Keywords
Hemorrhoidal disease; Visual analog scale; Bleeding; Medical device made of substances
Abbreviations
ADEs: Adverse Device Effects
AEs: Adverse Events
FAS: Full Analysis Set
HD: Hemorrhoidal Disease
IP: Investigational Product
LOCF: Last Observation Carried Forward
LS: Least Squares
MDMS: Medical Device Made of Substances
PP: Per Protocol
Qol: Quality of Life
SAEs: Serious Adverse Events
SAF: Safety Population
VAS: Visual Analog Scale
Introduction
HD is defined as the distal displacement of enlarged, congested anal cushions. It is a widespread and frequent pathology, though the true epidemiology of symptomatic disease is unknown as many people with symptoms do not seek medical care [1]. Disease pathogenesis and progression is undoubtedly multifactorial involving mechanical, vascular and inflammatory factors, which influence the presence and intensity of clinical manifestations. However how these factors are connected and the extent of their contribution in every single patient who develops the disease is still unclear [2-6]. In internal HD, distal displacement of anal cushions and the maintenance of their usual size depend, respectively, on the integrity and quality of fixation structures (i.e., the connective tissue surrounding hemorrhoidal sinusoids and the Treitz’s muscle) and on the balance between arterial perfusion and venous return from sinusoids [7,8]. Venous return may be limited by conditions that chronically raise intraabdominal pressure such as obesity, excessive time spent on the toilet and straining with defecation. Particularly in this latter case, the shear stress produced on hemorrhoidal tissues by descending stools, together with changes thought to be linked to aging, may lead to degeneration and dysfunction of the fixation structures, whose impairment may be further promoted by inflammatory events eventually induced by repeated elongation of tissues. While no classification of external hemorrhoids is used clinically, internal hemorrhoids are graded according to Goligher’s classification. The classification is based on the presence of prolapse outside of the anal canal during defecation and whether it may be reduced. Grade I hemorrhoids do not prolapse; grade II: prolapse and spontaneously reduce; grade III: prolapse and must be manually reduced; grade IV: hemorrhoids cannot be reduced [9]. Clinical manifestations of HD include bleeding, discomfort, anal leakage and symptoms of perianal irritation (i.e. itching and burning). These manifestations seem to occur after hemorrhoidal nodules have begun their sliding down process [10,11]. Current management of grade I and II HD is based on dietary and lifestyle modification combined with medical therapy or on interventional non-surgical procedures [3,4,12]. The topical medications available usually contain either a combination of local anesthetics with antiâ?ÂÂÂinflammatories or muscle relaxants [13], thus addressing a single mechanism of those that underlie signs and symptoms onset. Neofitoroid (Aboca S.p.a. – Sansepolcro, Arezzo – Italy) is a 100% natural class IIb Medical Device Made of Substances (MDMS) for topical treatment of HD. It is a product made of substances of natural origin in which the patented functional complex Helydol® (i.e., dry extract of Helichrysum lipophilic fraction) is combined together with a pool of functional molecules (i.e., extract of Ruscus, dry extract of Aloe Vera gel, aqueous solution of Helichrysum, oil of Hypericum, Shea butter, Jojoba oil, essential oils of Melaleuca, Peppermint and Cypres). The formulation has the required features thanks to all its components andadheres to the epithelial surface and establishes a physical protective barrier. The adhered formulation exerts a lubricating action which facilitates the transit of feces and reduces their rubbing on the mucosa. This broad physical and mechanical protection is complemented by an indirect antiinflammatory action dependent on the antioxidant properties of the system of molecules.
This was a prospective study aimed to evaluate the effect of NeoFitoroid ointment in reducing symptoms and signs of HD and the tolerability of treatment.
Materials and Methods
Ethical Procedures
The study protocol was approved by the competent Ethical Committee and was conducted in accordance with the principles of the Declaration of Helsinki. Written Informed Consent for the study was obtained at screening/baseline visit from all enrolled patients.
Study design, participants and intervention
This was a post-market clinical follow-up, prospective, single arm study to evaluate the effect of a CE marked class IIb MDMS in reducing symptoms and signs of HD, and the tolerability of treatment. Patients underwent an initial visit (visit 1–day 0) to verify selection criteria and to collect pre-treatment data. During treatment they received two phone contacts at days 3 and 7, followed by a post-treatment visit (visit 2–day 11) and a final telephone follow-up contact 30 days post-treatment initiation. During treatment patients were requested to fill in a detailed diary and record the evolution of their HD symptoms and signs. A quality of life questionnaire (Qol; EQ-5D-5L) was filled in before and after treatment.
Inclusion criteria were the following: patients of both sexes aged over 18 years, with non-thrombosed HD of grade I or II according to Goligher’s classification (diagnosed by anoscopy or rectoscopy at screening), not expected to require interventional or surgical treatment in the 31 days post enrollment, and with VAS score for HD-related discomfort ≥ 30 at baseline. Exclusion criteria included the presence of gastrointestinal disease or cancer or other acute or chronic medical conditions that could interfere with the interpretation of study results; mycosis or virologic disease with anal or perianal localization; concomitant abuse of drugs or alcohol; presence of conditions putting at risk compliance to study protocol; use of other topical preparations for treatment of HD in the 14 days preceding enrollment.
Patients were invited to follow their usual habits in hygiene and diet and had to apply the MDMS (tube containing 40 ml with endorectal applicator) twice a day for 10 days (in the morning and before going to sleep). In addition, they had to apply the product before and after each defecation. Patients were allowed to continue therapy for any pre-existing disease whereas any interventional or surgical treatment for HD or any topical treatment for HD other than the MDMS was not permitted and caused subject withdrawal from the study.
Study endpoints
The primary endpoint of the study was the reduction of discomfort, measured through a 0-100 VAS (from “no symptoms” to “overwhelming symptoms”) after 10 days of treatment compared to baseline.
Secondary endpoints included the following: reduction of discomfort at 3 and 7 days of treatment compared to baseline; reduction of pain, itching and burning symptoms measured through a 0-100 VAS at 3, 7 and 10 days of treatment compared to baseline; reduction of bleeding and anal leakage at 3, 7 and 10 days of treatment compared to baseline as measured by a 6-point scale (never, rarely, sometimes, often, very often, always); presence of prolapse after treatment compared to baseline; QoL index improvement from baseline to day 10. The evaluation of safety of study product was made by registering Adverse Events (AEs), Serious Adverse Events (SAEs) and Adverse Device Effects (ADEs).
Sample size calculation
Thirty-seven subjects achieve 90% power in detecting the expected mean difference of 2.2 (SD=4) on the primary endpoint (= difference in VAS score of discomfort at day 10 versus baseline), with alpha=0.05. Considering an estimate of 20% dropout rate, 45 subjects were recruited.
Statistical analysis
The following populations were considered for analysis. Safety (SAF) population: all subjects enrolled in the study who received at least one dose of the Investigational Product (IP). Full Analysis Set (FAS) population: all subjects from the safety set fulfilling the inclusion/exclusion criteria who successfully performed day 3 data collection. Per Protocol (PP) population: all subjects complying with the clinical investigation plan delivering a complete data set of measurements and evaluations regarding the primary endpoint.
In the analysis of the primary endpoint in the FAS population, missing data were replaced by “Last Observation Carried Forward” (LOCF) method.
In the analysis of the secondary endpoints, Poisson mixed model was used to evaluate variation in symptoms (VAS). Least Squares (LS) means derived from the model, their CI 95% and p value of comparisons between each time point and baseline were calculated. Bleeding and anal leakage were recoded as positive and negative in two different variables derived from their frequency as follows: Grade1: Negative: never; Positive: all others categories. Grade2: Negative: never, rarely, sometimes; Positive: all others categories. Frequencies of positive patients in each grade were compared by Logistic Mixed model. Mixed models were used to take in account repeated data for the same subject. Missing values in quantitative data analyzing by Mixed model were imputed by statistical interpolation. No imputation was expected for objective clinical signs but to evaluate bleeding, anal leakage and presence of prolapse in FAS population missing data where either replaced by LOCF method or imputed as positive (worst case). Subject Qol was a secondary efficacy outcome that was not repeatedly evaluated during treatment: instead, its change from baseline to day 10 was assessed. QoL data had missing data also in PP population but no imputation was made and the analysis population was represented by patients (N=32) who delivered both baseline and V2 QoL data. SAS 9.4 software was used for all analysis. A p value<0.05 was considered statistically significant.
Results
This prospective study was carried out in one Italian centre (Azienda Ospedaliero - Universitaria Bologna, Policlinico Sant’Orsola - Malpighi) where enrollment was conducted over an 8-month period.
Participants at baseline and analysis populations
Forty-five patients were enrolled in the study. Both SAF and FAS populations consisted of 42 patients. Three patients withdrew consent/discontinued participation without receiving any dose of IP, while 38 patients were included in the PP population. Demographic and clinical characteristics of the study population at baseline are summarized in Table 1. In almost all patients (93%) the anoscopy was the diagnostic procedure performed and 64% of patients were affected by Grade II haemorrhoids. Prolapse was evident in 22% of patients.
| |
Summary statistics |
Total (n=45) |
| Male |
% (n/Pts) |
73.3% (33/45) |
| Age |
Mean (± SD) |
50.5 (± 15.2) |
| |
Median (IQR) |
49.0 ( 36- 64) |
| |
Min-Max |
29 - 79 |
| Procedure |
| Anoscopy |
% (n/Pts) |
93.3% (42/45) |
| Rectoscopy |
% (n/Pts) |
6.7% (3/45) |
| Hemorrhoidal disease grade |
| Grade I |
% (n/Pts) |
35.6% (16/45) |
| Grade II |
% (n/Pts) |
64.4% (29/45) |
| Prolapse |
% (n/Pts) |
22.2% (10/45) |
| Concomitant medications |
| Blood pressure medications |
% (n/Pts) |
33.3% (15/45) |
| Lipid-lowering medications |
% (n/Pts) |
13.3% (6/45) |
| Mucosal protective compounds, antacid and anti-secretory drugs |
% (n/Pts) |
24.4% (11/45) |
| Aspirin |
% (n/Pts) |
11.1% (5/45) |
| Other nonsteroidal anti-inflammatory drugs |
% (n/Pts) |
8.8 % (4/45) |
| Anti-diabetic drugs |
% (n/Pts) |
11.1% (5/45) |
| Antibiotics |
% (n/Pts) |
4.4% (2/45) |
| Pain/fever reducer drugs |
% (n/Pts) |
6.7% (3/45) |
| Antidepressants/anxiety medications |
% (n/Pts) |
4.4% (2/45) |
| Alpha-blockers |
% (n/Pts) |
6.7% (3/45) |
| Anti-cancer drugs/ angiogenesis inhibitors |
% (n/Pts) |
4.4% (2/45) |
| Others (a different one per each patient) |
% (n/Pts) |
31.1% (14/45) |
Table 1: Demographic and Clinical characteristics of the study population at baseline.
Primary endpoint
The primary endpoint was the variation of discomfort between day 10 and baseline. In FAS population, data showed a significant reduction (p<0.001) of discomfort after 10 days of treatment when compared to baseline, irrespective of the non-parametric test used to test the differences (Figures 1 and 2). The average VAS score for discomfort decreased from 47.4 (SD 19.7) before treatment to 15.4 (SD 18.2) after treatment, showing a mean difference from baseline of -32.0 (SD 22.8) points, while the median decrease from baseline was of 30 (IQR 40; 15) points.
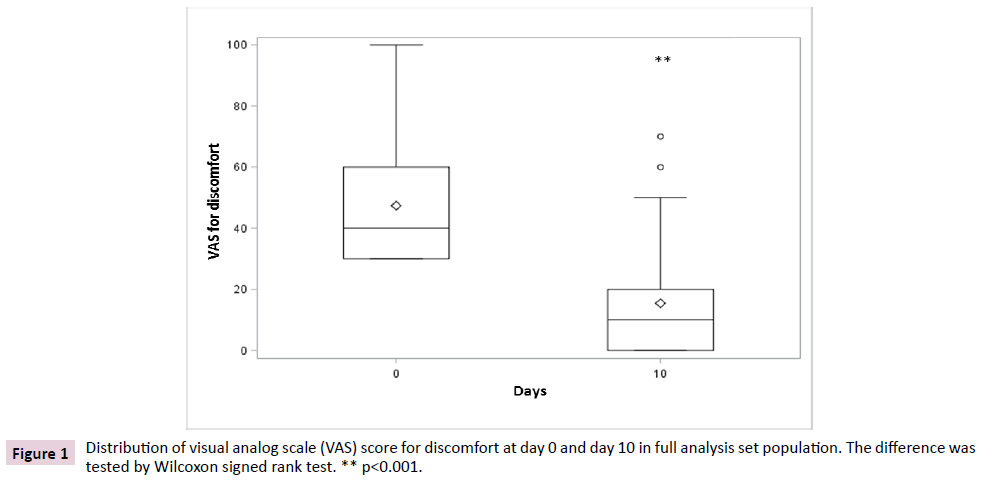
Figure 1: Distribution of visual analog scale (VAS) score for discomfort at day 0 and day 10 in full analysis set population. The difference was tested by Wilcoxon signed rank test. ** p<0.001.
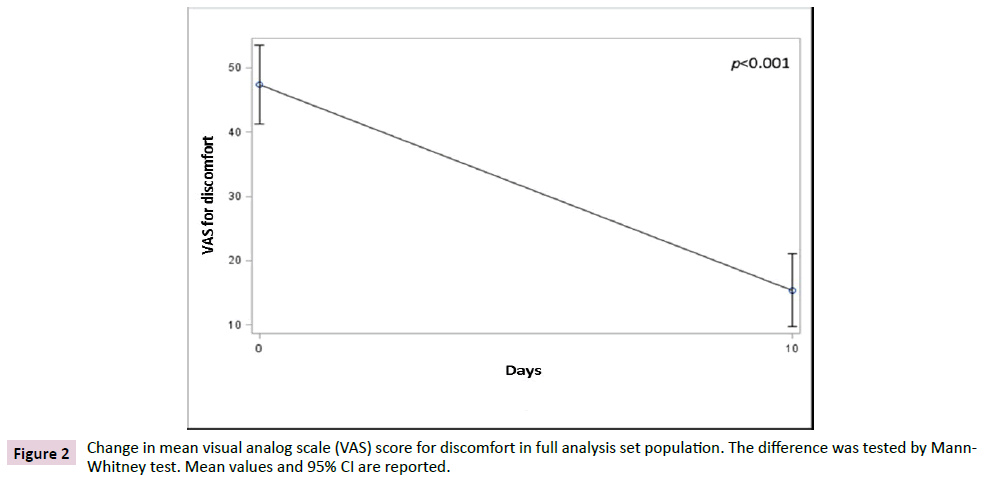
Figure 2: Change in mean visual analog scale (VAS) score for discomfort in full analysis set population. The difference was tested by Mann- Whitney test. Mean values and 95% CI are reported.
Patients belonging to the PP population experienced a similar significant reduction of discomfort, mean ( ± SD) going from 46.4 ( ± 19.1) to 13.9 ( ± 17.1) on day 10 (p<0.001), thus confirming the efficacy of the MDMS in reducing discomfort associated to HD
Secondary endpoints
Marginal mean of discomfort, pain, itching and burning at 0, 3, 7 and 10 days in FAS population are shown in Figure 3. Discomfort was the most disturbing symptom suffered by study subjects and significantly decreased (p<0.001) at each time point when compared to baseline in both the FAs and the PP populations (data not shown). Pain, itching and burning decreased in a similar way as discomfort, showing the same highly significant reduction (p<0.001) in both FAS and PP population. Results were obtained, as early as 3 days after treatment and were confirmed at later time points. Discomfort and pain had the most substantial relative decrement in the first days of treatment, showing a reduction by more than 40% and by nearly 60%, respectively, since day 3.
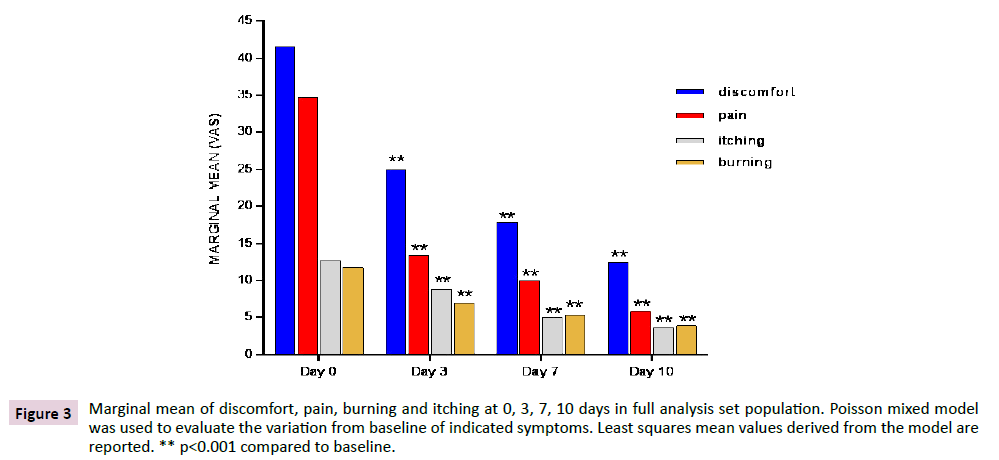
Figure 3: Marginal mean of discomfort, pain, burning and itching at 0, 3, 7, 10 days in full analysis set population. Poisson mixed model was used to evaluate the variation from baseline of indicated symptoms. Least squares mean values derived from the model are reported. ** p<0.001 compared to baseline.
Figure 4 shows the variation of proportions of patients in PP population with bleeding and anal leakage different from “Never” (i.e. patients positive in grade 1 of bleeding and anal leakage) from baseline to the end of treatment. Data showed a statistically significant reduction of patients positive for bleeding (p<0.001) and for anal leakage (p<0.05) at each time point compared to baseline.
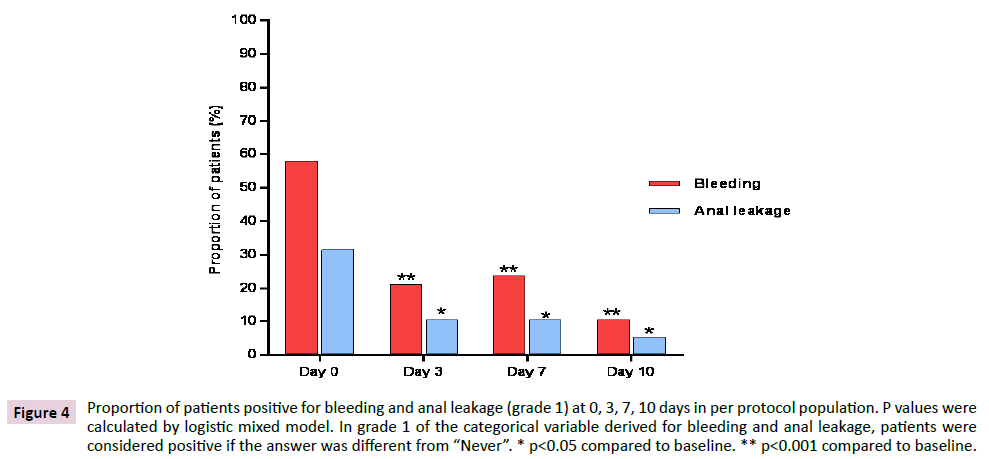
Figure 4: Proportion of patients positive for bleeding and anal leakage (grade 1) at 0, 3, 7, 10 days in per protocol population. P values were calculated by logistic mixed model. In grade 1 of the categorical variable derived for bleeding and anal leakage, patients were considered positive if the answer was different from “Never”. * p<0.05 compared to baseline. ** p<0.001 compared to baseline.
The total number of patients who were positive in grade 1 bleeding at 0, 3, 7 and 10 days were 22, 8, 9 and 4 respectively. Out of 22 patients who were positive at baseline, 8 were still positive at day 3, 7 were still positive at day 7 and 3 were still positive at day 10. As for anal leakage, the total number of patients who were positive in grade 1 anal leakage at 0, 3, 7 and 10 days were 14, 4, 4 and 2 respectively. Out of 14 patients who were positive at baseline, 2 were still positive at day 3, 1 was still positive at day 7 and no one was positive on day 10. In FAS population a similar evolution was observed, with significant reductions (p<0.001) at each time point for bleeding and reductions which were significant or tended to significance (p=0.055) for anal leakage, irrespective of the method applied for imputation of missing data (Figure 5).
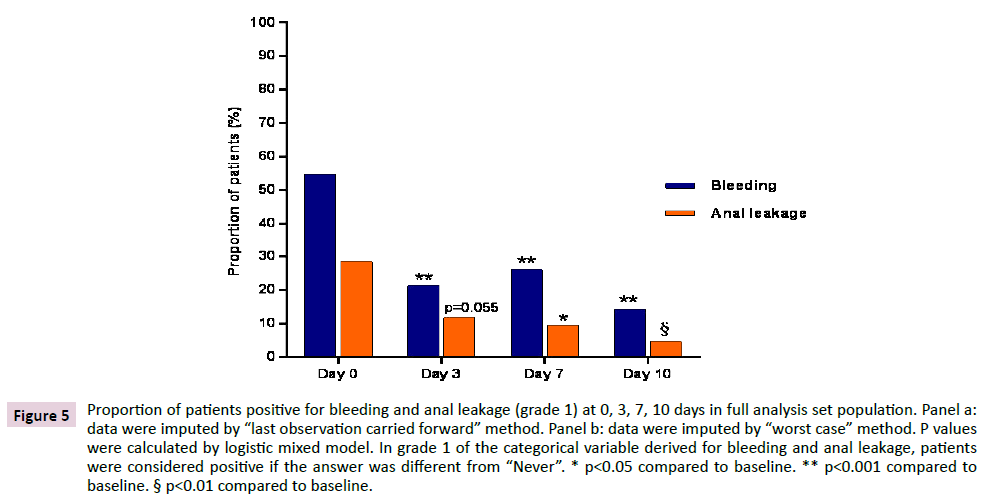
Figure 5: Proportion of patients positive for bleeding and anal leakage (grade 1) at 0, 3, 7, 10 days in full analysis set population. Panel a: data were imputed by “last observation carried forward” method. Panel b: data were imputed by “worst case” method. P values were calculated by logistic mixed model. In grade 1 of the categorical variable derived for bleeding and anal leakage, patients were considered positive if the answer was different from “Never”. * p<0.05 compared to baseline. ** p<0.001 compared to baseline. § p<0.01 compared to baseline.
Regarding grade 2 bleeding and anal leakage, data (PP population; data not shown) showed a reduction of positive patients (i.e. patients with bleeding or with anal leakage different from “Never, rarely, sometimes”) over the entire treatment period. Differences were not always statistically significant, probably due to the low number of observations. The reduction of patients positive in grade 2 bleeding was statistically significant at day 3 (p<0.05) and at day 7 (p<0.001), but not at the latest time point. As for anal leakage, only 2 patients were positive in grade 2 leakage at baseline and, despite no one was positive at either day 3, 7 or day 10, the difference was not statistically significant.
Presence of prolapse and subject Qol were outcomes that were not repeatedly evaluated during treatment: instead, their change from baseline was assessed at the end of treatment (data not shown).
At V1, 10 patients presented prolapse, and in 6 of them (60%) no prolapse was evident at V2. However no significant change was found between V1 and V2 when frequencies calculated on the total count of patients of analysis population were compared (PP population; Mc Nemar test).
The distribution of Qol levels regarding each of the 5 items explored by QoL questionnaire were compared in a population (N=32) composed by patients who delivered both baseline and V2 QoL data (data not shown). The number of patients with good QoL level (i.e, level 1) at baseline was very high in all items except for the item “pain discomfort” where patients belonging to level 1 increased from 53.1% before treatment to 68.8% after treatment (p<0.05).
Regarding AEs experienced by patients, six patients had one AE each (14.3%, CI 95% 5.4%-28.5), which in all cases were considered as not related to the use of the medical device.
Discussion
The present study provides evidence of the ability of the study product to significantly alleviate and reduce the frequency of many different clinical manifestations of HD. This clinical achievement reflects the product’s ability to directly and indirectly counteract the different pathophysiological factors/ events leading to symptom onset [2-6].
Distal displacement of enlarged hemorrhoids and the reduction of venous return from hemorrhoidal sinusoids are core pathological features of HD, almost always present even when the disease is not yet symptomatic [2]. Bleeding is a very common sign of internal disease, reported by many authors as the most common clinical manifestation. Consistent with this, more than 50% of studied patients resulted positive in grade 1 bleeding at baseline, thus reporting at least one episode of bleeding in the days immediately prior to enrollment. Bleeding is usually associated with bowel movements and is described by patients as brightred blood drips into the toilet bowl [14]. It is due to prolapsed hemorrhoidal nodules exposure to shear stress due to descending stools, particularly when stools are hard, and straining occurs with defecation. Consequently, sub-epithelial arterial vessels may become injured and prone to erosion during defecation with resultant hemorrhage [15]. Blood vessels’ vulnerability to erosion may be further promoted by inflammatory phenomena due to repeated mechanical stress of tissues. In this context, the MDMS allows to create an adherent layer on the surface of the epithelium, offering different levels of protection. A first level of protection comes from the lubricating and moistening features of the product, which facilitate the transit of feces and reduce the friction on the mucosa, while maintaining the mucosa and the perianal skin hydrated [16-18]. Furthermore, the radical scavenger properties of the device indirectly counteract the inflammatory process, which is commonly accompanied and sustained by excessive oxidative stress [19-21].
The protection emerging from adhesion of such a multivalent film to the mucosa and in the perianal area allows a safe and effective use of the product also in presence of lesions such as anal fissures. Lesions of anal mucosa are a common cause of anal pain in HD patients [22] who are expected to benefit from a topical treatment limiting repeated direct physical traumatisms, inflammatory stimuli and preventing microbial and fecal contamination of injured tissue. These properties are concomitantly conveyed to the product by the complex nature of its composition and, although an assessment of the presence and evolution of anal lesions was beyond the scopes of this study, lesion management may have been at the base of pain reduction in some patients.
Anal leakage is mainly responsible for the itching and burning associated to internal HD and it is recognized to be primarily dependent on mechanical factors. The internal hemorrhoidal vascular plexus is covered by a transitional, mucus-producing epithelium [23]. The downward displaced mucosa of hemorrhoidal nodules, therefore, permits a greater amount of mucus to be transferred outside the anus verge and to get in contact with the perianal skin, causing perinanal irritation, itching and burning. Furthermore, skin irritation may be the consequence of fecal soiling favored by the sliding down of nodules [24].
The protective barrier realized by the IP limits the occurrence of skin irritation by preventing the skin to come in contact with mucus and feces. Moreover, since inflammation enhances mucus production and vasodilatation [25], the radical scavenging properties of the MDMS, by counteracting inflammation, may have helped in reducing anal leakage and, as a consequence, itching and burning.
Among HD symptoms, discomfort has a much more unclear genesis but it is thought to be due to the combined effect of local hemodynamic alterations [14] together with the sensation of rectal fullness and/or the feeling of incomplete evacuation produced by prolapsed internal hemorrhoidal nodules [3]. At baseline 10 patients had evidence of prolapse, among them 6 patients had no clinical evidence of prolapse after treatment (V2). Although straining during a clinical examination is obviously much different from straining during defecation, study data suggest that treatment reduces prolapse appearance when a patient is asked to strain. This may have contributed to the alleviation of discomfort and other symptoms.
It appears reasonable to speculate that the frequency with which hemorrhoids prolapse outside the anal verge is linked to the frequency with which Treitz’s muscle undergoes elongation to such an extent to cause significant additional deterioration of this fixation structure, thus making it more likely that disease progresses to the point where hemorroidal nodules are not reducible. The dynamic of these interrelated phenomena is clearly complex and difficult to be unraveled, however, considering the multifaceted action of the product, in particular its lubricating and moistening features, it cannot be excluded that use of the study product may even help in slowing prolapse progression.
A key concept arising from what has been discussed so far is that the positive effect of the MDMS is provided by the integrated complex of natural substances combined in the product as an integral entity. All its components considered together allowing the final product to achieve the therapeutic benefit through a non-pharmacological mode of action.
HD is fairly common in the general population. This combined with the chronicity of the disease is at the base of the importance not only of using effective therapies, but also to closely consider the tolerability and risk of the conservative therapies available. There are several topical medications which contain active ingredients such as local anesthetics, corticosteroids, antibiotics and muscle relaxants. Evidence of efficacy of these drugs is still limited and not always do studies show the level of tolerability and safety expected and needed for topical treatment of HD [13,26]. For instance, results of the study by Tjandra et al. [27] supported the efficacy of a topical ointment composed by nitroglycerin 0.2% to relieve HD symptoms in selected patients with lowâ?ÂÂÂgrade hemorrhoids. However, 43% of patients experienced headache during treatment [27]. Moreover, corticosteroids, when used for long periods, may damage the perianal skin and even cause ulceration [28]. In the present study, 6 out of 42 patients (14.3%) of SAF population had one AE, none of which was considered as related to study treatment, providing evidence of a favorable risk-benefit ratio of the study product.
The present study has the limit of being uncontrolled. However, not only all clinical manifestations showed intensity or frequency reduction but, for those repeatedly evaluated, reductions were progressive over the course of treatment and statistically significant at each time point compared to baseline. It seems unlikely that knowing to be receiving a specifically developed treatment or the expectation of a treatment's effectiveness may have significantly contributed to these results, or that these results can be linked, to a significant extent, to a spontaneously favorable course of the disease.
Conclusion
The system of natural substances specifically combined into the MDMS seems to allow the product to intersect several different pathophysiological mechanisms underlying onset and magnitude of HD clinical manifestations. The achieved clinical benefit is coupled with a safety profile due to the product’s non-pharmacological mechanism of action, making the MDMS under study a valid non-interventional option for therapeutic management of grade I and II HD.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by Aboca S.p.A., the manufacturer of the medical device made of substances (brand name NeoFitoroid). Aboca S.p.A. was the Sponsor of the study. The Sponsor conceived the study design and was involved in interpretation of data and in writing of the clinical study report.
Trial Registration: NCT03545724 2018.06.04
36505
References
- Guttenplan M (2017) The evaluation and office management of hemorrhoids for the gastroenterologist. Curr Gastroenterol Rep 19: 30.
- Margetis N (2019) Pathophysiology of internal hemorrhoids. Ann Gastroenterol 32: 264–272.
- Lohsiriwat V (2012) Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol 18(17): 2009–2017.
- Ganz RA (2013) The evaluation and treatment of hemorrhoids: a guide for the gastroenterologist. Clin Gastroenterol Hepatol 6: 593–603.
- Jacobs D (2014) Clinical practice. Hemorrhoids. N Engl J Med 371: 944–951.
- Godeberge P (2004) Role of the venous component in the development of hemorrhoidal disease: pathophysiological insights and therapeutic implications. Medicographia 26: 178–181.
- Sandler RS, Peery AF (2019) Rethinking what we know about hemorrhoids. Clin Gastroenterol Hepatol 17: 8–15.
- Hyung KY (2014) The pathophysiology of hemorrhoids. In: Hyung Kyu Yng, eds. Hemorrhoids. Berlin Heidelberg: Springer-Verlag.
- Banov L Jr, Knoepp LF Jr, Erdman LH, Alia RT (1985) Management of hemorrhoidal disease. J S C Med Assoc 81: 398–401.
- Sun Z, Migaly J (2016) Review of hemorrhoid disease: presentation and management. Clin Colon Rectal Surg 29: 22–29.
- Riss S, Weiser FA, Schwameis K, Riss T, Mittlböck M, et al. (2012) The prevalence of hemorrhoids in adults. Int J Colorectal Dis 27: 215–220.
- Rivadeneira DE, Steele SR, Ternent C, Chalasani S, Buie WD, et al. (2011) Standards Practice Task Force of The American Society of Colon and Rectal Surgeons. Practice parameters for the management of hemorrhoids (revised 2010). Dis Colon Rectum 54: 1059–1064.
- Mott T, Latimer K, Edwards C (2018) Hemorrhoids: diagnosis and treatment options. Am Fam Physician 97: 172–179.
- Aigner F, Gruber H, Conrad F, Eder J, Wedel T, et al. (2009) Revised morphology and hemodynamics of the anorectal vascular plexus: impact on the course of hemorrhoidal disease. Int J Colorectal Dis 24: 105–113.
- Morgado PJ, Suárez JA, Gómez LG, Morgado P J Jr (1988) Histoclinical basis for a new classification of hemorrhoidal disease. Dis Colon Rectum 31: 474–480.
- Kraft JN, Lynde CV (2005) Moisturizers: what they are and a practical approach to product selection. Skin Therapy Lett 10: 1–8.
- Dal'Belo SE, Gaspar LR, Maia Campos PM (2006) Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res Technol 12: 241–246.
- Maisenbacher P, Kovar KA (1992) Analysis and stability of Hyperici Oleum. Planta Med 58: 351–354.
- Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Ríos JL (2002) Antioxidant activity of antiâ?ÃÂâÂÂÃÂÃÂinflammatory plant extracts. Life Sci 70(9): 1023–1033.
- Facino RM, Carini M, Franzoi L, Pirola O, Bosisio E (1990) Phytochemical characterization and radical scavenger activity of flavonoids from Helichrysum italicum G. Don (Compositae). Pharmacol Res 22(6): 709–721.
- Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395(2): 203–230.
- Schubert MC, Sridhar S, Schade RR, Wexner SD (2009) What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol 15(26): 3201–3209.
- Jose Marcio Neves Jorge, Habr-Gama A (2011) Anatomy and embryology of the colon, rectum, and anus. In: Beck D, Roberts P, Saclarides T, Senagore A, Stamos M, Wexne S, eds. ASCRS Manual of Colon and Rectal Surgery. 2nd edition. New York Dordrecht Heidelberg London: Springer.
- Lestar B, Penninckx F, Kerremans R (1989) The composition of anal basal pressure. An in vivo and in vitro study in man. Int J Colorectal Dis 4: 118–122.
- Rubanyi GM (1988) Vascular effects of oxygen-derived free radicals. Free Radic Biol Med 4: 107–120.
- Chong PS, Bartolo DC (1988) Hemorrhoids and fissure in ano. Gastroenterol Clin N Am 37: 627–644, ix.
- Tjandra JJ, Tan JJ, Lim JF, Murray-Green C, Kennedy ML, et al. (2007) Rectogesic (glyceryl trinitrate 0.2%) ointment relieves symptoms of haemorrhoids associated with high resting anal canal pressures. Colorectal Dis 9: 457-463.
- Acheson AG, Scholefield JH (2008) Management of haemorrhoids. Br Med J 336(7640): 380–383.











