Keywords
Phenylephrine; Ephedrine; Spinal anesthesia; Maternal hypotension; Cesarean section
Introduction
Low blood pressure during the administration of spinal anesthesia for surgical delivery is secondary to the sympathetic impediment, and it can be dangerous for both the fetus and the mother. Uncertain effects are abatement in uterine and placental perfusion, withdrawal of fetal oxygenation and fetal acidosis, and maternal symptoms of decreased cardiac output. Other side effects, such as nausea, vomiting or diversification of mindfulness may also occur [1].
The frequency of low blood pressure after administration of spinal anesthesia for surgical delivery may be about to eighty percent if preventive reinforcements such as precedent hydration, propelling the uterus to the left, and vasopressors, has not been considered [2].
Ephedrine has been contemplated the exclusive primary vasopressor for the treatment of low blood pressure as a result of the administration of anesthetic in the spine in contempt of the shortcomings of a confirmation of their preeminence that settled other vasoconstrictors [3]. Phenylephrine has been practiced for the prohibition or the treatment of spinal-induced hypotension in the surgical delivery. Boilerplate alternatives of vasopressor agents remains a contentious matter [4]. It is therefore necessary to analyse the effect of ephedrine and phenylephrine in prophylax and treatment of low blood pressure in patients undergoing spinal block, to figure out the side effects of these drugs and to determine fetal Apgar scores.
Background
A study was undertaken in Iran [5]. Eighty-three patients were recruited in the study and were randomly proportionally into 3 groups. Group one got phenylephrine infusion; Group two received ephedrine infusion while the group three is delivered as a placebo. Some reduction in BP about 20% from the baseline was treated with 50-100 μg phenylephrine in group one, or 5-10 mg of ephedrine in groups two and three. Blood pressure, heart rate and arterial oxygen saturation were recorded in time. Mother and newborn perioperative complications are monitored and recorded. Systolic and diastolic blood pressure was greater in phenylephrine group of recipients than the control group, but not higher than in the ephedrine group. Maternal arrhythmias were more prevailing in ephedrine and phenylephrine groups than the control group. Vomiting was more common in the ephedrine group (P<0.05). In addition, a five-minute Apgar scores were higher in newborns phenylephrine and ephedrine groups than in the placebo group (P<0.05). The newborn phenylephrine group had lower acidosis than the other groups. The authors concluded that prophylactic infusion of phenylephrine can effectively lower spinal anesthesia related hypotension without significant complication for the mother or fetus [5].
A study was managed in Brazil. Ninety pregnant women undergoing cesarean section were randomized to three groups "phenylephrine, metaraminol and ephedrine. The infusion dose is duplicated when the systolic blood pressure is transferred to eighty percent of the baseline criterion and a bolus was given when the systolic blood pressure dropped to below eighty percent. Infusions dose split when it systolic blood pressure increased to 120% and came close when it became higher. There was no difference in the incidence of hypotension, bradycardia, reactive hypertension, infusion discontinuation, atropine or Apgar. Recovery dose was higher only in the ephedrine group compared with metaraminol group. The incidence of nausea and vomiting and fetal acidosis was greater in the ephedrine group. The three drugs were effective to prevent hypotension, fetal effects were more current in the ephedrine group [6].
Purpose of Study
The purpose of the present trial is to analyze the effect of ephedrine and phenylephrine to prevent and treat low blood pressure in pregnant women undergoing abdominal surgical delivery under spinal anesthesia, to figure out the adverse effects of ephedrine and phenylephrine, and to assess fetal changes as measured by Apgar scores.
Methodology
Design
A prospective, randomized, double-blind clinical trial
Sample and sampling
The power of the study is calculated, at 80%, and the level of alpha as p<0.05, the sample size is calculated as 20 women in each group. To raise the power of our study, we have taken 30 women in each group as has been accompanied in previous studies.
Pre-enrollment assessment
Each patient must have undergone a complete blood count to examine hemoglobin levels and platelet counts to exclude all patients who had a low platelet count (less than 100 × 103), since the low number increasing contingency of epidural hematoma.
Randomization
Patients were at random prorated into two groups using consecutive closed envelopes with random numbers formerly processed by a health care provider who was note laborate with the study in any respect. Patients who greet the inclusion criteria were arbitrary receive either: Group (1) (n=27), Ephedrine: 10 mg i.v. bolus simultaneously with subarachnoid block. Group (2) (n=28), Phenylephrine: 80 μg i.v. bolus simultaneously with subarachnoid block. The study drugs were arranged in indistinguishable 10-mL syringes by an anesthesiologist not elaborated with data assemblage.
After recruitment, 27 patients designated to the ephedrine group of thirty because one of them withdrew their consent to participate, and two of them were indoctrinated to general anesthesia. In regard to phenylephrine group, 28 patients designated to the intervention of the thirty; two of the 30 patients reverted their consent to participate in the study.
Blindness
Participants in the study and anesthesia personnel involved in the operation were blind to group allotment.
Inclusion criteria
(1) Physical status ASA I or II (2) Full-term pregnancy of a single fetus; (3) electoral cesarean section; (4) 18-40 years old.
Exclusion criteria
(1) Rejection to engage in the study; (2) Patients less than 18 years; (3) preceding or pregnancy-induced systemic hypertension; (4) the occurrence of cardiovascular or cerebrovascular sickness; (5) fetal disorders; (6) allergy to the drugs used in the study, and contraindications to spinal block; (7) parturient woman with blood pressure of 135/95 mmHg and above; (8) parturient woman who has chronic hypertension; (9) parturient woman has a heart rate <60 bpm and >120 beats per minute.
Conceptual definition of the terms
Hypotension was characterized as a downturn in systolic arterial pressure >20% of baseline and treated with a bolus of 50% of the introductory dose of the vasopressor. Reactive hypertension was described as blood pressure 20% higher than the base level for the use of vasopressors. Heart rate below 60 beats per minute defined as bradycardia when accompanied by hypotension, and it was treated with 0.5 mg atropine. Apgar on the first and fifth minutes of all newborns were investigated and a score below eight was contemplate low. Tachycardia considered on a heart rate which is higher than 100 beats [7,8].
Procedure
After earning a study approval by the Institutional Review Board (IRB) of the University, written informed consent was achieved from all parturient women. Sixty women were entered into the study.
A data sheet includes the following information was accomplished for every woman: name, age, height, weight, place of residence, the body mass index, gestational age, arterial blood pressure, pulse, respiratory rate, electro- cardio gram and peripheral capillary oxygen saturation as the baseline
A physical assessment was implement for all patients. Laboratory tests were evaluated (complete blood count, specifically the platelet count). Intravenous cannula 16 Fr G was interpolate. Ringer's lactate (RL) solution (20 ml/kg) was instilled 30 minutes before spinal injection for the all women.
Patients were placed supine, or a sitting position, for a few minutes and blood pressure and heart rate were measured three times at 3-minute intervals and the arithmetic average of the values was calculated, which was considered the basal pressure of pregnant women and recorded on the data collection form.
Ephedrine or phenylephrine was administrated for spinal anesthesia. Patients in Group (1) disposed a prophylactic intravenous bolus of 10 mg at the same time of spinal block. Patients in group (2) disposed a prophylactic intravenous bolus of 80 mcg of phenylephrine at the same time of spinal block.
In the current study, the dose of 80 μg of phenylephrine was chosen depend on an earlier study [9], in which this dose was the effective dosage when administered as an intravenous bolus, without severe side effects. The dose of 10 mg of ephedrine was chosen depend on an earlier study [10], where this was the effective dose when conducted as an intravenous bolus, without serious side effects.
The syringes with the study drugs are produced by an anesthesiologist who was not being elaborated in the collection of data and analysis of results.
Spinal puncture was performed with a spinal needle by an anesthesiologist (pencil point spinal needle G 27 Fr) between the L3-4 or L4-5 (due to fused vertebrae as shown in Table 1 for few patients), when the patient was in the left lateral decubitus, and a Crawford wedge was located under her right hip to have the left uterine displacement.
| Anesthetic – surgical parameters |
Phenylephrine
(n=28) |
Ephedrine
(n=27) |
P-Value |
| Time from blockade to skin incision (min) (Mean ± SD) |
5.46 ± 2.912 |
5.89 ± 2.439 |
0.244 |
Time from blockade to uterine incision (min)
(Mean ± SD) |
11 ± 3.432 |
12.67 ± 4.243 |
0.110 |
| Time from blockade to fetal delivery (min) (Mean ± SD) |
13.54 ± 3.469 |
15.33 ± 4.566 |
0.124 |
Time from uterine incision to fetal delivery (min)
(Mean ± SD) |
2.46 ± 1.374 |
2.67 ± 1.414 |
0.507 |
Level of the block L3-4
n (%) |
26 (92.9%) |
24 (88.9%) |
0.609 |
Level of the block L4-5
n (%) |
2 (7.1%) |
3 (11.1%) |
0.609 |
| Total dose of vassopressors (Mean ± SD) |
125.71 µg ± 35.64 |
19.81 mg ± 5.46 |
------ |
| Number of patients who required rescue dose |
20 (71.4%) |
24 (88.9%) |
0.005* |
| Number of rescue doses: |
|
|
|
| 0 |
8 (28.6%) |
3 (11.1%) |
0.033* |
| 1 |
9 (32.1%) |
6 (22.2%) |
| 2 |
10 (35.7%) |
8 (29.6%) |
| 3 |
1 (3.6%) |
9 (33.3%) |
| 4 |
0 (0.0%) |
1 (3.7%) |
| Number of rescue drug combinations |
0 (0 %) |
6 (22.2 %) |
|
| Total intravenous fluids given (20 ml/kg) |
1494.21 ± 361.570 |
1605.56 ± 246.395 |
0.337 |
| Total urine output (ml) during operation |
137.50 ± 51.379 |
124.07 ± 49.858 |
0.266 |
| Total estimated blood loss (ml) |
625 ± 143.049 |
666.67 ± 159.928 |
0.388 |
Anesthesia time (min)
From spinal block to PACU |
38.07 ± 13.379 |
42 ± 7.937 |
0.566 |
Surgical time (min)
From surgical incision to PACU |
33.25 ± 11.844 |
36.63 ± 7.088 |
0.493 |
*Significant at p<0.05 level. Data are Mean ± SD with P-values derived from Mann-Whitney U test or Frequencies and Percentages (%) with P-values
derived from Chi Square test.
Table 1 Anesthetic and surgical parameters in phenylphrine and ephedrine groups.
A solution consist of Hyperbaric Marcaine (0.5%, 2 ml and 10 mcg Fentanyl) was administered. Patients were placed in a supine position immediately after spinal block.
The progress of the block was registered, whereupon oxygen therapy administered to all patients; 6 l/min, conveyed via a face mask until delivery. Heart rate and blood pressure were recorded immediately from the time of induction of spinal anesthesia then every 3 minutes until skin closure.
The number of trials and lumbar level of the block was recorded. All patients were noticed for the block parameters of an anesthesiologist, and hemodynamic changes and complications after spinal anesthesia. Assess dermatome levels after administration of a subarachnoid block (SAB) each minute after puncture by a swap immersed in alcohol were performed. The alcohol sponge to test the level of a block dedicated by Rocco et al. [11]. Permission for surgery, received only when the level of the blockade reached
The time from blockade to the incision of the skin, the section of the uterus, and removal of the fetus recorded. The incidence of maternal hypotension, reactive hypertension, bradycardia, nausea and vomiting, and the total dose vasoconstrictor were also analyzed. Apgar at the first and fifth minutes of all newborns were steadfast and a score less than eight was considered low.
Rescue medication for hypotension
Maternal hypotension was defined as a blood pressure equal to or lower than 20% of baseline values and it was treated with a bolus of 50% of the initial dose of the vasopressor (5 mg of ephedrine for group (1); 40 μg of phenylephrine for group (2). First time (min) rescue medication drug given was noted.
Rescue medication for bradycardia
Atropine was administered in 0.5 mg stepwise on any occasion of bradycardia (heart rate <60 beats/min) was associated with a systolic pressure of less than baseline or if the heart rate was <45 beats/min regardless of arterial pressure. Requirements of atropine treatment were noted.
The incidence of maternal tachycardia and reactive hypertension
The presence of maternal tachycardia (heart rate >100 beats/ min) and reactive hypertension (increase in systolic pressure over baseline by 20% after using the vasopressor) was recorded. The number of vasopressor doses required, total dose of vasopressor administered, time of first administration of vasopressor and requirements to vasopressor administration were noted [12].
Data collection
We were engrossed in what adverse effects a patient senses and to obtain an appraisal of the pervasiveness after giving the ephedrine and phenylephrine drugs. To explore what had been reported earlier we searched on MedLine of studies reporting the ultimate side effects of ephedrine and phenylephrine. This was used as a base when progressing the data collection sheet. Data collection sheet was validated by the expert group that including, two anesthesiologists, two certified nurse anesthetist (CRNA), a post-operative nurse and a statistician. Small comments had been feedback which had been concerns about at the final version of the data collection sheet.
Vital signs (BP, Pulse, electro- cardio gram and peripheral capillary oxygen saturation, and RR) was recorded upon arrival and every 15 minutes in the PACU until discharge from the PACU and Apgor score evaluated by a pediatrician at the first minute and 5 minutes. All variables were documented (nausea, vomiting, headache, shivering, restlessness, arrhythmias, reactive hypertension, back pain, pain at the surgical incision, atropine needed, time from spinal puncture to skin incision, time to uterine incision, time from uterine incision to fetal delivery, and rescue dose of ephedrine and phenylephrine).
Data analysis plan
SPSS version 20 was used for data analysis. The results were performed only for patients enrolled in and completed the study. Descriptive statistics (frequencies, percentages) were used. The student t test, Mann-Whitney test and Chi-square test were used. A p<0.05 was contemplated significant.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki and was permitted by the institutional review board (IRB) of the university. Consent forms were attained from the patients proceeding to participation.
Results
Table 2 shows that there are no significant differences between the phenylephrine group and the ephedrine group in all general characteristics of patients exhibited in the table at the 0.05 level (the p-values>0.05).
| General characteristics |
Phenylephrine
(n=28) |
Ephedrine
(n=27) |
P-Value |
| Age (years) |
31.640 ± 3.369 |
30.48 ± 5.550 |
0.403 |
| Weight (kg) |
78.2 ± 14.38 |
80.27 ± 12.3197 |
0.613 |
| Height (cm) |
164.14 ± 7.347 |
161.70 ± 5.075 |
0.151 |
| Body mass index (kg.m2) |
28.696 ± 5.0004 |
30.978 ± 4.5249 |
0.081 |
| Gestational age (weeks ) |
38.586 ± 1.819 |
39.011 ± 1.1768 |
0.128 |
| Baseline systolic pressure (mmHg) |
123.29 ± 9.63 |
120.56 ± 9.4 |
0.255 |
| Baseline heart rate (beats/min) |
89.18 ± 10.86 |
88.37 ± 12.2 |
0.919 |
*Significant at 0.05 level. Data are Mean ± SD with P-values derived from Mann-Whitney U test or Frequencies and Percentages (%) with P-values derived from Chi Square test.
Table 2 Demographic data of the patients in both phenylephrine and ephedrine groups.
The Table 1 shows that there is a significant difference at the 0.05 level between the phenylephrine group and the ephedrine group in the number of patients who required rescue doses (phenylephrine n=20/28 (71.4%), ephedrine n=24/27 (88.9%), p-value=0.005<0.05. This indicates that the number of patients who required rescue medication in the ephedrine group is significantly more than the number of patients in the phenylephrine group; results are in favor of phenylephrine. The Table 1 shows also that there is a significant difference in the number of rescue doses between the two drugs; for the phenylephrine group there is only one patient (3.6%) that received 3 rescue doses, which is less than the expected number, and for the ephedrine group, there are 9 patients (33.3%) that received 3 rescue doses, which is more than the expected number; the p-value=0.033<0.05.
On the other hand, the Table 1 shows that there are no significant differences between the phenylephrine group and the ephedrine group in the other parameters and variables exhibited in the Table 1 at the 0.05 level (the p-values>0.05).
The Table 3 shows that there is a significant difference at the 0.05 level between the phenylephrine group and the ephedrine group in the reactive hypertension episodes variable (phenylephrine=7.7%, ephedrine=14.5%); the p-value= 0.006<0.05 (Figure 1). This means that patients in the ephedrine group have significantly more reactive hypertension than the patients in the phenylephrine group; results are in favor of phenylephrine
| Side effects |
Phenylephrine
(n=28) |
Ephedrine
(n=27) |
P-Value |
| Hypotension episodes |
53 (15.7%) |
48 (14.5%) |
0.993 |
| Hypotension patients (number) |
17 ( 60.7 %) |
18 ( 66.7 %) |
0.646 |
| Reactive hypertension patients (number ) |
15 (53.6%) |
11 (40.7%) |
0.341 |
Reactive hypertension
(episodes) |
26 (7.7%) |
48 (14.5%) |
0.005* |
| Arrhythmias |
7 (25%) |
10 (37%) |
0.334 |
| Tachycardia (episodes ) |
101 (29.9%) |
100 (30.1%) |
0.845 |
| Tachycardia patients(numbers) |
21 (75.0%) |
21 (77.8%) |
0.808 |
| Bradycardia (episodes) |
6 (1.8%) |
5 (1.5%) |
0.345 |
| Bradycardia (number) |
6 (21.4%) |
3 (11.1%) |
0.301 |
| Vomiting |
0 (0%) |
4 (14.8%) |
0.034* |
| Nausea |
3 (10.7 %) |
6 (22.2%) |
0.249 |
| Nausea and vomiting (together) |
3 (10.7%) |
10(37%) |
0.018* |
| Headache |
4 (14.3%) |
4 (14.8%) |
0.956 |
| Shivering |
3 (10.7%) |
2 (7.4%) |
0.670 |
| Restlessness |
3 (10.7%) |
8 (30.8%) |
0.068 |
| Patients needing Atropine because of bradycardia |
4 (14.3%) |
2 (7.4%) |
0.413 |
| Number of trials of spinal needle insertion of more than one time |
1.64 ± 0.826) |
1.56 ± 0.847) |
0.574 |
| Number of patients that have been stuck with spinal needle more than one time |
13 (46.4%) |
10 (37.03%) |
0.480 |
| Back pain |
0 (0%) |
0 (0%) |
------ |
| Pain at the surgical incision n(%) |
0 ) 0%) |
1 (3.7 %) |
0.304 |
*Significant at 0.05 level. Data are Mean ± SD with P-values derived from Mann-Whitney U test or Frequencies and Percentages(%) with P-values derived from Chi Square test.
Table 3 The percentage and number of episodes of side effects in phenylphrine and ephedrine groups.
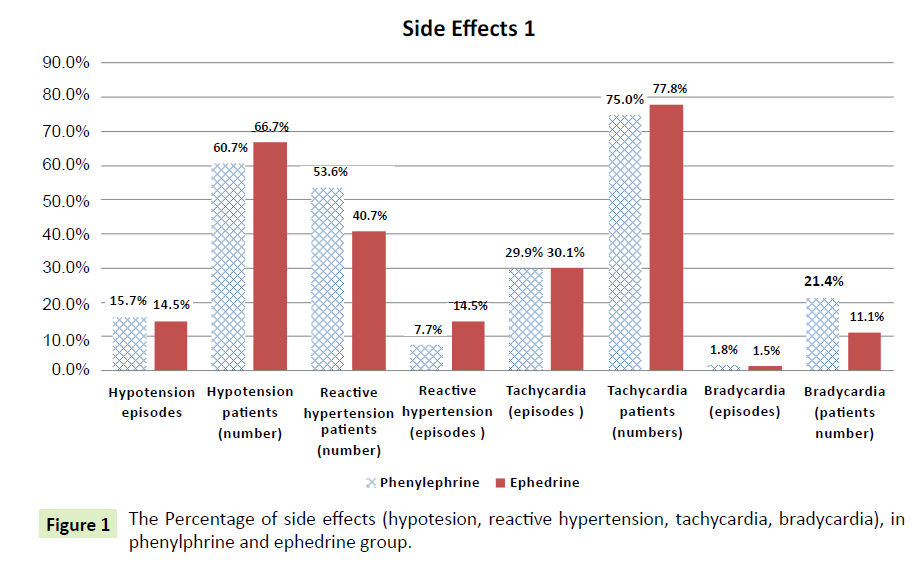
Figure 1: The Percentage of side effects (hypotesion, reactive hypertension, tachycardia, bradycardia), in phenylphrine and ephedrine group.
The Table 3 also shows that there is a significant difference at the 0.05 level between the phenylephrine group and the ephedrine group in the vomiting variable (phenylephrine=0.0%, ephedrine=14.8%); the p-value=0.034<0.05 (Figure 2). This indicates that the patients in the ephedrine group experienced significantly more vomiting than the patients in the phenylephrine group; results are in favor of phenylephrine.
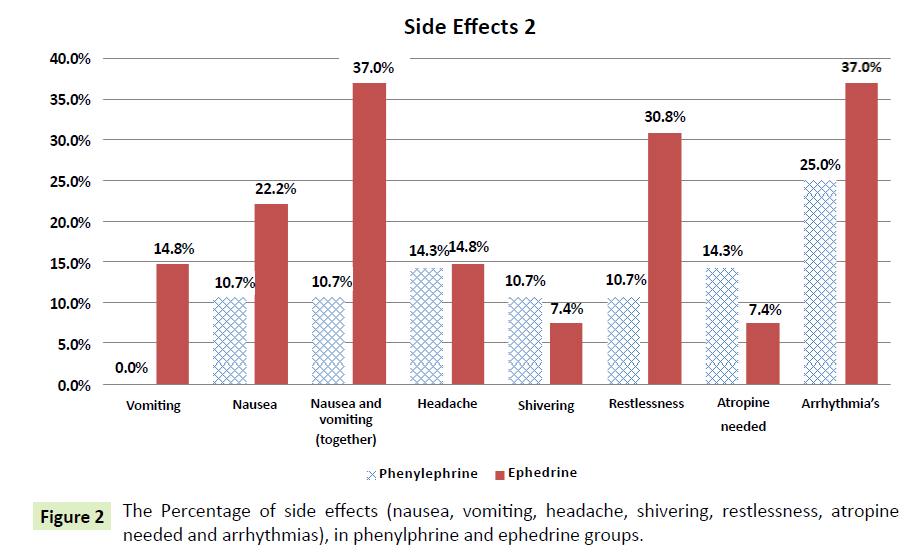
Figure 2: The Percentage of side effects (nausea, vomiting, headache, shivering, restlessness, atropine needed and arrhythmias), in phenylphrine and ephedrine groups.
The Table 3 shows that there is a significant difference at the 0.05 level between the phenylephrine group and the ephedrine group in the nausea and vomiting variable (phenylephrine=10.7%, ephedrine=37%); the p-value=0.018<0.05 (Figure 2). This illustrates that the patients in the ephedrine group had significantly more episodes of nausea and vomiting than the patients in the phenylephrine and the results are in favor of phenylephrine.
On the other hand, the Table 3 shows that there are no significant differences between the phenylephrine group and the ephedrine group in the other variables exhibited in the Table 3 at the 0.05 level (the p-values>0.05).
The percentage of side effects (hypotesion, reactive hypertension, tachycardia, and bradycardia) in phenylphrine and ephedrine groups is summarized in (Figure 1) and the percentage of side effects (nausea, vomiting, headache, shivering, restlessness, atropine needed and arrhythmias) in phenylphrine and ephedrine groups is summarized in (Figure 2).
There are no significant differences between the phenylephrine group and the ephedrine group in the two measurements of Apgar score at 1 minute and 5 minutes at the 0.05 level (the p-values are >0.05).
There is a significant difference at the 0.05 level between the phenylephrine group and the ephedrine group in the postoperative systolic blood pressure (phenylephrine mean=111.31, ephedrine mean=116.84); the p-value=0.027<0.05. On the other hand, there are no significant differences between the phenylephrine group and the ephedrine group regarding systolic blood pressure, diastolic blood pressure and heart rate changes before and after spinal anesthesia, and after administration of vasopressors.
The result shows also there is no significant difference at 0.05 level between the Phenylephrine group and the Ephedrine group in the First time (min) rescue medication drug given (Phenylephrine: Mean=15.8, Ephedrine: Mean=11.58), the P-Value=0.167>0.05.
Discussion
Techniques to control maternal blood pressure
After subarachnoid block for cesarean section, low blood pressure can be reduced by treatment of IV fluid, averting aortocaval limiting and reasonable use of vasopressors. It has been found that the decrease of placental perfusion is related to the reduction of maternal artery pressure [13]. In the present study, all patients were hydrated with 20 ml/kg of Ringer's lactate, which was launched preceding to the spinal anesthesia. However, some studies have shown inadequacy of previous hydration due to hasty redistribution [14]. However, the dehydration is made despite the fact that it has been contentious results [15]. Crystalloids and colloid are used to prevent or treat maternal hypotension in addition to vasopressors [16]. Moreover, the left uterine displacement, combined with fluid preload to prevent maternal hypotension, although vasopressors are also often necessary [10].
In the current study uterine was directed to the left to decrease aortocaval restrictive, and the blockade was perpetuated at the same level in all patients. This management is compatible with another study, which confirmed that the left uterine displacement is known to reduce the effects of aortocaval compression [15]. Despite all the conservative measures, a vasoconstrictor drugs are often required to prevent low blood pressure during anesthesia in the spinal canal [17].
Maintenance of blood pressure
In the present study, 10 mg of ephedrine and 80 μg of phenylephrine given to preserve systolic arterial blood pressure of 100 mmHg. Our study is congruent with Moran et al. [18] that gave 10 mg of ephedrine or 80 phenylephrine to maintain systolic arterial pressure of 100 mm Hg. our study is also congruent to Thomas, et al. [19]. Additionally, our results are consistent with a Prakash et al. [20] which confirmed that 100 micrograms bolus doses of phenylephrine are as effective as the 6-mg bolus doses of ephedrine for treatment of hypotension after spinal anesthesia in women undergoing caesarean section. Our results are also consistent with a systematic review of randomized controlled trials conducted by Lee et al. [9] showed that phenylephrine and ephedrine have similar effect to prevent or treat hypotension. Furthermore, our results coincide with the study of Bhardwai et al. [21] where phenylephrine, ephedrine, and metaraminol used separately to maintain maternal BP during spinal anesthesia for caesarean section. They concluded that all three vasopressors were equally effective in maintaining maternal BP without any harmful effect on maternal or fetal outcomes [22].
The current study is not consistent with the study of Magalhaes et al. [10], They concluded that ephedrine was more effective than phenylephrine in the prevention of hypotension. This may have been because a lower dose of phenylephrine was used in their study compared to this study. On the other hand, clinical trials have shown that phenylephrine may be more beneficial than ephedrine when used to prevent or treat spinal anesthesiainduced hypotension during caesarean section [23]. According to one study, phenylephrine is the preferred drug for treatment of hypotension after spinal anesthesia for elective caesarean section [24], which disagrees with our study.
Incidence of hypotension
In the current study was spinal anesthesia associated with hypotension in phenylephrine 17 (60.7%), and ephedrine 18 (66.7%) groups. The current study is consistent with study of Gunda et al. [25] showed that all patients had treatment for hypotension.
Many studies have compared the efficacy of phenylephrine and ephedrine in different doses and administration methods. A meta-analysis of four randomized clinical trials of Lee, et al. [26] showed that ephedrine could not be used as a prophylaxis against hypotension. This is because it cannot prevent hypotension in low doses and in high doses can cause high blood pressure that may be problematic [26]. In other study, the author showed that prophylactic infusion of phenylephrine was more effective than other methods of prevention of spinal anesthesia-induced hypotension [27].
In the current study found no statistical differences in systolic and diastolic blood pressure in both the ephedrine and phenylephrine groups. This conclusion is consistent with part of the study by Brooker et al. [28] compared the effects of phenylephrine and ephedrine to maintain blood pressure in caesarean section after spinal anesthesia. Their results showed that both systolic and diastolic pressure was maintained well, but the diastolic pressure was better maintained with phenylephrine than with ephedrine.
Incidence of bradycardia
In the current study, 6 (21.4%) women who received phenylephrine and 3 (11.1%) who received ephedrine developed bradycardia. This difference was not statistically significant. Our study is not consistent with Magalhaes, et al. [10] reported comparable number of bradycardia with ephedrine and phenylephrine. Our results are similar to that of a study by Thomas et al. [19] of women receiving phenylephrine who were more likely to develop bradycardia than those treated with ephedrine [19].
Our results are not compatible with other studies that found that phenylephrine causes significant reduction in heart rate after bolus [29]. Moreover, our results are not consistent with the results of the study by Lee et al. [9], in which they reported significantly higher incidence of bradycardia in patients receiving phenylephrine compared to patients who received ephedrine to prevent hypotension during spinal anesthesia for cesarean section.
In another study of concern performed by Nazir et al. [12], it was found that maternal bradycardia were more frequent with phenylephrine than with ephedrine. The authors explained that this can be expected due to an increase in blood pressure, where α agonist can lead to reactive bradycardia. This result is in line with our findings that 6 (21%) patients developed bradycardia in phenylephrine group and treated with atropine. The incidence of isolated phenylephrine related maternal bradycardia (heart rate ≤ 60 beats per minute) was highest (58%) in one study at doses of phenylephrine were used [19]. The authors suggested that maternal bradycardia contributed to the cardiac sympathetic denervation because the sensory block was high. Therefore, ephedrine, phenylephrine combination prevent maternal bradycardia, as the onomatopoeic effect of ephedrine would embarrassed this mechanism.
Incidence of tachycardia
In the current study, 21 (75.0%) patients in phenylephrine group and 21 (77.8%) of patients in the ephedrine group developed tachycardia. Our study is discordant with other study conducted by Gunda et al. [25] suggested that the incidence of tachycardia was significantly higher in ephedrine groups.
Incidence of reactive hypertension
This study shows that there is a significant between phenylephrine (7.7%) and ephedrine (14.5%) groups in the reactive hypertension episodes p=0.006. This means that patients in the ephedrine group have significantly more reactive hypertension episodes than patients in phenylephrine group. Our results are not in agreement with the study of Loughery et al. [30], who found no cases of rebound hypertension with ephedrine. However Magalhaes et al. [10] reported comparable number of reactive hypertension with ephedrine and phenylephrine.
Previous studies have shown that a bolus of 30 mg intravenous ephedrine would be more effective in the prevention of hypotension, but with an increased incidence of reactive hypertension [31]. In contrast, a prospective observational study of the intravenous administration of 15 to 20 mg of ephedrine reduced the incidence of maternal hypotension without increasing the incidence of reactive hypertension [32].
A meta-analysis by Lee et al. [33] concluded that doses above 14 mg ephedrine does not reduce the incidence of maternal hypotension, but they caused reactive hypertension in the mother and a small reduction in cord blood PH.
In the study of Magalhaes et al. [10], where a dose of 10 mg of ephedrine considered to be effective and at the same time had some side effects that do not confirm to our study, a dose of 10 mg of ephedrine caused the 11 (40.7%) patients develop reactive hypertension. On the other hand, even for patients who were administered 80 μg of phenylephrine, 15 (53.6%) patients developed reactive hypertension, but the difference was not significant. However, Lougher et al. (2002) found no cases of rebound hypertension with ephedrine, this finding is not consistent with our study.
Incidence of nausea and vomiting
This study shows that there is a significant difference between groups phenylephrine and ephedrine in vomiting (phenylephrine=0.0%, ephedrine 4 (14.8%); p=0.034. This suggests that the patients in the ephedrine group had significantly more vomiting than patients in the phenylephrine group. Moreover, the present study shows that there is a significant difference between the group of phenylephrine and ephedrine group of nausea and vomiting together (phenylephrine 3(10.7%, ephedrine 10(37%); p=0.018. This illustrates that the patients in the ephedrine group has significantly more nausea and vomiting than patients in phenylephrine group. Our results are consistent with a number of studies indicate that significantly higher incidence of nausea/vomiting with ephedrine use [22,25]. Yet Magalhaes et al. [10] reported a higher incidence of nausea/ vomiting in patient receiving phenylephrine compared to those who received ephedrine. They suggest that in all cases, administration of a second dose of vasopressin resulted in the occurrence of nausea and/or vomiting.
Rescue medication
In the current study, there is significant between phenylephrine and ephedrine groups in the number of patients who required rescue doses (phenylephrine n=20/28 (71.4%), ephedrine n=24/27 (88.9%), p-value=0.005. This indicates that the number of patients who required rescue medication in the ephedrine group was significantly more than the number of patients in phenylephrine group.
This study also shows that there is a significant difference in the number of rescue doses of the two drugs; for phenylephrine group, there was only one patient (3.6%) who received three doses of rescue, which is less than the expected number, and for the ephedrine group were nine patients (33.3%) who received three doses, more than the expected number; p-value=0.033.
Apgar score
The current study shows that there are no significant differences between group phenylephrine and ephedrine group in the two measurements of the Apgar score at one minute and 5 minutes. It does not seem to have any adverse neonatal effects in healthy fetuses. This study is in line with Moran et al. [4] study which concluded that there were no adverse neonatal effects in healthy fetuses. Our results also coincides with the study of Prakash et al. [20] who demonstrated that Apgar score at 1 and 5 minutes was similar between the two groups of phenylephrine and ephedrine.
One study showed that even high doses of phenylephrine (over 2000 micrograms) were not associated with adverse effects on the fetus, as determined by the Apgar score [34]. In the current study, the dose was 80 micrograms of phenylephrine chosen based on a previous study that showed that this was the effective dose when administered as an intravenous bolus, without side effects on the fetus. Our findings are identical to that of a study by Lee et al. [9].
Evaluation of the first and fifth minute Apgar score values showed that the 5th Apgar scores were better in phenylephrine and ephedrine groups than the control group in a study of Moslemi et al. [5]. According to many studies, neonatal outcome is not affected by the prophylactic use of phenylephrine or ephedrine, and in some, the neonatal condition maintained well with prophylactic vasopressors [12].
Conclusion
We conclude from this study that phenylephrine 80 μg had similar vasopressor effect of ephedrine 10 mg for the prevention or treatment of maternal hypotension during spinal anesthesia for elective caesarean section and there was no difference in neonatal clinical outcomes as measured by Apgar score. The clinical significance of bradycardia, reactive hypertension and intraoperative nausea and vomiting should not be neglected. Phenylephrine administration before spinal anesthesia is superior to ephedrine to reduce reactive hypertension, nausea, vomiting, and the need for vasopressors rescue medication.
Relevance to Clinical Practice
Considering maternal complications, the most noticeable complication was short bradycardia (reflex bradycardia), who needed treatment with 0.5 mg of intravenous atropine. Nausea and vomiting that responded quickly to antiemetic medication was somewhat high in the ephedrine group.
18143
References
- Rout CC, Rocke DA (1994) Prevention of hypotension following spinal anesthesia for cesarean section. Int Anesthesiol Clin 32: 117-135.
- Riley ET, Cohen SE, Rubenstein AJ, Flanagan B (1995) Prevention of hypotension after spinal anaesthesia for caesarean section: 6% hetastarch versus lactated Ringer’s solution. Anesth Analg 81: 838-842.
- Ralston DH, Shnider SM, DeLorimier AA (1974) Effects of equipotent ephedrine, metaraminol, mephentermine, and methoxamine on uterine blood flow in the pregnant ewe. Anesthesiology 40: 354-370.
- Moran DH, penillo M (1991) A comparative study of phenylephrine and ephedrine. J Clin Anesthesia 3: 301-305.
- Moslemi F, Rasooli S (2015) Comparison of Prophylactic Infusion of Phenylephrine with Ephedrine for Prevention of Hypotension in Elective Cesarean Section under Spinal Anesthesia: A Randomized Clinical Trial. Iran J Med Sci 40: 19-26.
- Aragão FF, Aragão PW, Martins CAS, Filho NS, Barroqueiro ESB (2014) Comparison of metaraminol, phenylephrine and ephedrine in prophylaxis and treatment of hypotension in cesarean section under spinal anesthesia. Rev Bras Anestesiol: 64: 299-306.
- Kunter MA, Nachtsheim CJ (2005) Applied linear statistical model. 5th edition, USA, MCgraw.
- Neves JFP, Monteiro GA, Almeida JR (2010) UtilizaUtilizac,¸ãodafenilefrinapara controle da pressão arterial emcesarianasele-tivas: dose terapêutica versus profilática. Rev Bras Anestesiol 60: 391-398.
- Lee A, Warwick D, Kee N, Gin T (2002) Trails of ephedrine versus phenylephrine for the management of hypotension during spinal anaesthesia for caesarean section. Anaesth Analg 94: 920-926.
- Magalhaes E, Goveia CS, Ladeira L, Nascimento B, Kluthcouski S (2009) Ephedrine versus phenylephrine: prevention of hypotension during spinal block for Cesarean section and effects on the fetus. Rev Bras Anestesiol 59: 11-20.
- Rocco AG, Raymond SA, Murray E (1985) Differential spread of blockade of touch, cold and pinprick during spinal anesthesia. Anesth Analg 64: 917-923.
- Nazir I, Bhat MA, Qazi S, Buchh VN, Gurcoo SA (2012) Comparison between phenylephrine and ephedrine in preventing hypotension during spinal anesthesia for cesarean section. J Obstet Anaesth Crit Care 2: 92-97.
- Corke BC, Dutta S, Ostheiner GW, Weiss JB, Alper MH (1982) Spinal anaesthesia for caesarean section. The influenceof hypotension on neonatal outcome. Anaesthesia 37: 658-662.
- Ueyama H, He YL, Tanigami H, Mashimo T, Yoshiya I (1999) Effects of crystalloid and colloid preload on blood volume in the parturient undergoing spinal anesthesia for elective cesarean section. Anesthesiology 91: 1571-1576.
- Kinsella SM (2003) Lateral tilt for pregnant women. Why 15 degrees? Anaesthesia 58: 835-836.
- Olang PO (2010) Effects of spinal anesthesia during elective Cesarean section on neonatal outcome at the Kenyatta national hospital.
- Erler I, Gogarten W (2007) Prevention and treatment of hypotension during caesarean delivery. Anasthesiol Intensivemed Notfallmed Schmerzther 42: 208-213.
- Moran DH, Dutta S, Perillo M, Laporta RF, Bader A (1991) Phenylephrine in the prevention of hypotension following spinal anaesthesia for caesarean delivery. J Clin Anaesth 3: 301-305.
- Thomas DG, Robson SC, Redfern N, Hughes D, Boys RJ (1996) Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anesthesia for Caesarean section. Br J Anaesth 76: 61-65.
- Prakash S, Pramanik V, Chellani H, Salhan S, Gogia AR (2010) Maternal and neonatal effects of bolus administration of ephedrine and phenylephrine during spinal anaesthesia for caesarean delivery: a randomized study. Int J Obstet Anesthesia 19: 24-30.
- 21 Bhardwai N, Jain K, Arora S, Bharti N (2013) A comparison of three vasopressoes for tight control of maternal blood pressure during spinal anesthesia: Effect on maternal and fetal outcome. J Anaesthesiol Clin Pharmacol 29: 26-31.
- Macarthur A, Riley ET (2007) Obstetric anesthesia controversies: vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin 45: 115-32.
- NganKee WD, Khaw KS (2006) Vasopressors in obstetrics: what should we be using? Curr Opin Anaesthesiol 19: 238-243.
- Veeser M, Hofmann T, Roth LR, Klöhr S, Rossaint R, et al. (2012) Vasopressors for the management of hypotension after spinal anesthesia for elective caesarean section. Systematic review and cumulative meta-analysis. Acta Anaesthesiol Scand 56: 810-816.
- Gunda CP, Malinowski J, Tegginmath A, Venkatesh G, Suryanarayana VG, et al. (2010) Vassopresor choice for hypotension in elective Cesarean section: Ephedrine or Phenylephrine. Arch Med Sci 6: 257-263.
- Lee A, Ngan K, Werwidk D, Gin T (2004) A dose-response meta-analysis of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for elective cesarean delivery. Anesth Analg 98: 483-490.
- NganKee WD, Khaw KS, Ng FF, Lee BB (2004) Prophylactic phenylephrine infusion for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg 98: 815-821.
- Brooker RF, Butterworth JF, Kitzman DW, Berman JM, Kashtan HI, et al. (1997) Treatment of hypotension after hyperbaric tetracaine spinal anesthesia: A randomized, double-blind, cross-over comparison of phenylephrine and epinephrine. Anesthesiology 86: 797-805.
- Sahu D, Kothari D, Menrotra A (2003) Comparison of bolus phenylephrine, ephedrine and mephentermine for maintenance of arterial pressure during spinal anaesthesia in caesarean section. A clinical study. Indian J Anaesth 47: 125-128.
- Loughrey JP, Walsh F, Gardiner J (2002) Prophylactic intravenous bolus ephedrine for elective Caesarean section under spinal anaesthesia. Eur J Anaesthesiol 19: 63-68.
- NganKee WD, Khaw KS, Lee BB, Lau TK, Gin T (2000) A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg 90: 1390-1395.
- Simon L, Provenchère S, de Saint Blanquat L, Boulay G, Hamza J (2001) Dose of prophylactic intravenous ephedrine during spinal anesthesia for cesarean section. J Clin Anesth 13: 366-369.
- Lee A, NganKee WD, Gin T (2003) A Dose-Response Meta-Analysis of Prophylactic Intravenous Ephedrine for the Prevention of Hypotension during Spinal Anesthesia for Elective Cesarean Delivery. Anesth Analg 98: 483-439.
- Emmett RS, Cyna AM, Andrew M, Middleton P, Simmons SW (2002) Techniques for preventing hypotension during spinal anesthesia for caesarean section. Cochrane Database Syst Rev 18: CD002251.







