Introduction
There are several species of microorganisms surrounding us in which some are beneficial and some are harmful to our health. The word ‘probiotic’ comes from Greek word to describe substance secreted by one microorganism that stimulate the growth of another, first used by Lilley and Stillwell [1]. The history of probiotics began with the history of man by consuming fermented foods that is well known in Greeks and Romans [2, 3]. The small and large intestines naturally enclose bacteria, often referred to as our ‘normal flora’. Our normal flora contains more than 400 species of bacteria that endow with many beneficial functions [4]. So Probiotics can be simply defined as naturally happening microorganisms consumed as a food component or dietary supplement that provide good health. A large amount of probiotic bacteria is present in milk or milk products which are mainly lactic acid bacteria (LAB). Lactic acid bacteria (LAB) including Lactobacillus spp. are Generally Recognized as Safe (GRAS) bacteria, that have been used in the processing of fermented food for centuries [5]. LAB can ferment different carbohydrate and generate lactic acid that helps in decrease pH. In addition lactic acid production has beneficial effect on formation of texture, aroma and flavor in different milk products [6]. Most probiotic bacteria belong to the group of lactic acid bacteria (LAB) and among them lactobacilli and bifidobacteria apparently play a significant role in maintaining the intestinal bionetwork and in stimulating the immune system of the host [7].
As a source of animal protein our people consume different types of milk and milk related products, among them cow, buffalo and goat milk are very common. Research works on cow milk probiotic was conducted numerous times in our country but on buffalo milk probiotic was not so familiar. Whereas in rural area buffalo is a common domestic animal so, buffalo milk can be potential source for probiotic bacteria. Buffalo milk represents an important animal product, due to its nutrient content. Buffalo milk also being a source of antimicrobial metabolite such as lactic acid, and bacteriocins. It is significantly lower in cholesterol and higher in calcium than cows, sheep or goats milks. As probiotic bacteria have abundant therapeutic or prophylactic effects [7] so development of various probiotic products with defined probiotic culture from buffalo milk can be innovative. Being a developing country like Bangladesh, considerable number of people lives below the poverty line and a substantial population remains malnourished. Development of various probiotic products, such as fermented milk drinks, yoghurt, cheese, ice-cream etc. with defined probiotic culture from buffalo milk would be able to confer health benefits of mass and common people of Bangladesh. Therefore, to bring about part of the probiotic product development buffalo milk samples were considered in this study as a potential source of probiotic bacteria. The main objectives of our study is to isolation and characterization of lactic acid bacteria from buffalo milk sample through different biochemical tests, and end with analysis of probiotic properties of those isolates.
Materials and Methods
Sample collection
Four milk samples were collected from different buffalo species of buffalo regeneration & development farm Bagherhat, Khulna division of Bangladesh. After collection, the samples were stored aseptically at 4°C in refrigerator to defend from deterioration and contamination. Sample was collected in autoclaved plastic vials. At each time of collection, provision was taken to prevent or avoid cross contamination of samples.
Isolation Lactic Acid Bacteria (LAB)
One gram of sample was dissolved in 9 ml of 0.15% buffered peptone water solution and diluted up to ten logarithmic (10- 10) fold. The diluted sample was then inoculated into the Lactobacillus MRS Agar plate by ensuring the criteria of PH 6.5, incubation temperature 37°c and incubation time 48 hr. After incubation single colony was obtained by streaking. Well isolated bacterial strains were picked up and stored in MRS broth for further studies.
One gram of sample was dissolved in 9 ml of 0.15% buffered peptone water solution and diluted up to ten logarithmic (10- 10) fold. The diluted sample was then inoculated into the Lactobacillus MRS Agar plate by ensuring the criteria of PH 6.5, incubation temperature 37°c and incubation time 48 hr. After incubation single colony was obtained by streaking. Well isolated bacterial strains were picked up and stored in MRS broth for further studies.
Bacterial characterization
Morphological characterization
Bacterial plates were more purified by subcultured continually on MRS agar media, and the colony morphologies (color, shape and size) were examined in nude eye, however microscopic observation was needed to separate one colony to another. According to the protocol of Erkus, gram staining was done with some modifications [8]. At first single colony was taken aseptically then smeared on to a clean dry slide and heat-fixed. Heat fixed smear was flooded with crystal violet solution for 30 sec and rinsed with water for 5 sec. Then grams iodine solution was used to cover over the slide for 1minute and rinsed with tap water for 5 sec. The slide was then decolorized with 95% ethanol for 15 to 30 sec and rinsed with 5 sec. Finally safranin was used as counter stains for 60-80 sec and rinsed with water, and then scrutinizes the isolates under light microscope.
Catalase Test
Slide method is used to perform catalase test. In this method a clean glass slide was divided into two sections with lubricant pencil, one should be labeled as test and the other as control. A small drop of normal saline on each area was placed with a sterilized and cooled inoculating loop; a small amount of the culture from the petri plate was picked up. One or two colonies were emulsified on each drop to make a level suspension. One drop of hydrogen peroxide was given over the test smear and the other drop on control part. The fluid over the smears was observed for the appearance of gas bubbles.
Probiotic Properties Analysis
Determination of Sugar Fermentation
Sugar fermentation tests were done according to modified protocol of Erkus [8]. Five different sugars; Glucose, Fructose, Sucrose, Maltose and D-sorbitol, were used for sugar fermentation assay. At first every sugar was dissolved in deionized water at a final concentration of 5% (w/v), then sterilization of sugar solutions were done by filter paper with 0.22 μm pore diameter. MRS broth (pH 6.5) was taken into screw cap test tube and phenol red (0.01 g/L) was added into the tube as a pH indicator. Inverted Durham’s tubes were placed and the medium was autoclaved at 121°C for 15 min. 1 ml different sugar solutions were inoculated into different tubes and 200 μl overnight liquid cultures were inoculated into the broth medium. Incubation was performed anaerobically at 37°C for 24 h. Sugar fermentation was observed, as the acid production change the color of medium from its original to yellow and formation of gas in test tubes.
Assay for NaCl Tolerance
For the determination of NaCl tolerance of isolated LAB, test tubes containing MRS broth were adjusted with different concentrations (1-10%) of NaCl. After sterilization, each test tube was inoculated with 1% fresh overnight culture of LABs and incubated at 37°C for 24 h. After 24 h of incubation their growth were determined by observing their turbidity [9].
Phenol and Bile Salt Tolerance Test
For the determination of phenol tolerance, test tubes containing MRS broth were adjusted with different concentration (0.1-0.4%) of phenol. After sterilization, each test tube was inoculated with 1% (v/v) fresh overnight culture of LABs and incubated at 37°C for 24 h. After 24 h of incubation their growth were determined by 620 nm filter absorbance of cell concentration by spectrophotometer [9].
Bile salt tolerance test of isolated lactic acid bacteria from selected buffalo milk sample were assayed using the protocol by Zinedine and Faid with some modification [10]. MRS broths with different concentrations (0.05%, 0.15% and 0.3%) of bile oxgall (Sigma Laboratories, UK) were used to find out the tolerance and growth rate of isolated LABs. Final PH of the medium was adjusted to 6.5 and autoclaved at 121°C. Then 1% overnight culture of isolated LABs were inoculated into the MRSO (MRS-Oxgall) broth medium and incubated at 37°C for 24h under anaerobic condition. Then the survival rates of the isolates were measured by taking absorbance at 620 nm filter of the MRSO with bacterial culture by spectrophotometer.
Milk Coagulation Test and Antimicrobial Activity
To determine the milk coagulation prototype 1% (v/v) culture of probiotic bacteria was inoculated into sterilized cow’s milk and incubated for 24 hr. Milk coagulation due to involvement of lactic acid forming bacteria was observed.
The possible probiotic potentials or the antagonistic activity of the isolate against the selected pathogens was investigated by well diffusion method on a solid medium [11]. In the well diffusion assay, isolated colonies of probiotic cultures were inoculated in 5 ml LB broth and grown at 30°C on a shaking incubator at 150 rpm for 72h, and cells were removed by centrifugation at 8,000 rpm for 5 min and the culture supernatant were sterilized by passage through 0.45 μm pore size filters (Millipore). Four embattled pathogenic bacteria Salmonella typhi, Vibrio cholera, E. coli, Shigella spp. were precultured in LB broth medium, incubated at 30°C for two days and the culture was swabbed over the nutrient agar plate. Wells (4 mm in diameter) were punched into the swabbed nutrient agar plate and 80 μl of culture supernatants from the probiotic isolate were added. The plates were then incubated at 37°C for 24 h. Antibacterial activity was estimated as the diameter (mm) of the clear inhibitory zone formed around the wells.
Results
Growth morphology
A total four probiotic strains were isolated named as A1, A2, B1 and D2 that formed round, creamy white colonies on MRS agar plate. Morphological, cultural, physiological and biochemical characteristics of the isolates were examined for their identification and further experiments. Among four isolates all were gram positive but A1, A2 revealed rod shaped while B1 and D1 were cocci shaped.
Biochemical characterization
Four isolates were performed by catalase test in which all of them show negative result. Sugar fermentation test was performed to consider their capability to ferment different sugars. In this experiment five sugars maltose, sucrose, fructose, sorbitol and dextrose were used. The results of sugar fermentation test were described in Table 1 that confirmed all the isolates were able to ferment given sugars.
| Isolate |
Sugar |
| |
Maltose |
Sucrose |
Fructose |
Sorbitol |
Dextrose |
| A1 |
++ |
++ |
++ |
++ |
+/- |
| A2 |
++ |
++ |
++ |
++ |
++ |
| B1 |
+/- |
++ |
+/- |
++ |
+/- |
| D1 |
+/- |
++ |
+/- |
++ |
+/- |
‘+’ Represents good fermentation; ‘+/-’ represents moderate fermentation; ‘-’ represents no fermentation.
Table 1: List of sugar fermentation results given by different isolate.
Probiotic properties analysis
Nacl and Phenol Tolerance Test
In this study all the isolated probiotic candidates were able to grow at 1-6% NaCl concentration, whereas fairly grow at 7% concentration but completely failed at 8-10% NaCl concentration (Table 2). The isolated probiotic candidates were screened for their aptitude to endure phenolic environment. Isolates were detected in 0.1%, 03% and 0.4% phenol solution throughout interval of 2, 6 and 24 hours. The optical density was measured by spectrophotometer; according to the results all the isolates are defiant to phenol at different concentrations. At 0.1% concentration all isolates showed higher level of tolerance whereas in 0.3% they were moderate but in 0.4% tolerance was much lower (Figure 1).
| Isolates |
1% NaCl |
2% NaCl |
3% NaCl |
4% NaCl |
5% NaCl |
6% NaCl |
7% NaCl |
8% NaCl |
9% NaCl |
10% NaCl |
| A1 |
+ |
+ |
+ |
+ |
+ |
+ |
+/- |
- |
- |
- |
| A2 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
- |
| B1 |
+ |
+ |
+ |
+ |
+ |
+ |
+/- |
- |
- |
- |
| D1 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
- |
‘+’ indicates normal level growth; ‘+/-’ indicates moderate growth; ‘-’ indicates no growth.
Table 2: NaCl tolerance test of isolates in MRS broth.
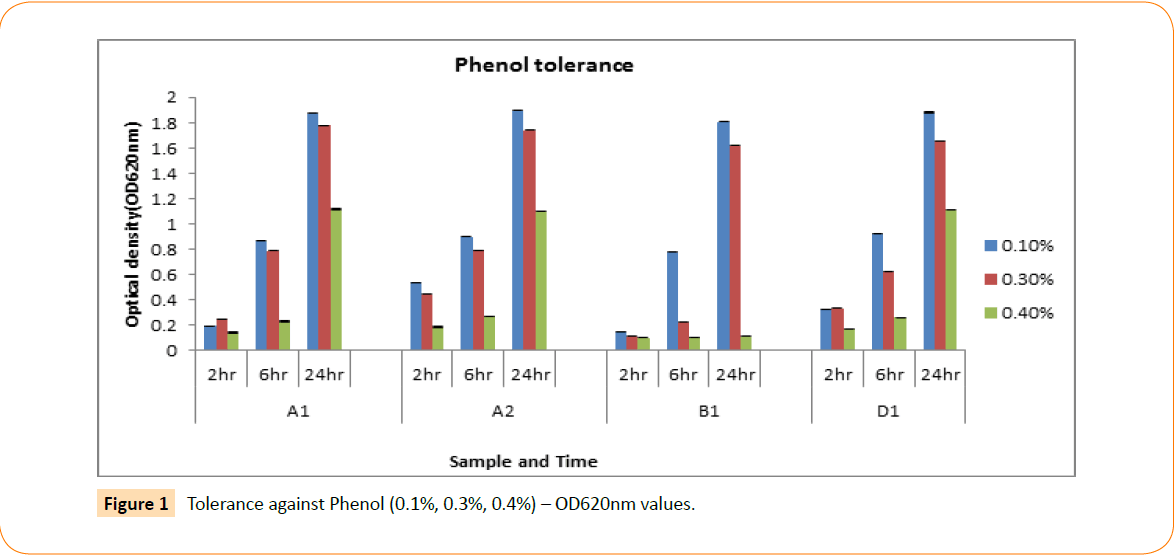
Figure 1: Tolerance against Phenol (0.1%, 0.3%, 0.4%) – OD620nm values.
Bile Salt Tolerance Test
The isolates were screened for their capacity to tolerate bile salt, although the bile concentration of the human gastro intestinal tract varies. Experimented isolates were able to grow at 0.05%, 0.15% and 0.50% of bile salt concentrations. The optical density is measured by spectrophotometer after 2 hr, 4 hr and 24 hr intermission. Their ability to tolerate different concentrations of bile salt in different time interval showed in Figure 2.
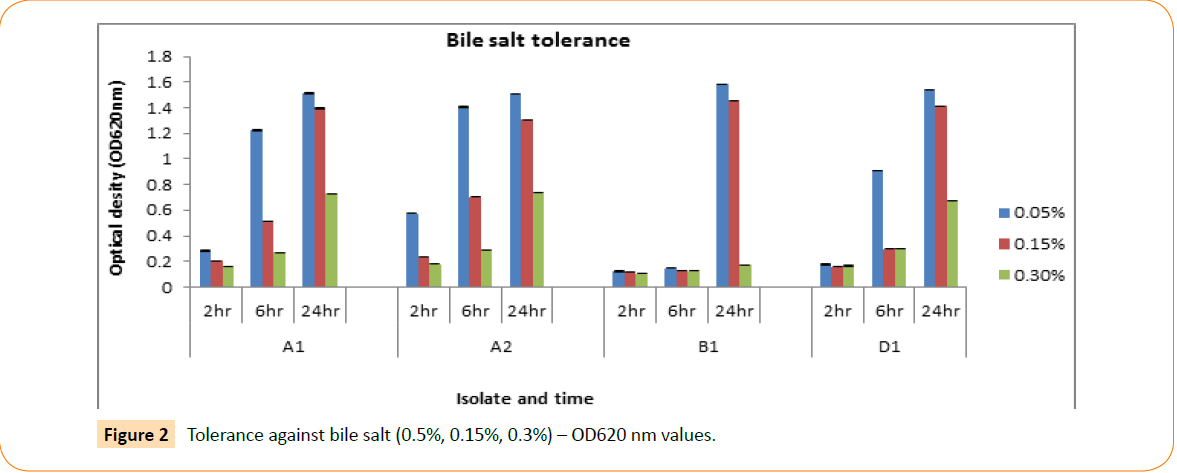
Figure 2: Tolerance against bile salt (0.5%, 0.15%, 0.3%) – OD620 nm values.
Antimicrobial Activity Test
The selected isolates were examined for their antimicrobial commotion against the indicator microorganisms’ viz. Salmonella thyphimurium, Escherichia coli, Shigella spp. and Vibrio cholera. The diameter of inhibition zones confirmed that all the isolates have antibacterial consequence against the indicator microorganisms (Figure 3). The averages of diameters of inhibition zones were given in Table 3. All the probiotic candidates also have the ability to coagulate raw cow’s milk that confirmed milk coagulation test.
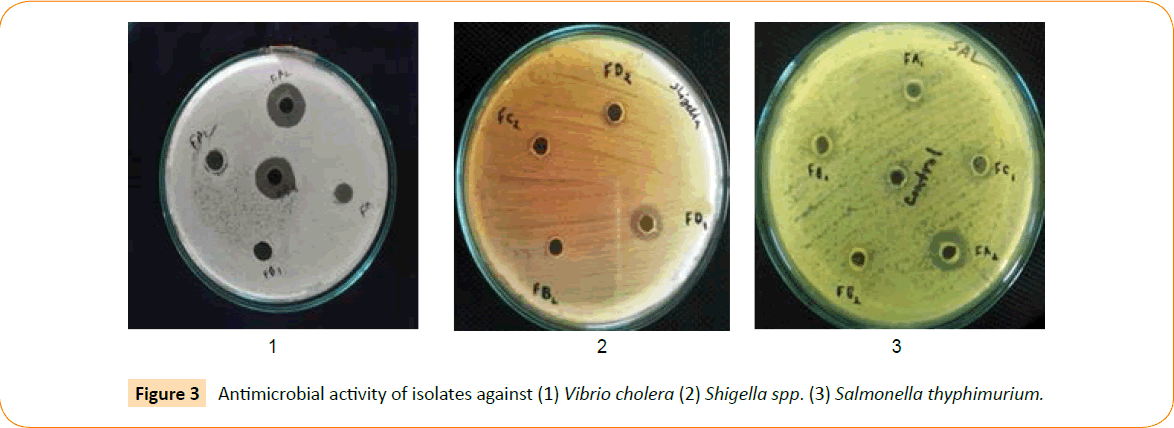
Figure 3: Antimicrobial activity of isolates against (1) Vibrio cholera (2) Shigella spp. (3) Salmonella thyphimurium.
| Isolate |
Indicator microorganisms
Zone of inhibition (mm) |
| Salmonella thyphimurium |
Shigellaspp |
V. cholera |
E. coli |
| A1 |
20 |
22 |
14 |
11 |
| A2 |
18 |
12 |
12 |
14 |
| B1 |
15 |
10 |
12 |
12 |
| D1 |
16 |
12 |
10 |
11 |
Table 3: Diameter of inhibition zones (mm).
Discussions
The study was designed for characterization and determination of probiotic properties of some lactic acid bacteria (LAB) isolated from buffalo milk collecting from buffalo regeneration farm, Bagherhat, Khulna, Bangladesh. MRS agar media is used for cultivation of lactic acid bacteria (LAB). After growing the isolates on MRS agar media they were subculture and colonies are selected for physical and biochemical characterization. After that isolates of four samples were selected for determination of probiotic properties. Selected isolates were gram positive confirmed by gram staining and catalase negative.
L. fermentum and L. casei were rod shaped, gram positive, facultative anaerobic, non endospore forming, non motile and catalase negative bacteria that occurs in chains, pairs or singly [6,12]. In this study a similar result was observed for A1 and A2 isolates after morphological and biochemical study. Bifidobacterium longum and L. acidophilus isolated from buffalo milk found coccus shaped, gram positive, facultative anaerobic, non endospore forming, non motile, catalase negative that occurs in chains, pairs or singly [13,14]. In this study similar result was also found for B1 and D1 isolate after morphological and biochemical study.
The isolated probiotic bacteria were capable to grow optimally at 37°C. Pundir et al. isolated lactic acid bacteria from fermented foods which were able to grow at 25, 37 and 40°C [15]. All probiotic isolates were capable to grow at 1-6% NaCl concentration but failed at 7-10% NaCl concentration. Lactobacillus strains from river buffalo milk cheese [16], which as well found to tolerate 1-7% NaCl. The bile concentration of the human gastrointestinal territory varies, the mean intestinal bile concentration is believed to be 0.3% w/v and the staying time is suggested to be 4 h [17]. According to our findings, every probiotic isolates were able to grow up in 0.05 to 0.3% bile salt concentration. Biochemical description of probiotic strains showed that resistant and tolerance to bile salts was not dependent on species; however,they are different among the strains of the same species observed was reported by additional researchers [18-20]. Resistance to bile salt toxicity is one of the criteria used to select probiotic strains that would be potentially capable of performing effectively in human gastrointestinal tract [21]. In our study, all probiotic isolates were competent to tolerate 0.1 to 0.4% phenol concentrations which is a toxic metabolite produced by deamination of some amino acids during disintegration by intestinal bacteria [22].
Milk coagulation aptitude was observed by all probiotic isolates of Lactobacillus spp. and Befidobacterium spp. Isolated probiotic strains also showed antimicrobial activity against Vibrio cholerae, Salmonella typhi, E. coli, and shigella species by producing zones of inhibition ranged from 10 mm to 22 mm diameter. That means isolated probiotic strains can produce antimicrobial product which can restrain the growth of pathogenic bacteria. The antimicrobial effect of probiotics could be due to the production of acetic and lactic acids that lowered the overall pH [23,24]. Probiotic bacteria may also have competed for nutrients concurrently by producing hydrogen peroxide and bacteriocins that act as antibacterial agents [25]. Other than bacteriocins, a few are also competent of producing reuterine that is identified to act as an antibacterial composites [26,27]. From all the conducted experiments it was observed that, isolated lactic acid bacteria could be used as an excellent candidate for probiotics and finally for probiotic product development.
Conclusion
Recently it has been discovered that probiotics have effect on anticancer agent. Therefore, some future studies should be performed to use these isolates reliably including molecular techniques like 16S rRNA sequencing for accurate identification of lactic acid bacterial species and multiplex RAPD-PCR technique could be used to reveal the complete metabolic potential of each of the probiotic strain which opens future research works to study for better efficacy and advancement of food biotechnological research in the food and dairy industries.
- Lilly DM, RH Stillwell (1965) Probiotics: growth-promoting factors produced by microorganisms. Science 147: 747-748.
- Gismondo M, L Drago, A Lombardi (1999) Review of probiotics available to modify gastrointestinal flora. Int J Antimicrob Agents 12: 287-292.
- Guarner F, et al. (2005) Should yoghurt cultures be considered probiotic? Br J Nutr 93: 783-786.
- Mahan LK, Escott-Stump S (2004) Food, nutrition and diet therapy. Saunders USA
- Gharaei-Fathabad E, Eslamifar M (2011) Isolation and Applications of one strain of Lactobacillus paraplantarum from tea leaves (Camellia sinensis). Am J Food Technol 6: 429-434.
- Roos S, Engstrand L, Jonsson H (2005) Lactobacillus gastricus sp. nov., Lactobacillus antrisp.nov., Lactobacillus kalixensis sp. nov.and Lactobacillus ultunensis sp. nov., isolated from human stomach mucosa. Int J SystEvolMicrobiol 55: 77-82.
- Saarela M, et al.(2002) Gut bacteria and health foods—the European perspective. Int J Food Microbiol78: 99-117.
- Erkus O (2007) Isolation, phenotypic and genotypic characterization of yoghurt starter bacteria. Izmir Institute of technology
- Hoque M, et al. (2010) Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World Journal of Dairy & Food Sciences 5: 39-46.
- Zinedine A, Faid M (2007) Isolation and characterization of strains of Bifidobacteria with probiotic proprieties in vitro. World Journal of Dairy & Food Sciences 2: 28-34.
- Rahman S, et al. (2009) Application of probiotic bacteria: a novel approach towards ensuring food safety in shrimp aquaculture. Journal of Bangladesh Academy of Sciences 33: 139-144.
- Hammes WP, RF Vogel, (1995) The genus lactobacillus, in The genera of lactic acid bacteria 19-54.
- Moreno,MF et al.(2006) The role and application of enterococci in food and health. Int J Food Microbiol106: 1-24.
- Shafakatullah N, Chandra M(2014) Screening of Raw Buffalo’s Milk from Karnataka for Potential Probiotic Strains. Res.J.RecentSci3: 73-78.
- Pundir RK et al. (2013) Probiotic potential of lactic acid bacteria isolated from food samples: an in vitro study Journal of Applied Pharmaceutical Scienc 3: 85-93
- Jeronymo-Ceneviva AB, et al.(2014) Probiotic properties of lactic acid bacteria isolated from waterbuffalomozzarella cheese. Probiotics antimicrob proteins 6: 141-156.
- Prasad J, et al. (1998) Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. International Dairy Journal 8: 993-1002.
- Liong M, Shah N(2005) Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J Dairy Sci 88: 55-66.
- Pennacchia C, et al. (2004) Selection of Lactobacillus strains from fermented sausages for their potential use as probiotics. Meat sci67: 309-317.
- Mishra V, D Prasad(2005) Application of in vitro methods for selection of Lactobacillus caseistrains as potential probiotics. Int J Food Microbiol 103: 109-115.
- Begley M, Gahan CG, HillC (2005) The interaction between bacteria and bile. FEMS Microbiol Rev 29: 625-651.
- Sathyabama S, Vijayabharathi R, Priyadarisini VB(2015) Screening for probiotic properties of strains isolated from feces of various human groups. J Microbiol 50: 603-612.
- Bezkorovainy A (2001) Probiotics: determinants of survival and growth in the gut. The American journal of clinical nutrition 73: 399-405.
- Mobarez A, et al.(2008) Antimicrobial effects of bacteriocin like substance produced by L. acidophilus from traditional yoghurt on P. aeruginosa and S. aureus. J BiolSci 8: 221-224.
- 25.Marteau P, et al. (1990) Effect of chronic ingestion of a fermented dairy product containing Lactobacillus acidophilus and Bifidobacteriumbifidum on metabolic activities of the colonic flora in humans. Am J ClinNutr52:685-688.
- Ray, B. and A. Bhunia, Fundamental food microbiology. 2007: CRC press.
- Marteau P, et al. (1990) Effect of chronic ingestion of a fermented dairy product containing Lactobacillus acidophilus and Bifidobacteriumbifidum on metabolic activities of the colonic flora in humans. Am J ClinNutr52:685-688.
7867








