Keywords
Primary ileal adenocarcinoma; Anemia; Small bowel malignancy; Contrast enhanced CT; Rare disease
Introduction
Most of the gastrointestinal (GI) malignancies occur in stomach and colon. Although the small bowel makes up 75% of length and 90% of absorption area of digestive tract, malignancy in small intestine is very rare, accounting for only 0.6% of total cancer cases and 3.3% of digestive system cancers in the United States [1]. There are four histological types of small bowel cancer. Adenocarcinoma and carcinoid tumors are the most common types, each accounting for proximately 40% of small bowel malignancies. Lymphoma and stromal sarcoma make up the remaining 20% [2].
Small bowel adenocarcinoma is usually diagnosed at advanced stage [3]. The most common symptoms are weight loss, abdominal pain, and gastrointestinal bleeding [3,4]. Early clinical presentations including fatigue, abdominal pain, vomiting, nausea, and weight loss are often overlooked or misdiagnosed. It’s common that diagnosis is not established until the appearance of its emergency complications such as obstruction, perforation, and overt GI bleeding.
More than half of small bowel adenocarcinoma involves duodenum (55–57%), followed by jejunum (25-29%) [5,6].Small bowel adenocarcinoma in ileum is very rare, only accounts for 10-13% of cases [5,6]. Herein, we reported a case of small bowel adenocarcinoma at ileum with chronic anemia, occult bloody stool, and elevated tumor markers. Diagnosis is difficult due to the non-specific symptoms, broad spectrum of differential diseases, and the complication of concurrent benign GI diseases.
Case Report
A 68 years old male patient came to our clinic with half year history of progressive fatigue, dizziness and palpitation. He had been diagnosed with anemia about 6 months ago in a local clinic, and treated with some Chinese traditional herbal medications without any improvement. Medical and family history is otherwise insignificant. He denied any abdominal pain, nausea, vomiting, diarrhea, hematemesis, black or bloody stool, or any changes in eating habit. Physical examination featured anemia appearance. No other abnormal findings were revealed throughout the physical examination.
On admission tests, routine blood test confirmed microcytic hypochromic anemia with hemoglobin 70 g/L. The stool occult blood test showed weak positive. The tumor markers profile revealed elevated non- small cell lung cancer associated antigen CYFRA21-1 21.05 ng/mL (0.10-3.30 ng/ml)?elevated Carbohydrate antigen (CA) 72-4 19.66 U/ml (0.00-6.90 U/ml), and normal Carcinoembryonic antigen (CEA) and CA19-9 level. Patient was admitted to gastrointestinal ward for further workup and given oral iron supplement treatment for anemia at the mean time.
Upper and lower GI endoscopies were performed in search for blood loss in stomach or colon. The only gastroscopy finding was chronic non-atrophic gastritis. And a 0.6cm Yamada type II polyp in sigmoid colon was revealed by colonoscopy. Electrical resection of the polyp was performed under colonoscopy, and pathological examination of the polyp tissue confirmed tubular adenoma. Contrast-enhanced CT enteroclysis revealed a space occupying lesion at right side of mid-abdomen, probably representing a lymphoma (Figure 1). In order to confirm the nature and organ origin of the lesion, contrast-enhanced MRI enterograph was conducted. Based on the image findings, the mid-abdominal mass was probably arising from intestinal mesentery or conduit, with higher possibility of intestinal lymphoma, but stromal tumor could not be ruled out.
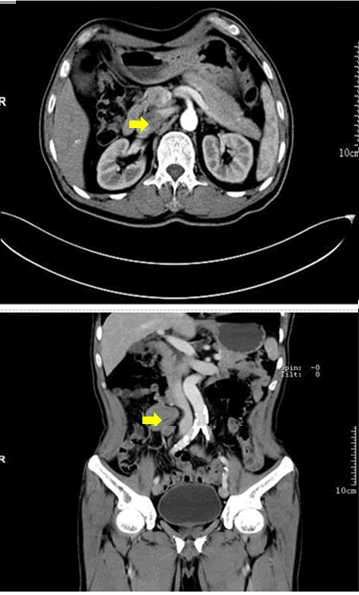
Figure 1: Axial and coronal projections of the computerized tomography of the abdomen. Arrows point fusion of swelling lymph nodes.
Because the diagnosis of the tumor could not be established, laparotomy was performed under patient's consent. A 3 cm × 2 cm shrinkage narrow growth mass with serosal invasion was observed at the ileum conduit wall about 50 cm proximal from ileocecal valve (Figure 2A). An 8 cm × 5 cm × 3 cm mass represen ti n g fusion of lymph nodes found on the mesentery root at the opposite side (Figure 2B). Intestinal resection followed by anastomsosis and enterolysis was carried out to remove the two lesions. Post-operative pathology examination of the resected tissues revealed poorly differentiated adenocarcinoma (Figure 3), and involvement of all the five retrieved lymph nodes. The final pathological diagnosis is primary ileal adenocarcinoma with lymph node invasion. Patient was referred to internal GI department for chemotherapy.
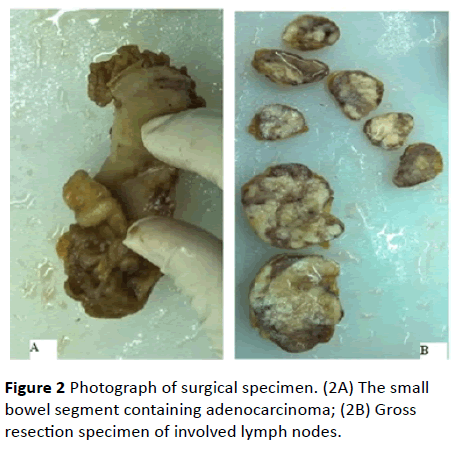
Figure 2: Photograph of surgical specimen. (2A) The small bowel segment containing adenocarcinoma; (2B) Gross resection specimen of involved lymph nodes.
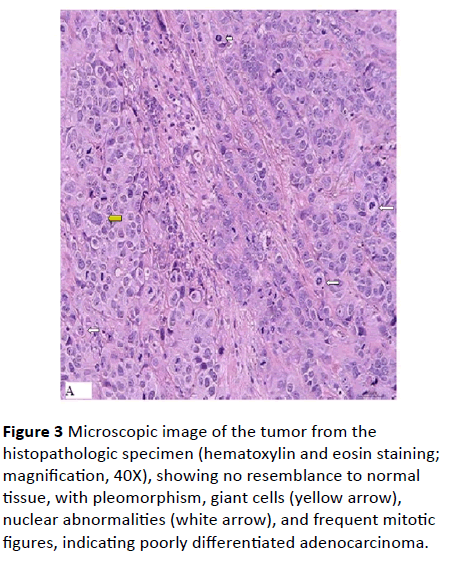
Figure 3: Microscopic image of the tumor from the histopathologic specimen (hematoxylin and eosin staining; magnification, 40X), showing no resemblance to normal tissue, with pleomorphism, giant cells (yellow arrow), nuclear abnormalities (white arrow), and frequent mitotic figures, indicating poorly differentiated adenocarcinoma.
Discussion
Even though some advanced techniques have been available to investigate the small bowel, the majority of small bowel cancers are not revealed until it develops to a late stage. 35% of the patients had synchronous metastases and 39% had tumors with lymph-node invasion at diagnosis. Diagnosis is often made after the development of emergency complications such obstruction, perforation, and gastrointestinal bleeding [7]. Recent years, in advanced countries, diagnoses were obtained by upper gastrointestinal endoscopy (28%), surgery (26%), small bowel barium transit (22%) and CT scan (18%) [8]. On average, diagnosis was made more than 6-18 months after the first symptom presentation.
Early diagnosis of small bowel adenocarcinoma is crucial for appropriate clinical management and good prognosis. However the broad spectrum of differential diseases and difficulty in small bowel investigation often impede diagnosis. In our reported case, patient presents with chronic fatigue and anemia which is non-specific and contains a broad spectrum of differential diseases. Lab work revealed occult blood in stool, and elevated two tumor markers CYFRA21-1 and CA72-4 which are often elevated in patient with tumor, inflammation, or smokers. The constellation of symptom and signs in an old patent is suggestive of malignancy in GI system. Upper and lower endoscopy was conducted to rule out stomach cancer and colon cancer, which were two most common causes of iron deficiency in men and postmenopausal woman [9]. Instead of malignancies benign lesions were found in stomach and colon. Gastritis may cause iron anemia and occult bleeding in stool. Colonic polyps could be also associated with GI bleeding. Fortunately, we didn’t stop investigation after the finding of those benign lesions. Abdominal CT imaging was carried out to investigate small bowel leading to revealing of the involved lymph nodes.
Anemia has been reported to be the only clinical presentation in another reported small bowel adenocarcinoma, which takes two years and four colonoscopies to arrive at the diagnosis [10]. Fallah et al. reported a jejunum adenocarcinoma occurred in the background of celiac disease presenting with severe anemia, bleeding, nausea and vomiting. Celiac disease was taken granted to be responsible for symptoms, investigation for rarer small small bowel disease was delayed until the fourth emergency admission for hematemesis [11]. In our reported case, positive test results of occult GI bleeding and tumor markers are suggestive for malignancy. The findings of concurrent benign diseases didn’t stop us from further investigations. In a summary, in chronic anemic patients, even benign disease could explain anemia, investigation are helpful to rule out the rare malignancy in mall bowel.
CT, MIR, capsule endoscopy and small bowel endoscopy are common tools to investigate small bowel. CT enteroclysis was reported to have sensitivity of 85–95% for the diagnosis of small bowel tumour, and MRI enteroclysis and capsule endoscopy are slightly more sensitive and specific [8]. Small bowel endoscopy is the most powerful tool plus the advantage of its biopsy option. But it’s not as convenient as the others. In our case, fusion of involved lymph nodes were revealed by contrast enhanced CT and NMR enterography. However, neither of the two image examinations was able to reveal the primary lesion situated at ileum. Neither could distinguish the secondary lymph involvement and primary lymphoma. Our experience suggested that, at least in low differentiated small bowel adenocarcinoma with early lymph invasion, CT and NMR imaging is more sensitive to the involved lymph nodes and metastasis. This might limit their use in screening small bowel adenocarcinoma in its early stage when the primary lesion is small and invasion outside intestinal conduit is not yet visible in the images. Small bowel endoscopy or capsule endoscopy might be superior in screening for primary lesion in small intestinal conduit before surgery in the present case. However capsule endoscopy is contraindicated in subobstruction and thus should be used with caution as restrictive growth and complication of obstruction is common in adenocarcinoma of lower intestine.
Diagnosis before surgery is critical to plan for appropriate surgery procedure. It has been reported that retrieval of 9 and more lymph nodes gives better prognosis [12]. We would have had more lymph nodes retrieved for better prognosis. Biopsy can be carried out under single balloon or double balloon small bowel endoscopy, which makes endoscopy the best choice of examination before surgery. The diagnosis might have established before surgery if small bowel endoscopy had been conducted on our patient before surgery.
Due to the rarity of this disease, few reports have been published on the association of tumor-markers with small bowel adenocarcinoma. CEA and CA 19-9 levels have been reported to be elevated in about 40% of small bowel adenocarcinoma patients [4]. They were within normal range in this reported case. Instead, tumor markers CYFRA 21-1 and CA 72-4 were elevated. Both markers are reported to be related to stomach cancer. In a study on the association of 5 tumor markers with stomach cancer, CYFRA 21-1 was the most sensitive one, and CA 72-4 are significantly related to lymph node involvement, metastasis or stage [13,14]. Those markers elevations are often seen in non-specific scenario of malignancy, inflammation, or in smokers. There are no data yet on CYFRA and CA 72-4 association with small bowel adenocarcinoma. Investigation of those tumor markers profile in small bowel adenocarcinoma cases might contribute to early detections of this rare malignancy.
11110
References
- Siegel RL, Jemal A (2015) Cancer statistics 2012. CA Cancer J Clin 65: 87-108.
- Pan SY, Morrison H (2011) Epidemiology of cancer of the small intestine. World journal of gastrointestinal oncology 3: 33-42.
- Natthakan TB, Pitulak A, Udom K, Varayu P (2015) Clinical, radiologic, and endoscopic manifestations of small bowel malignancies: Afirst report from Thailand. Asian Pacific Journal of Cancer Prevention 16.
- Zaanan A, Costes L, Gauthier M, Malka D, Locher C, et al. (2010) Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Annals of oncology 21: 1786-1793.
- Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J (2004) Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 101: 518-526.
- Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF (2010) A single-institution experience with 491 cases of small bowel adenocarcinoma. American journal of surgery 199: 797-803.
- Negoi I, Paun S, Hostiuc S, Stoica B, Tanase I, et al. (2015) Most small bowel cancers are revealed by a complication. Einstein 13: 500-505.
- Aparicio TZ, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, et al. (2014) Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Digestive and Liver Disease 46: 97-104.
- Goddard AF, James MW, McIntyre AS, Scott BB (2011) British Society of G. Guidelines for the management of iron deficiency anaemia. Gut 60: 1309-1316.
- Popa B, Constantinescu G, Ilie M, Badila E, Beuran M, et al. (2014) Ileum adenocarcinoma, a missed cause of anemia. Journal of gastrointestinal and liver diseases: JGLD 23: 362.
- Fallah J, Afari ME, Cordova AC, Olszewski AJ, Minami T (2015) Small bowel adenocarcinoma as the cause of gastrointestinal bleeding in celiac disease: A rare malignancy in a common disease. Case reports in oncological medicine.
- Wilhelm A, Muller SA, Steffen T, Schmied BM, Beutner U, et al. (2015) Patients with adenocarcinoma of the small intestine with 9 or more regional lymph nodes retrieved have a higher rate of positive lymph nodes and improved survival. Journal of Gastrointestinal Surgery 20: 401-410.
- Gwak HK, Lee JH, Park SG (2014) Preliminary evaluation of clinical utility of CYFRA 21-1, CA 72-4, NSE, CA19-9 and CEA in stomach cancer. Asian Pacific Journal of Cancer Prevention 15: 4933-4938.
- Sun Z, Zhang N (2014) Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World journal of surgical oncology 12: 397.








