in their synthesis [41]. Our results agree with the study of Vardi et al. [42] who reported that MTX caused a significant decrease in antioxidant enzyme parameters (CAT) in the rat’s small intestine.
Gastrointestinal toxicity is one of the most serious side effects of MTX treatment. It has been known that Methotrexate causes severe damage to the intestinal mucosa [43,44] . It increases the incidence of apoptosis in the area of rapidly dividing cells, including gastrointestinal tract mucosa [45]. The proliferation of small intestinal epithelial cells occurs in the crypts. The crypt cells, which rapidly regenerate, migrate to the villus tip, and replacement of intestinal epithelium is complete in about 2 days in rats [45], and about 3 days in humans [46].
The results of the present study have revealed that MTX-induced small intestinal injury is characterised by villous shortening, variable fusion degrees and epithelial atrophy (Figure 6C). Our results are similar to other studies in which MTX was reported to cause severe damage in the small intestine [10,47]. The histopathological findings of our study showed that ethanol fraction of cactus cladode ameliorated the mucosal destruction and preserved the intestinal epithelium morphology (Figure 6D). The results of this experimental study showed that cactus administration protected against the oxidative and morphological intestinal damage caused by MTX-treatment. The mechanism of this protection was probably related to the decrease in oxidative stress. Oxidative damage is the most potent threat facing any living organism. The intracellular accumulation of reactive oxygen species can arise from toxic insults and can perturb the cells natural antioxidant defence system resulting in damage to all major classes of macromolecules.
Over the last decades, oxidative stress has been shown to be a major component of several biological and pathological processes like aging, inflammation, carcinogenesis, wound healing activity [28] and several other diseases including Parkinson’s, Huntington’s etc. [47,48]. Our study shows that ethanol fraction of cactus cladode has a protective effect against MTX-induced oxidative damage. This protection was evidenced in all tested oxidative damage endpoints. And it is certainly associated with the presence of several antioxidants such as ascorbic acid, vitamin E, carotenoids, reduced glutathione, flavonoids and phenolic acids actually detected in the fruits and vegetables of various cactus [31,49-51]. In addition, more recently, significant antioxidant properties of the most frequent cactus betalains have been revealed and numerous in vitro studies have demonstrated their ability to neutralize reactive oxygen species [24,52,53]. Lee et al. [54] investigated the anti-oxidant activity of cactus cladodes and concluded that this anti-oxidant property was due to several compounds, particularly flavonoids (quercetin, myricetin), and vitamins. Our results are in accordance with other published studies, which underlined the relevant preventive potential of cactus cladode extracts [55-57].
Acknowledgements
This research was funded by the Tunisian Ministry of Scientific Research and Technology through the Research Unit of Macromolecular Biochemistry and Genetics (BMG), Faculty of Sciences of Gafsa. (cmcu 10G0807) and through the Research unit of “Valorisation des Biomolecules Actives” VBA Higher Inst. of Applied Biology Medenine-University of Gabes Tunisia.
7715
References
- Wollina U, Ständer K, Barta U (2001) Toxicity of methotrexate treatment in psoriasis and psoriatic arthritis--short- and long-term toxicity in 104 patients. Clin Rheumatol 20: 406-410.
- Doolittle GC, Simpson KM, LindsleyHB (1989) Methotrexate-associated, early-onset pancytopenia in rheumatoid arthritis. Arch Intern Med 149: 1430-1431.
- Kremer JM, AlarcónGS, Lightfoot RW, WillkensRF, Furst DE, et al. (1994) Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum 37: 316-328.
- Naruhashi K, Nadai M, Nakao M, Suzuki N, Nabeshima T, et al. (2000) Changes in absorptive function of rat intestine injured by methotrexate. Clin Exp Pharmacol Physiol 27: 980-986.
- Ki ntzel PE (2001) Anticancer drug-induced kidney disorders. Drug Saf 24: 19-38.
- HershEM, Wong VG, Henderson ES, FreireichEJ (1966) Hepatotoxic effects of methotrexate. Cancer 19: 600-606.
- Loehry CA, Creamer B (1969) Three-demensional structure of the rat small intestinal mucosa related to mucosal dynamics. I. Mucosal structure and dynamics in the rat after the administration of methotrexate. Gut 10: 112-116.
- VenhoVM (1976) Effect of methotrexate on drug absorption from the rat small intestine in situ and in vitro. Acta Pharmacol Toxicol (Copenh) 38: 450-464.
- Takeuchi H, Kosakai Y, Tsurui K, Horie T, Awasu S (1989) Change in small intestinal brush border membranes of rats followings methotrexate administration. Pharmacol Toxicol 65: 269-273.
- Tsurui K, Kosakai Y, Horie T, Awazu S (1990) Vitamin A protects the small intestine from methotrexate-induced damage in rats. J Pharmacol ExpTher 253: 1278-1284.
- Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA (1983) The pharmacology and clinical use of methotrexate. N Engl J Med 309: 1094-1104.
- VOGEL WH, SNYDER R, SCHULMAN MP (1963) The inhibition of dehydrogenases by folic acid and several of its analogs. BiochemBiophys Res Commun 10: 97-101.
- Caetano NN, Campello AP, CarnieriEG, Kluppel ML, Oliveira MB (1997) Effect of methotrexate (MTX) on NAD(P)+ dehydrogenases of HeLa cells: malic enzyme, 2-oxoglutarate and isocitrate dehydrogenases. Cell Biochem Funct 15: 259-264.
- Neuman MG, Cameron RG, Haber JA, Katz GG, Malkiewicz IM, et al. (1999) Inducers of cytochrome P4502E enhance methotrexate-induced hepatocytoxicity. Clin Biochem 32: 519-536.
- Yuncu M, Eralp A, Koruk M, Sari I, BaÄŸci C, et al. (2004) Effect of vitamin A against methotrexate-induced damage to the small intestine in rats. Med PrincPract 13: 346-352.
- Uz E, Oktem F, YilmazHR, Uzar E, Ozgüner F (2005) The activities of purine-catabolizing enzymes and the level of nitric oxide in rat kidneys subjected to methotrexate: protective effect of caffeic acid phenethyl ester. Mol Cell Biochem 277: 165-170.
- Cetiner M, Sener G, Sehirli AO, EkÅŸioÄŸlu-Demiralp E, Ercan F, et al. (2005) Taurine protects against methotrexate-induced toxicity and inhibits leukocyte death. Toxicol Appl Pharmacol 209: 39-50.
- Yüncü M, Eralp A, Celik A (2006) Effect of aged garlic extract against methotrexate-induced damage to the small intestine in rats. Phytother Res 20: 504-510.
- Gentile C, Tesoriere L, Allegra M, Livrea MA, D'Alessio P (2004) Antioxidant betalains from cactus pear (Opuntiaficus-indica) inhibit endothelial ICAM- expression. Ann N Y AcadSci 1028: 481-486.
- Barbera G, Inglese P (1993) La colturadelfecodindia.edagricole-edizioniagricoledallacalderinis.r.l. Bologna.
- Hegwood DA (1990) Human health discoveries with Opuntia sp. (prickly pear). HorticultSci 25: 1515-1516.
- Ramadan MF, Morsel JT (2003) Recovered lipids from prickly pear [Opuntiaficus-indica (L.) Mill] peel: A good source of polyunsaturated fatty acids, natural antioxidant vitamins and sterols. Food Chem83 :447-456.
- Stintzing FC, Carle R (2005) Cactus stems (Opuntia spp.): a review on their chemistry, technology, and uses. MolNutr Food Res 49: 175-194.
- Tesoriere L, Fazzari M, Allegra M, Livrea MA (2005) Biothiols, taurine, and lipid-soluble antioxidants in the edible pulp of Sicilian cactus pear (Opuntiaficus-indica) fruits and changes of bioactive juice components upon industrial processing. J Agric Food Chem 53: 7851-7855.
- ZouDM, Brewer M, Garcia F, FeugangJM, Wang J, et al. (2005) Cactus pear: a natural product in cancer chemoprevention. Nutr J 4: 25.
- Ahmad A, Davies J, Randall S, Skinner GR (1996) Antiviral properties of extract of Opuntiastreptacantha. Antiviral Res 30: 75-85.
- LoroJF, del Rio I, Pérez-Santana L (1999) Preliminary studies of analgesic and anti-inflammatory properties of Opuntiadillenii aqueous extract. J Ethnopharmacol 67: 213-218.
- Park EH, KahngJH, Lee SH, Shin KH (2001) An anti-inflammatory principle from cactus. Fitoterapia 72: 288-290.
- Tesoriere L, Butera D, Pintaudi AM, Allegra M, Livrea MA (2004) Supplementation with cactus pear (Opuntiaficus-indica) fruit decreases oxidative stress in healthy humans: a comparative study with vitamin C. Am J ClinNutr 80: 391-395.
- Wolfram R, Budinsky A, Efthimiou Y, Stomatopoulos J, Oguogho A, et al. (2003) Daily prickly pear consumption improves platelet function. Prostaglandins Leukot Essent Fatty Acids 69: 61-66.
- Park EH, KahngJH, PaekEA (1998) Studies on the pharmacological action of cactus: identification of its anti-inflammatory effect. Arch Pharm Res 21: 30-34.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52: 302-310.
- Mercier Y, Gatellier P, Renerre M (2004) Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci 66: 467-473.
- Clairbone A (1985) Catalase activity. Handbook of methods for oxygen radical research. CRC. Press Boca. Raton. Florida.
- Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods enzymol 2: 764- 817.
- Hall PD, Jenner MA, Ahern MJ (1991) Hepatotoxicity in a rat model caused by orally administered methotrexate. Hepatology 14: 906-910.
- RoenigkHHJr, BergfeldWF, St Jacques R, Owens FJ, Hawk WA (1971) Hepatotoxicity of methotrexate in the treatment of psoriasis. Arch Dermatol 103: 250-261.
- Naik SR, Panda VS (2007) Antioxidant and hepatoprotectiveeffects of Ginkgo bilobaphytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int 27: 393-399.
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329: 23-38.
- AteÅŸÅŸahin A, CeribaÅŸi AO, Yilmaz S (2007) Lycopene, a carotenoid, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rats. Basic Clin Pharmacol Toxicol 100: 372-376.
- Vardi N, Parlakpinar H, Ozturk F, Ates B, Gul M, et al. (2008) Potent protective effect of apricot and beta-carotene on methotrexate-induced intestinal oxidative damage in rats. Food ChemToxicol 46: 3015-3022.
- Robinson JW, Antonioli JA, Vannotti A (1966) The effect of oral methotrexate on the rat intestine. BiochemPharmacol 15: 1479-1489.
- Jolly LE Jr, Fletcher HP (1977) The effect of repeated oral dosing of methotrexate on its intestinal absorption in the rat. Toxicol Appl Pharmacol 39: 23-32.
- Barry MA, Behnke CA, Eastman A (1990) Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol 40: 2353-2362.
- Lipkin M, Sherlock P, Bell B (1963) Cell proliferation kinetics in the gastrointestinal tract of man. Ii. Cell renewal in stomach, ileum, colon, and rectum. Gastroenterology 45: 721-729.
- Kosakai Y, Horie T, Awazu S (1991) Protective effect of vitamin A against the methotrexate-induced damage to small intestine: a study on the crypt cells. Pharmacol Toxicol 69: 291-295.
- Calabrese V, Renis M, Calderone A, Russo A, Reale S, et al. (1998) Stress proteins and SH-groups in oxidant-induced cellular injury after chronic ethanol administration in rat. Free Radic Biol Med 24: 1159-1167.
- Kuti JO (2004) Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem 85: 527-533.
- Panico AM, Cardile V, Garufi F, Puglia C, Bonina F, et al. (2005) Protective effect of Capparisspinosa on chondrocytes. Life Sci 77: 2479-2488.
- Shin HC, Hwang HJ, Kang KJ, Lee BH (2006) Anantioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch Pharm Res 29: 165-171.
- Stintzing FC, Herbach KM, MosshammerMR, Carle R, Yi W, et al. (2005) Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J Agric Food Chem 53: 442-451.
- Siriwardhana N, JeonYJ (2004) Antioxidative effect of cactus pear fruit (Opuntiaficus-indica) extract on lipid peroxidation inhibition in oils and emulsion model systems. Eur Food Res Technol 219: 369-376.
- Lee JC, Kim HR, Kim J, Jang YS (2002) Antioxidant property of an ethanol extract of the stem of Opuntiaficus-indica var. saboten. J Agric Food Chem 50: 6490-6496.
- Ncibi S, Ben Othman M, Akacha A, Krifi MN, Zourgui L (2008) Opuntiaficusindica extract protects against chlorpyrifos-induced damage on mice liver. Food ChemToxicol 46: 797-802.
- Zourgui L, Golli EE, Bouaziz C, Bacha H, Hassen W (2008) Cactus (Opuntiaficus-indica) cladodes prevent oxidative damage induced by the mycotoxinzearalenone in Balb/C mice. Food Chem Toxicol 46: 1817-1824.
- Brahmi D, Bouaziz C, Ayed Y, Ben Mansour H, Zourgui L, et al. (2011) Chemopreventive effect of cactus Opuntiaficusindica on oxidative stress and genotoxicity of aflatoxin B1. NutrMetab (Lond) 8: 73.












