Abstract
Background: Surgical intervention for metastatic bone lesions has shown to have a satisfactory outcome. However, several factors can affect the survival of patients with metastatic bone disease. This review aims to evaluate the prognostic factors that affect the survival of patients with bone metastatic disease.
Methods: A retrospective study was conducted on 40 patients with bone metastatic disease who underwent surgical treatment between 2007 and 2015 at the oncology unit of the orthopaedic department at Hospital Universiti Sains Malaysia. Prognostic factors affecting the median survival rate were evaluated. The performance status questionnaire of the Eastern Cooperative Oncology Group (ECOG) was used to assess the patient's quality of life at three, six, and twelve months post-operatively. The survival rate was calculated using the Kaplan-Meier method.
Results: After evaluating 250 patient folders with metastatic bone disease, 40 cases met the inclusion criteria for this study. The study population consisted of 29 females and 11 males, with 70% of patients being under 60 years of age. The majority of patients were Malays (36 patients) and Chinese (4 patients). The most common primary tumour was breast cancer (42.5%), followed by thyroid cancer (17.5%, n=7).
The median survival for all patients was 36 months. Survival analysis revealed that age (p=0.028), chemotherapy (p=0.003), location of metastasis (p=0.021), surgical treatment for bone lesions (p=0.038), and quality of life assessed by ECOG questionnaire at three, six, and twelve months after surgery (p=0.001) were significant prognostic factors affecting survival of patients with metastatic bone disease.
Conclusions: Surgical intervention is a significant prognostic factor affecting survival in patients with metastatic bone disease. Patients with shorter life expectancies may require less invasive surgery, whereas those with longer survival estimates may require more extensive and durable reconstructive surgery. Other factors, such as age, location of lesions, number of bone lesions, and chemotherapy, also influence survival. The study further revealed that the ECOG performance status (0-2 and 3-5) of patients at three, six, and twelve months post-surgery is a statistically significant factor affecting survival.
Overall, the study underscores the importance of considering various prognostic factors when managing patients with metastatic bone disease. These findings may help guide clinical decision-making and improve the overall prognosis and quality of life of patients with this condition.
Keywords
Metastatic; Bone; Cancer; Chemotherapy; Surgery; Survival
period
INTRODUCTION
In recent years, the National Cancer Registry unit of
Malaysia has reported a significant increase in cancer
incidence. In 2007, a total of 18,219 new cancer cases were
registered, with 57.6% of cases already at advanced stages
at the time of diagnosis [1]. Breast cancer was found to
be the most common cancer in females, while colorectal
cancer was the second most common cancer overall, and
lung cancer was the most common cancer in males and the
third most common cancer in the general population [2].
Metastasis to the bone from carcinomas is a major medical
and social issue. Approximately 50% of primary cancers
tend to disseminate to the bone, which is the third most
frequent site of metastatic spread after the lung and liver
[3]. Prostate, breast, and kidney cancers have the highest
predilection to disseminate to the bone, followed by the
lung and thyroid. The most common sites of involvement
are the spine, pelvis, ribs, skull, and proximal long bones [4- 5]. While six-month survival rates have been reported for
patients with primary solid tumours such as prostate cancer
(98%), breast cancer (89%), lung cancer (50%), and kidney
cancer (51%), management of metastatic bone disease
remains challenging [6]. Treatment options for metastatic
bone disease include medical treatment, radiation therapy,
chemotherapy, and surgical intervention. Among these
options, surgical intervention for metastatic bone lesions
has shown promising outcomes. Bone pain is frequently
the first sign of metastatic disease, and approximately 80%
of all breast cancer patients will have one episode of bone
pain that requires treatment. Pathologic fractures are a
major cause of prolonged disability [7]. Skeletal-related
events (SREs) significantly impact morbidity, performance
status, quality of life (QOL), functional capacity, and
survival. Despite numerous studies and scoring systems
that have been developed to identify prognostic factors in
metastatic bone disease, none have been able to provide
definitive conclusions on the exact factors contributing to
the severity and survival of the disease [8-9]. It should be
noted that not all cases of metastatic bone disease require
surgical intervention. The present study was conducted at
the orthopaedic oncology and reconstructive surgery centre
of University Sains Malaysia (USM) in Kelantan, the only
tertiary centre for this specialty in the region. USM covers
the entire east coast of Malaysia and receives referrals
from other states, thus providing a comprehensive source
of information on patients with metastatic bone disease. The study examined recorded patient folders from 2008
to 2016 to identify prognostic factors affecting the survival
of patients with bone metastatic disease who were treated
surgically.
METHODS
This retrospective study aimed to analyse the survival
prognostic factors and quality of life outcomes in patients
who underwent surgical intervention for metastatic bone
disease. The study population was identified by reviewing
the records of 250 patients with metastatic bone disease who
were treated between 2008 and 2015 at the orthopaedic
oncology unit of Hospital Universiti Sains Malaysia [10].
Inclusion criteria for the study were patients who underwent
surgical intervention related to metastatic bone disease,
had a history of solid tumour, presented with bone lesions,
had metastatic lesions at any site (limbs, spine, and pelvic),
and were followed up at our clinic for at least 18 months
post-operation. Exclusion criteria included patients who
died intra-operatively, patients with multiple myeloma and
lymphoma, patients treated conservatively, and patients
who underwent surgery solely for spine metastasis [11].
After applying the inclusion and exclusion criteria, a total of 40 patients were included in the study. The
data collected from the patient records included age at
presentation, type of primary tumour, date of diagnosis for
primary tumour, treatment modes of the primary tumour
(chemotherapy, radiotherapy, type of surgery), presence of
visceral or cerebral metastases, solitary or multiple skeletal
metastases, presence of solitary or multiple lesions of
the spine, presentation of the patient (pain, pathological
fracture, neurological problems), date of diagnosis for
bone metastasis, type of surgery, date of the surgery, date
of death taken from Malaysian registry centre, and survival
time calculated from the date of the surgery to the date of
death [12].
The performance status of the survived patients was assessed
using the Eastern Cooperative Oncology Group (ECOG)
questionnaire to evaluate their quality of life at three, six,
and twelve months after the surgery. The survival analysis
was performed using the Kaplan-Meier method [13].
Descriptive statistics, including means, standard deviations,
and frequency distributions, were calculated for continuous
and categorical variables, respectively. The correlation
between survival time and various prognostic factors was
analysed using Cox regression analysis.
All statistical analyses were performed using SPSS version
26.0 (IBM Corp., Armonk, NY, USA). The level of
significance was set at p < 0.05.
RESULTS
A total of 40 patients (29 female and 11 male) were
included in this study, with a mean age of 54.43 years at
presentation. Age was equally distributed, with 30% of
patients (12/40) above 60 years and 70% below 60 years
(28/40). The largest ethnic group was Malay (90%, n=36),
followed by Chinese (10%, n=4) (Tab. 1.).
Variables |
n. patients |
Survival Status n (%) |
Median |
p-values* |
| |
|
Survive |
Died |
Survival until 1-9-2015 |
|
| Age |
| Below 60 |
26 |
14(%46.15) |
12 |
53 |
0.028 |
| Above 60 |
11 |
9(18.2%) |
2 |
14 |
|
| Types of tumor |
| Rapid growth |
18 |
12(33.3%) |
6 |
46 |
0.674 |
| Slow growth |
19 |
11(42.1%) |
8 |
16 |
|
| Chemotherapy |
| Yes |
20 |
14(30.0%) |
8 |
15 |
0.003 |
| No |
17 |
9(47%) |
6 |
58 |
|
| Radiotherapy |
| Yes |
27 |
18(33.3%) |
9 |
53 |
0.304 |
| No |
10 |
5(50%) |
5 |
36 |
|
| Metastatic lesion |
| Solitary |
24 |
14(41.7%) |
10 |
16 |
0.38 |
| multiple |
13 |
9(30.8%) |
4 |
58 |
|
| Location |
| Appendicular |
8 |
8(0%) |
0 |
12 |
0.021 |
| Axial bone |
23 |
10(56.5%) |
13 |
58 |
|
| Combine both |
6 |
5(16.7%) |
1 |
14 |
|
| Organ metastasis |
| Yes |
19 |
13(31.6%) |
6 |
46 |
0.212 |
| No |
18 |
10(44.4%) |
8 |
36 |
|
| Surgery |
| Harrington |
5 |
5(0%) |
0 |
36 |
0.038 |
| Nailing |
1 |
1(0%) |
0 |
16 |
|
| Endoprosthesis |
23 |
0(56.5%) |
13 |
58 |
|
| Allograft |
5 |
5(0%) |
0 |
10 |
|
| curettage& bone cement |
3 |
2(33.3%) |
1 |
15 |
|
| ECOG post op. |
| 3 month (0-2) |
25 |
11(65%) |
14 |
58 |
0.001 |
| 3 month (3-5) |
12 |
12(0%) |
0 |
10 |
|
| 6 month (0-2) |
25 |
11(56.0%) |
14 |
58 |
0.001 |
| 6 month (3-5) |
12 |
12(0%) |
0 |
9 |
|
| 1 year (0-2 ) |
19 |
5(73.7%) |
14 |
75 |
0.001 |
| 1 year (3 - 5 ) |
18 |
8(0%) |
0 |
12 |
|
Tab.1. Summary of results that show the association between survival and prognostic factors and median survival of patients post operation.
*Pearson Chi-square applied
The most common primary tumour was breast cancer
(42.5%, n=17), followed by thyroid cancer (17.5%, n=7),
renal cancer (15.0%, n=6), unknown primary tumour
(7.5%, n=3), lung cancer (5%, n=2), and other primary
tumours (prostate cancer, testicular cancer, nasopharyngeal
cancer, and ovarian cancer, each n=1, 2.5%).
Prognostic factors evaluated included pathological fracture
(76.92%, n=30), neurological deficit (5.13%, n=2), and
major surgeries such as resection and endoprosthesis
placement surgery (58.97%, n=24). The second most
common surgery performed was Harrington procedure
for pelvic involvement (17.95%, n=7), followed by minor
surgeries such as allograft and osteosynthesis (12.36%,
n=5), curettage and bone cementing (6.82%, n=3), and
intramedullary nailing for pathological fracture (2.27%,
n=1). Quality of life was evaluated using the ECOG
questionnaire at 3 and 12 months after surgery. Patients
were divided into two groups based on ECOG scores:
ECOG 0-2 (good quality of life) and ECOG 3-5 (poor
quality of life). At 3 months, 33.33% of patients (n=13)
had an ECOG score of 0-2, while 66.67% (n=27) had an
ECOG score of 3-5. At 12 months, 48.72% of patients
(n=19) had an ECOG score of 0-2, while 51.28% (n=20) had an ECOG score of 3-5. Survival was analysed using
the Kaplan-Meier method. After 18 months of follow-up,
14 patients (37.8%) survived, while 23 (62.16%) died.
The global median survival for all patients was 36 months
(Fig.1.1). Survival was significantly different between age
groups, with 46.2% of patients under 60 years surviving
compared to 18.2% of patients over 60 years (p=0.028).
Histopathological investigation showed that 18 patients
had a rapid-growing tumour, of whom 12 died and 6
survived (33.3%). For the slow-growing tumour, 19
patients were identified, of who 11 died and 8 survived
(42.1%). The difference was not statistically significant
(p=0.674) (Fig.1.2). Regarding metastasis, 24 patients
had solitary metastasis, of whom 14 died and 10 survived
(41.7%), with a median survival of 58 months. Multiple
metastases were found in 13 patients, of whom 9 died and
4 survived (30.8%), with a median survival of 16 months.
The difference was not statistically significant (p=0.38).
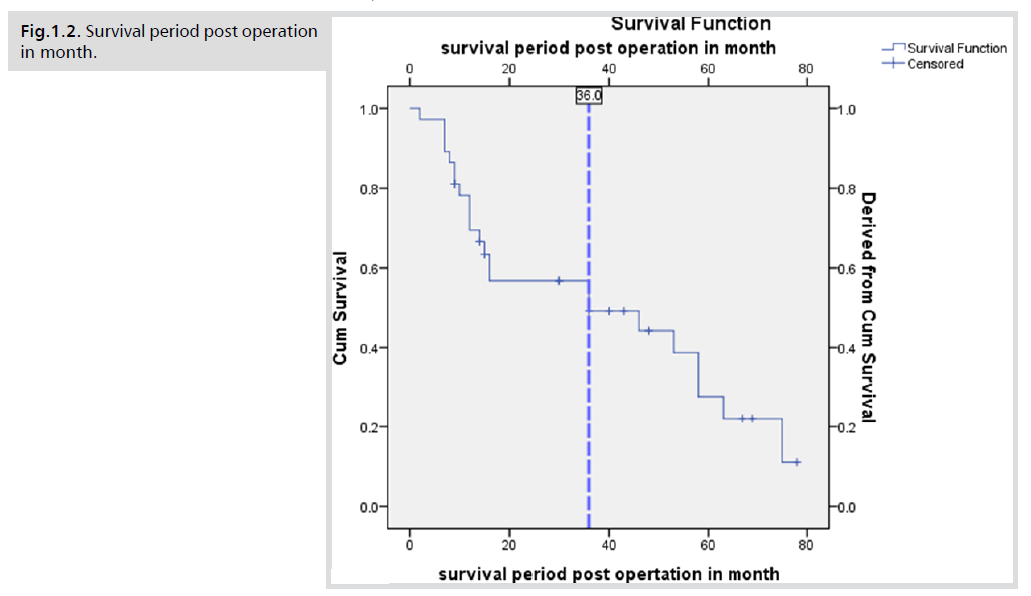
Fig.1.1. Survival period post operation in month.
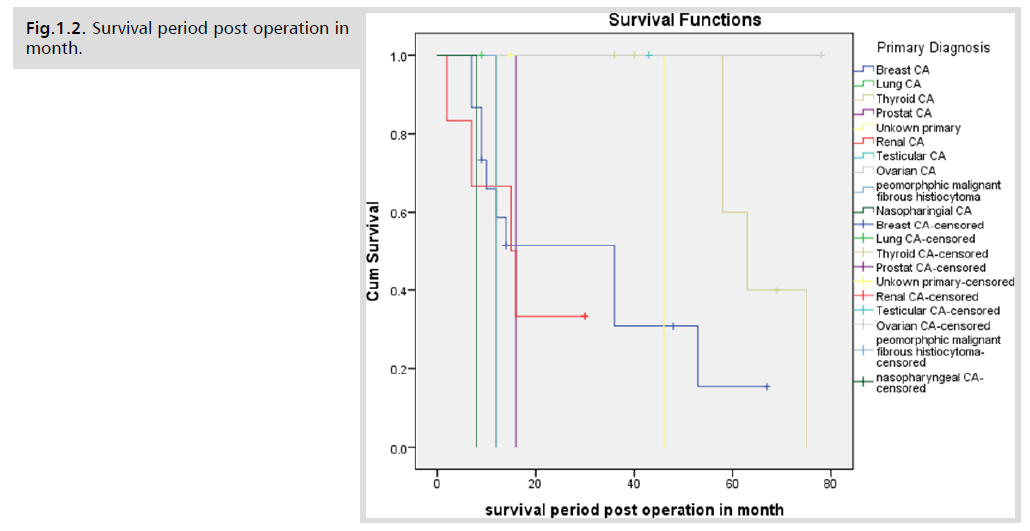
Fig.1.2. . Survival period post operation in month.
The location of metastasis was significantly associated with
survival (p=0.021). Eight patients had appendicular bone.
DISCUSSION
The present review examined the clinical characteristics,
treatment approaches, and prognostic factors of surgically
treated patients with bone metastases (Fig.2.1). The study
sample consisted of individuals with a mean age of 54.43
years, with a male-to-female ratio of 1:3. Our findings
showed a slightly lower mean age at presentation compared
to other studies, while gender distribution was consistent
with the literature [14].
Fig.2.1. The present review examined the clinical characteristics, treatment approaches, and prognostic factors of surgically treated patients with bone metastases.
Breast cancer was found to be the most common primary
solid tumour, followed by thyroid, renal, and lung cancer.
This contrasts with previous studies that reported varying
tumour types as the most common. In terms of bone
involvement, the proximal femur was the most frequently
affected site, followed by the pelvic, lower limb, and
spine metastasis. However, other studies have reported
the axial bones to be more commonly involved than the
appendicular skeleton [15].
Treatment approaches varied based on the extent and
location of the lesions. Internal fixation was the most
common treatment for long bone lytic lesions, while
intramedullary nailing was only performed in a few cases.
Delayed presentation of patients was associated with
increased bone damage and the need for replacement
surgery [16] The Harrington procedure, allograft, and
osteosynthesis were used for pelvic involvement, see the figure
(Fig.3.1). Resection and endoprosthesis placement were
frequently performed for proximal humerus and proximal
femur, distal femur, and proximal tibia lesions (Fig.3.2).
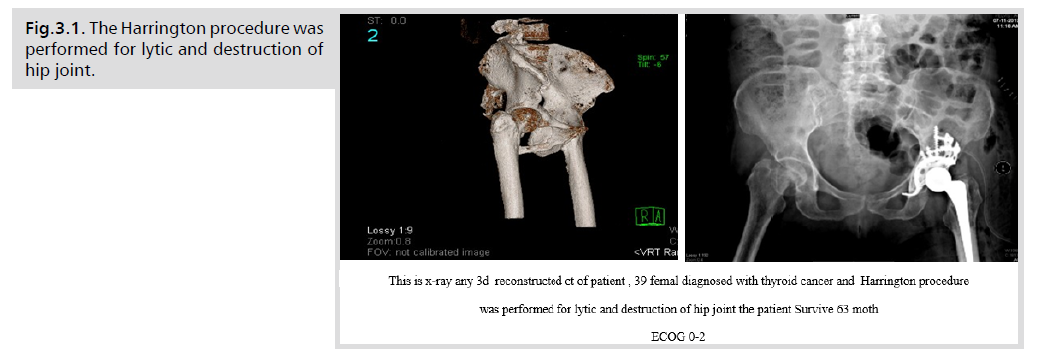
Fig.3.1. The Harrington procedure was performed for lytic and destruction of hip joint.
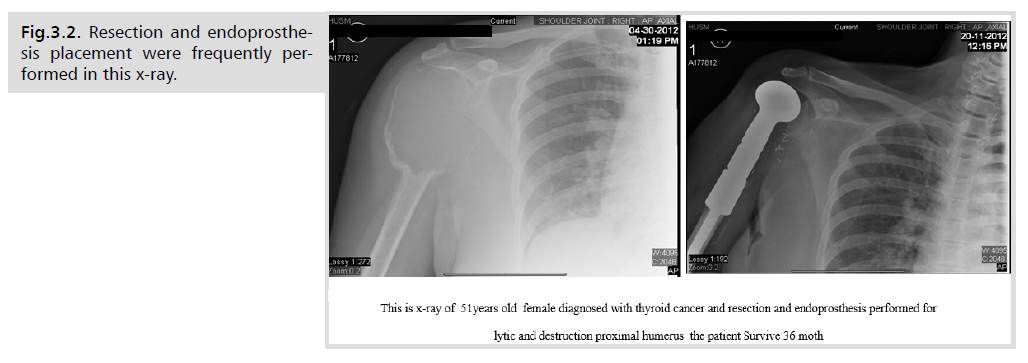
Fig.3.2. Resection and endoprosthesis placement were frequently performed in this x-ray.
The quality of life of the patients was evaluated using
the ECOG questionnaire, which was administered three,
six, and twelve months after surgery. The ECOG results
significantly correlated with survival, with patients with
a performance status of 3 or 4 having a 50% higher
risk of dying than those with better performance status.
Pathological fracture was a negative prognostic factor,
although it was not found to significantly influence survival.
Previous chemotherapy was a significant prognostic factor,
likely due to patients with advanced disease at the initial
treatment of the primary lesion being more likely to receive chemotherapy. The type of surgery was found to
significantly affect survival, while the site of the primary
tumour, radiotherapy, and pathological fracture were not
significantly associated with survival. The performance
status was affected by age and the type of surgery, but not
by spine metastasis or the site of the primary tumour [17].
CONCLUSION
The present study provides important insights into
the clinical characteristics, treatment approaches, and
prognostic factors of surgically treated patients with bone
metastases. These findings highlight the need for tailored
treatment approaches based on the location and extent of
bone involvement, as well as the importance of considering
prognostic factors when making treatment decisions.
Further research is needed to better understand the optimal
treatment strategies for this patient population [18].
In conclusion, the study found that surgery was a significant prognostic factor affecting survival in patients
with metastatic bone disease. Patients with short life
expectancies may require less invasive surgery, while those
with longer survival estimates may require more durable
reconstructive and extensive surgery to improve their
quality of life and survival. Age, location, number of bone
lesions, and chemotherapy were also significant prognostic
factors affecting survival. The study also identified the
performance status ECOG (0-2 and 3-5) after surgery as
a statistically significant factor affecting the survival range
of patients. However, some limitations of the study were
identified, including incomplete clinical records and a
relatively small number of patients. As a recommendation,
national-level collaboration is needed to evaluate the
prognostic factors of patients with metastatic bone disease
with other centers in Malaysia and possibly in South East
Asia to obtain larger data and patient numbers for more
robust analysis.
ACKNOWLEDGEMENT
None
CONFLICT OF INTEREST
None
REFERENCES
- Kachnic LA, Pugh SL, Tai P, et al. RTOG 0518: Randomized Phase III Trial to Evaluate Zoledronic Acid for Prevention of Osteoporosis and Associated Fractures in Prostate Cancer Patients. Prostate Cancer Prostatic Dis. 2013; 16(2): 1-10.
Indexed at, Google Scholar, Crossref
- Adler RA, Gill RS. Clinical utility of denosumab for treatment of bone loss in men and women. Clin Interv Aging. 2011; 6(6): 119-124.
Indexed at, Google Scholar, Crossref
- Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000; 31(7): 515-528.
Indexed at, Google Scholar, Crossref
- Bauer HC. Controversies in the surgical management of skeletal metastases. J Bone Joint Surg Br. 2005; 87(7): 608-617.
Indexed at, Google Scholar, Crossref
- Toma CD, Dominkus M, Nedelcu T, et al. Metastatic bone disease: a 36-year single centre trend-analysis of patients admitted to a tertiary orthopaedic surgical department. J Surg Oncol. 2007; 96(5): 404-410.
Indexed at, Google Scholar, Crossref
- Loprinzi DP, Frith E. Physical activity, mortality and prostate disease. J Mol Pathophysiol. 2016; 5(8): 81-84.
Indexed at, Google Scholar, Crossref
- Coleman RE, Lipton A, Roodman GD, et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev. 2010; 36(9): 615-620.
Indexed at, Google Scholar, Crossref
- Jayasekera J, Onukwugha E, Bikov K, et al. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. J Pharmacoeconomics. 2014; 32(2): 173-191.
Indexed at, Google Scholar, Crossref
- Weber KL, Randall RL, Grossman S, et al. Management of lower-extremity bone metastasis. J Bone Joint Surg Am. 2006; 88(4): 11-19.
Indexed at, Google Scholar, Crossref
- Oboma YI, Susan BE, Elesha SO, et al. Breast cancer biomarkers at Niger delta University hospital: Comparisons with national and International trends and clinical significance. J Patphy. 2017; 903(7): 1-6.
Indexed at, Google Scholar, Crossref
- Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014; 3(7): 1359-1367.
Indexed at, Google Scholar, Crossref
- Ruggieri P, Mavrogenis AF, Casadei R, et al. Protocol of surgical treatment of long bone pathological fractures. Injury. 2010; 41(6): 1161-1167.
Indexed at, Google Scholar, Crossref
- Forsberg JA, Sjoberg D, Chen QR, et al. Treating metastatic disease: Which survival model is best suited for the clinic? Clin Orthop Relat Res. 2013; 471(6): 843-850.
Indexed at, Google Scholar, Crossref
- Biermann JS, Holt GE, Lewis VO, et al. Metastatic bone disease: diagnosis, evaluation, and treatment. J Bone Surgery. 2009; 91(8): 1518-1530.
Indexed at, Google Scholar
- Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Critical reviews in oncol hematol. 2005; 56(8): 365-378.
Indexed at, Google Scholar, Crossref
- Harrington KD, Sim F, Enis J, et al. Methylmethacrylate as an adjunct in internal fixation of pathological fractures. Experience with three hundred and seventy-five cases. J Bone Joint Surgery. 1976; 58(9): 1047-1055.
Indexed at, Google Scholar
- Dey YN, Mahor S, Kumar D, et al. Gastrokinetic activity of Amorphophallus Paeoniifolius Tuber in rats. J Intercult Ethnopharmacol. 2016; 5(1): 36-42.
Indexed at, Google Scholar, Crossref
- Kumar S, Kashyap P. Antiproliferative activity and nitric oxide production of amrthanolic extract of Fraxicus micrantha on michigan cancer foundation-7 mammalian breast cancinoma cell line. J Intercult Ethnopharmacol. 2015; 4(8): 109-113.
Indexed at, Google Scholar, Crossref










