Keywords
Doxorubicin; Naringin; Rat; Bone marrow; Antioxidants
Introduction
Doxorubicin (Adriamycin or DOX)), a topoisomerase II targeting drug, is one of the most effective chemotherapeutic agents used in the clinic to treat cancer since its isolation in 1960s from Streptomyces peucetius [1,2]. DOX is as one the most effective chemotherapy used either alone or in conjunction with other drugs or radiotherapy to treat different types of malignant neoplasia. DOX has been used to treat various malignant tumours including breast cancer, childhood solid cancers, multiple myelomas, gastric carcinoma, thyroid carcinomas, myeloblastic leukemias, ovarian cancer, small cell lung cancer, Hodgkin's and non-Hodgkin's lymphomas, Kaposi’s sarcoma, neuroblastoma, osteosarcoma, nephroblastoma, Wilms tumour and soft tissue sarcomas [3-6]. The DOX has been successfully used to treat hepatic cancers [7]. The combination of DOX with lurbinectedin (PM01183) has been found to treat small cell lung cancer in phase I clinical trial [8].
The DOX cancer cells during the specific stage of cell cycle, especially the cells in the S phase of the cell cycle are highly sensitive to its effect [9]. The use of DOX in clinics is associated with the induction of numerous adverse side effects that includes alopecia, bone marrow aplasia, mouth ulcers, dizziness, myelosuppression, local aggressivitye nausea, vomiting, hallucinations, vertigo, hepatotoxicity, nephrotoxicity and life-threatening cardiotoxicity, which limits its success [10,11]. DOX administration also leads to lacrimation, hypersensitivity (fever, chills, and urticaria), hyperpigmentation of the nails, and conjunctivitis [12]. Immunosuppression is another adverse effect that ensues DOX therapy making the patients more vulnerable to fatigue, microbial infections, gingival bleeding and reduced healing time [13].
The use of DOX leads to the development of life threatening cardiomyopathy both in children and adults who receive DOX for their cancer treatment [14-17]. The DOX induces cardiotoxicity in the patients employing many mechanisms. One of the predominant mechanisms of cardiotoxicity by DOX is the generation of oxygen free radicals in the cell mitochondria [14,18-20]. The DOX generates formation of reactive oxygen species (ROS), including superoxide anion radical and H2O2 [21,22], which in turn are produced due to redox cycling of DOX in vivo. The superoxide radicals undergo reaction with H2O2 to form highly reactive hydroxyl radicals by the iron catalyzed Haber-Weiss reaction. These secondarily derived hydroxyl radicals are highly reactive and damage proteins and DNA by triggering the onset of lipid peroxidation that stimulate a cascade of toxic reaction leading to cytotoxicity of cells and tissues [23]. The DOX intercalates into the cellular DNA and suppresses the activities of DNA and RNA polymerases leading to inhibition of nucleotide replication. It also leads to inhibition of topoisomerase II, which are crucial in the formation of DNA-cleavable complexes and the relegation of DNA during DNA replication, leading to cytocidal effect [24,25]. Further, reductive conversion of DOX is an important mechanism of cytotoxicity and is also the main cause of drug resistance in cancer cells [26,27]. This reductive conversion of DOX is triggered by one-electron reduction of the quinone moiety of doxorubicin, by the action of NADPH and the cytochrome P450 reductase into a semiquinone radical that exerts the cytotoxic effect, or it can be oxidized back into the quinine form [28,29]. The additional oxidative stress triggered by doxorubicin may not be counteracted by endogenous system and supplementation with of exogenous antioxidants may be helpful to reduce the DOX-induced oxidative stress.
Naringin (Naringenin 7-O-neohesperidoside) is a dietary flavonoid synthesized by a variety of citrus fruits including Citrus nobilis, Citrus paradise, Citrus junos, Artemisia selengensis, Citrus unshiu, Citrus sinensis, Artemisia stolonifera Citrus tachibana, roots of Cudrania cochinchinensis, aerial parts of Thymusherba barona, fruits of Pon cirus and other citrus species [30]. The name naringin is derived from Sanskrit ‘narangi’ meaning ‘orange’. The grape fruit juice contains usually 800 mg/L of naringin [31]. Various preclinical studies have reported the anticancer, cardioprotective, antiviral, hepatoprotective, and neuroprotective activities of Naringin [32-36]. Naringin scavenges free radicals, chelates metals and acts as an antioxidant agent [37-45]. Naringin treatment has been found to alleviate the radiation and DOX-induced chromosome damage in earlier studies [41,46,47]. Preclinical studies in mice revealed that naringin has been able to attenuate the benzo-a-pyrene-induced forestomach carcinoma [48]. Naringin protected against the DOX-induced cardiotoxicity in vitro and in vivo [35,49]. It has been reported to be active against diabetes, inflammation, lipodystrophy, cognitive damage, osteoporosis, dyslipidemia and fibrotic disease [50-53]. Naringin reduced iron-induced toxicity in vitro and in vivo [42,54].
The cytotoxic activity of naringin has been reported against breast cancer and HeLa cells and Walker’s carcinoma in rats [55-57]. The effect of naringin on the doxorubicin-induced biochemical lesions in rat bone marrow has not been not studied, therefore, present study was designed to investigate the protective effect of naringin against doxorubicin-induced biochemical damage in the bone marrow of albino rats.
Materials and Methods
Chemicals
Doxorubicin hydrochloride (Biochem Pharmaceutical Industries, Mumbai, India) was procured from a local supplier, whereas naringin was purchased from Acros Organics Ltd, Geel, Belgium. Cacodylic acid, 5,5-dithio2-nitrobenzoic acid ethylene-diaminetetraacetic acid, diethylene diethylenetriaminepentaacetic acid, glutathione (reduced), nitroblue tetrazolium, phenozine methosulphate, NADH, trichloroacetic acid, and 1-chloro,2,4-dinitrobenzene were supplied by Sigma Chemicals Co, St. Louis, MO, USA. Hydrogen peroxide, potassium dihydrogen phosphate, pyrogallol, disodium hydrogen phosphate, dipotassium hydrogen phosphate, and other routine chemicals were procured from Merck India Ltd., Mumbai, India.
Animal handling
The animal care and handling were carried out in accordance with the guidelines issued by the World Health Organization, Geneva, Switzerland and the INSA (Indian National Science Academy, New Delhi, India). Generally, six to eight weeks old male albino rats weighing 45-60 g were purchased from a local source, acclimatized to laboratory conditions. The animals were kept in the polypropylene cages in the controlled conditions of temperature and light (12 h of light and dark each). The entire experiments were approved by Institutional Animal Ethics Committee (IAEC) of Mizoram University, Aizawl, India.
Preparation of drugs and mode of administration
Doxorubicin hydrochloride (DOX) and naringin (NIN) were freshly dissolved in sterile double distilled water (DDW), immediately before use. The animals were injected intraperitoneally either with 0.01 ml/g body weight (b. wt.) NIN or DOX.
Experimental
The antioxidant action of naringin was studied in the rat bone marrow, where the animals were divided into the following groups:
• Control: The animals of control group received 0.01 ml/g distilled water intraperitoneally.
• Naringin: The animals from this group were intraperitoneally administered with 2 mg/kg b. wt. of naringin [43].
• Doxorubicin: This group of animals was injected with 5 mg/kg b. wt. of doxorubicin intraperitoneally [43].
• Naringin + Doxorubicin: The animals of this group were administered with 2 mg/kg b. wt. of naringin intraperitoneally once daily consecutively for three days and one hour after the last administration of naringin, the animals of this group were intraperitoneally injected with 5 mg/kg b. wt. of doxorubicin [43].
The rats from all the groups were killed by cervical dislocation at ½, 1 and 2 hours post DOX administration. The animals were dissected and perfused with ice cold saline transcardially for the determination of glutathione (GSH), glutathione-s-transferase (GST), catalase, superoxide dismutase (SOD) and lipid peroxidation (LOO) in the rat bone marrow. A minimum of five animals were used for each dose of group in each group at each time point and a total of 60 animals were used for the whole experiment. The bone marrow cells were extracted by removing the femora of each animal at the above specified posttreatment times and placed onto a wet Whatman filter paper. The femora were freed from muscles and other tissues, cleaned and the bone marrow was extracted individually from each animal.
Preparation of homogenate
The bone marrow was weighed and 10% homogenate was prepared in phosphate buffered saline for the estimation of glutathione, glutathione-s-transferase, catalase, superoxide dismutase and lipid peroxidation.
Total proteins
The protein contents were determined using the modified method of Lowry.
Glutathione
The glutathione contents in the bone marrow of rats were determined as described earlier [58]. In brief, proteins in the homogenate were precipitated by 25% TCA, and centrifuged. The supernatant was collected transferred into another tube. It was mixed with 0.2 M sodium phosphate buffer (pH 8.0) and 0.06 mM DTNB. The whole mixture was incubated for 10 minutes at room temperature. The absorbance of the sample/s was read against the blank at 412 nm in a UV-VIS double beam spectrophotometer (Shimadzu Corporation, Tokyo, Japan) and the GSH concentration has been calculated from the standard curve.
Glutathione-S-transferase
Glutathione-S-transferase (GST) was determined as described earlier [59]. Briefly, the bone marrow homogenate was mixed with 0.1 M potassium phosphate buffer, 1 mM EDTA, glutathione reductase, and 10 mM GSH, 12 mM tertbutyl- hydroperoxide. The resultant mixture was incubated for 10 min at 37°C in water bath. The absorbance was read against the blank at 340 nm using a double beam UV-VIS spectrophotometer.
Catalase
The catalytic reduction of hydrogen peroxide was used as a measure of catalase activity by the method described earlier [60]. In brief, the samples were mixed with hydrogen peroxide and incubated at 37°C. The decomposition of hydrogen peroxide was monitored every 30 seconds by recording the absorbance against the blank at 240 nm using a UV-VIS double beam spectrophotometer.
Superoxide dismutase
Total SOD activity, was determined by the pyrogallol autooxidation method [61]. Briefly, the samples were mixed with 62.5 mM tris-cacodylic acid buffer, containing 1 mM diethylenetriaminepentaacetic acid (DETAPA). This was followed by the addition of 4 mM pyrogallol. The autooxidation of pyrogallol was monitored against the blank at 420 nm using a UV-VIS double beam spectrophotometer.
Lipid peroxidation
Lipid peroxidation (LOO) was estimated according to the standard protocol [62]. Briefly, the samples were incubated with a mixture of trichloroacetic acid (15%), thiobarbituric acid (0.375%), and butylated hydroxytoluene (0.01%) in 0.25 N HCl at 95°C for 25 min. The reaction mixture was allowed to cool to room temperature and was centrifuged at 8,000 g. The supernatant was collected, and the absorbance was recorded against the blank using a UV-VIS double beam spectrophotometer. The lipid peroxidation has been determined against a standard curve prepared with tetraethoxypropane. For all biochemical estimations duplicate samples were used from each animal for various estimations listed above.
Statistical analysis
The significance between the treatments was determined using the Student’s ‘t’ test. A p value of <0.05 was considered statistically significant. The Solo 4 Statistical Software (BMDP Statistical Software Inc, Los Angeles, CA, USA) was used for statistical analyses.
Results
The results are shown as mean ± standard error of the mean in Table 1 and Figures 1-5.
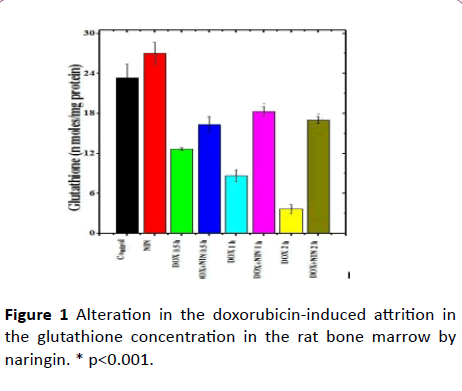
Figure 1: Alteration in the doxorubicin-induced attrition in the glutathione concentration in the rat bone marrow by naringin. * p<0.001.
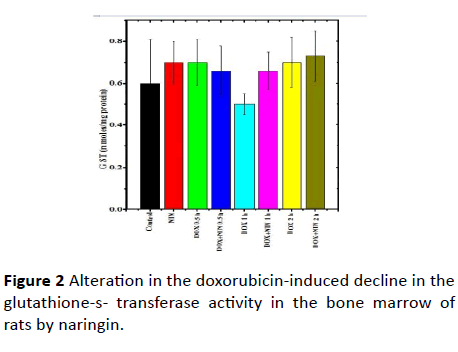
Figure 2: Alteration in the doxorubicin-induced decline in the glutathione-s- transferase activity in the bone marrow of rats by naringin.
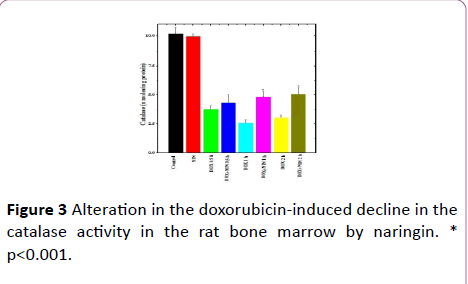
Figure 3: Alteration in the doxorubicin-induced decline in the catalase activity in the rat bone marrow by naringin. * p<0.001.
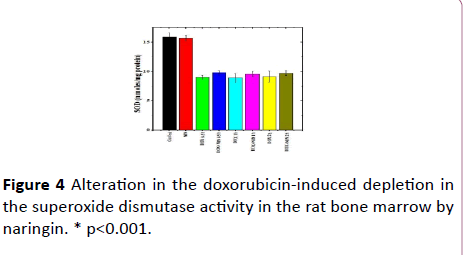
Figure 4: Alteration in the doxorubicin-induced depletion in the superoxide dismutase activity in the rat bone marrow by naringin. * p<0.001.
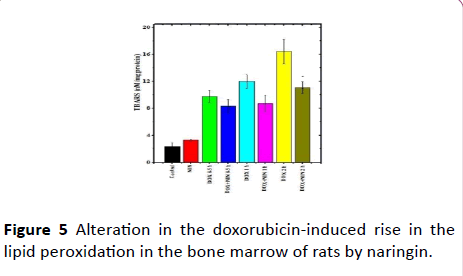
Figure 5: Alteration in the doxorubicin-induced rise in the lipid peroxidation in the bone marrow of rats by naringin.
| Treatment |
Assay time (h) |
nmol/mg protein |
LOO |
| Glutathione |
GST |
Catalase |
SOD |
nM TBARS |
| Control |
23.33 ± 2.03 |
0.6 ± 0.21 |
10.2 ± 0.52 |
15.93 ± 0.66 |
2.33 ± 0.58 |
| NIN |
27.00 ± 1.73 |
0.7 ± 0.1 |
9.96 ± 0.24 |
15.73 ± 0.49 |
3.33 ± 0.09 |
| DOX |
0.5 |
12.66 ± 1.20b |
0.7 ± 0.11 |
3.73 ± 0.33c |
9.03 ± 0.33c |
9.73 ± 0.93c |
| NIN+DOX |
0.5 |
16.33 ± 1.20 a |
0.66 ± 0.12 |
4.33 ± 0.69c |
9.8 ± 0.35c |
8.33 ± 0.97b |
| DOX |
1 |
8.66 ± 0.88c |
0.5 ± 0.05 |
2.57 ± 0.29c |
8.93 ± 0.73c |
11.96 ± 1.03c |
| NIN+DOX |
1 |
18.33 ± 0.67a* |
0.66 ± 0.09 |
4.83 ± 0.46c** |
9.6 ± 0.47c |
8.73 ± 1.24b |
| DOX |
2 |
3.66 ± 0.68c |
0.7 ± 0.12 |
3.03 ± 0.24c |
9.17 ± 0.93c |
16.4 ± 1.84c |
| NIN+DOX |
2 |
17 ± 0.53a** |
0.73 ± 0.12 |
5.07 ± 0.56b** |
9.73 ± 0.43c |
11.06 ± 0.89** |
Level of significance: *p<0.05. **p,0.01; §p<0.0001 when Doxorubicin is compared with Naringin + doxorubicin group. a p,0.01, bp<0.001 and cp<0.0001 when Doxorubicin is compared with control group.
DOX: Doxorubicin; NIN: Naringin; GST: Glutathione-s-transferase; SOD: Superoxide Dismutase; LOO:Lipid Peroxidation.
Table 1: Effect of naringin on the doxorubicin-induced alteration in various antioxidants (Mean+standard error of the mean) at different post-DOX-treatment times in the rat Bone marrow of rats.
Glutathione
The spontaneous glutathione concentration in lungs of nondrug treated control albino rats was estimated as 23.33 ± 2.03 nmol/mg protein. Administration of 2 mg/kg b. wt. of naringin treatment did increase the glutathione contents up to 27 ± 1.73 nmol/mg protein. However, this elevation in GSH concentration was not statistically significantly in the rat bone marrow when compared to control (Table 1). Doxorubicin treatment resulted in a significant decline in the GSH concentration as early as 0.5 h post treatment and this decline was approximately 2 folds lower than the control GSH concentration (Table 1 and Figure 1).
The GSH concentration continued to decline with assay time and almost 2.7 and 6.4-fold attrition was recorded in the GSH concentration at 1 and 2 h post-DOX treatment, respectively in the bone marrow of rats receiving 5 mg/kg b. wt. DOX alone (Table 1 and Figure 1). The administration of 2 mg/kg naringin before DOX treatment led to a rise in the GSH concentration in naringin+DOX treated group at all post DOX treatment times.
However, a significant elevation in the GSH concentration was found only at 1 and 2 h post-DOX-treatment in naringin +DOX treated group when compared to DOX-treatment alone (Table 1 and Figure 1).
Glutathione-S-transferase
The glutathione-s-transferase activity of non-drug treated control rat bone marrow was 0.6 ± 0.21 nmol/mg/protein and naringin treatment alone did not alter the spontaneous glutathione-s-transferase activity significantly when compared to control (Table 1). Doxorubicin treatment did not lead to a significant alteration in the GST activity, at all post-DOXtreatment times (Table 1 and Figure 2). Administration of Naringin before DOX treatment marginally elevated the GST activity at all post-DOX-treatment times in the naringin+DOX group when compared to DOX treatment alone. However, this elevation was statistically non-significant (Table 1 and Figure 2).
Catalase
The spontaneous catalase activity in the control rat bone marrow was found to be 10.2 ± 0.52 nmol/mg protein and naringin pretreatment did not significantly alter the spontaneous catalase activity (Table 1 and Figure 3). The administration of DOX to rats resulted in a significant decline in the catalase activity at all post-DOX-treatment assay times when compared to control (Table 1 and Figure 3). The DOX treatment caused approximately 2.7, 4 and 3.4-fold decline in the catalase activity at ½, 1 and 2 h post-DOX-treatment as compared to spontaneous catalase activity (Table 1). Naringin treatment before doxorubicin administration increased the catalase activity, when compared to DOX treatment at all posttreatment times (Figure 3). A significant increase in the catalase activity in the bone marrow of rats was observed at all post DOX treatment times, except ½ h where this rise was statistically non-significant (Table 1).
Superoxide dismutase
The spontaneous superoxide dismutase activity in the rat bone marrow was 15.93 ± 0.66 nmol/mg protein and naringin treatment alone did change the SOD activity significantly (Table 1 and Figure 4). The administration of doxorubicin alone in rats significantly reduced the SOD activity at all post- DOX-treatment assay times as compared to control (Table 1). The DOX treatment led to approximately 1.7-fold reduction in the SOD activity at all post-DOX-treatment times when compared to the spontaneous SOD activity (Table 1). The naringin pretreatment could not increase the SOD activity significantly in the naringin+ DOX group at all post DOX treatment times when compared to DOX treatment alone (Figure 4).
Lipid peroxidation
The results of the present study show that the rate of lipid peroxidation (LOO) was 2.33 ± 0.58 nM/mg protein in the bone marrow of control albino rat. The pretreatment of rats with naringin alone did not significantly alter the spontaneous lipid peroxidation (Table 1). DOX treatment of rats led to a significant rise in the lipid peroxidation when compared to spontaneous levels at all assay times and a highest rise in LOO was recorded at 2 h post-DOX-treatment, where it was of 16.4±1.84 nM TBARS (Table 1 and Figure 5). The DOX treatment alone increased the LOO by 4, 6 and 8 folds when compared to control LOO (Table 1). Naringin treatment before DOX administration significantly reduced the rate of lipid peroxidation only at 2 h post DOX treatment when compared to DOX treatment alone (Figure 5). Despite this decline, the lipid peroxidation was greater than the control group (Figure 5 and Table 1).
Discussion
Doxorubicin is an important antibiotic which is in frequent clinical use to treat different cancers either alone or in combination with other chemotherapeutic drugs [6]. Bone marrow plays an important role in blood formation and immunity and it is an essential tissue in the sustenance of life. Bone marrow suppression is one of the important adverse side effects of DOX apart from cardiomyopathy and other toxic effects [10,11,63,64]. This indicates that there is a need for the pharmacological agent/s that can reduce or prevent bone marrow damage in the patients receiving DOX. Therefore, the present study was planned to study the role of naringin treatment in protecting the biochemical lesions induced by doxorubicin in the bone marrow of rats.
Gamma-glutamylcysteinylglycine or glutathione (GSH), a tripeptide thiol is ubiquitously synthesized by all eukaryotic cells, which protects cells against the oxidative stress induced by various oxygen radicals [65-67]. The antioxidant action of GSH is well proven and depletion of GSH leads to structural and functional disturbances in cells and rise in the oxidative stress [65-67]. The DOX treatment led to a time dependent attrition in the GSH concentration in the rat bone marrow which reached to a lowest level at 2 h post-DOX treatment. A similar effect has been reported earlier in the mice bone marrow [44]. These results are also in conformation with the earlier reports where DOX has been found to decrease glutathione contents in rat and mice liver and heart [35,43,45,68]. The treatment of rats with Naringin before DOX treatment alleviated the doxorubicin depletion in the rat bone marrow is in agreement with earlier studies, where naringin has been reported to increase the GSH concentration in rat liver and mouse liver and bone marrow treated with DOX [35,43-45,68-70]. Likewise, the extract of Aegle mermelos has also been reported to increase the GSH contents in mice heart in an earlier study [68]. An amino acid glutamine has been reported to protect rats against DOX-induced attrition in GSH contents [71].
The enzyme glutathione-S-transferases (GSTs) are ubiquitously synthesized by all organisms and their main function is to conjugate various endogenous and exogenous electrophilic substances with GSH. GSTs are involved in cell proliferation, and cell mortality. Apart from these functions GSTs also play an important role in detoxification and protection of DNA against various toxic insults [72,73]. The neoplastic tissues overexpress GST and it is directly linked to resistance to chemotherapy [74]. The DOX treatment did not alter the GST activity significantly in the rat bone marrow. However, earlier studies have reported a decrease in the GST activity in mice and rats [43-45]. Treatment of rats with naringin also did not affect the GST activity. In contrast, earlier studies have reported a rise in the GST activity in mouse liver, bone marrow and heart [35,44,45]. This may be due to bone marrow cell death as GSTs are in involved in cell mortality.
The non-oxygen radical hydrogen peroxide has emerged as one of the major redox signalling molecule that is responsible for increased oxidative stress in the cells. It acts as a signalling molecule for cell proliferation, differentiation, inflammation and apoptosis [75]. The catalase or oxidoreductase enzymes are ubiquitously synthesized by cells and their main function is to neutralize hydrogen peroxide after it formation. These enzymes convert H2O2 into water and molecular oxygen [76,77]. Their reduced activity increases accumulation of H2O2 and oxidative stress. The DOX administration reduced the activity of catalase enzyme in the bone marrow of rats, which was lowest at 2 h post-DOX treatment. In earlier studies DOX treatment has been found to reduce the catalase activity in mouse and rat heart liver and bone marrow [35,43-45,68,71]. Catalase activity showed an increase in the rat bone marrow treated with naringin before DOX administration is in agreement with earlier studies, where a similar effect has been observed in the liver and hearts of rats and mice liver and bone marrow cells [35,43-45, 69,70]. Likewise, the extract of Aegle marmelos has been found to protect mice heart against the DOX-induced depletion in the catalase activity [68].
Superoxide dismutase are redox metalloenzymes (SOD), which are produced by all organism that use oxygen for their energy requirements. The SODs are located inside the cell as well in the extracellular matrix [78,79]. They provide protection against the superoxide anion produced during respiration by converting it into less harmful product H2O2. The SODs degrade H2O2 into harmless products like water and molecular oxygen [78,79]. DOX administration has led to a time dependent depletion in the SOD activity in the bone marrow cells of rat and lowest activity was recorded at 2 h post-DOX treatment in the present study. The cardiotoxic, hepatotoxic and nephrotoxic action of DOX is mediated through its interaction with eNOS that in turn elevates the superoxide radical generation [80]. This may have resulted in the of reduced SOD activity in our study. The DOX treatment has been reported to alleviate SOD activity in mice bone marrow, liver and heart earlier [35,44,45,68]. DOX treatment has also decreased the SOD activity in the rat liver [43]. Naringin treatment marginally alleviated the DOX-induced decline in rat lungs however the increase in SOD activity was not statistically significant. Naringin treatment has been found to decrease the DOX-induced attrition in the SOD activity in rat liver and heart and mice bone marrow, liver and heart in earlier studies [35,43-45, 69,70]. Likewise, mice heart receiving extract of Aegle mermelos before DOX administration showed elevation in the SOD activity [68].
Lipids in the form of triglycerides are used to store energy and they are also integral part of cell membrane [81]. The lipids are targeted by free radicals and H2O2 via Fenton reaction and produce lipid peroxides, which are index of cytotoxicity [82]. DOX induces ROS, which produce lipid peroxides to trigger cancer cell killing [28]. This may be the reason for time dependent increase in the lipid peroxidation in the rat bone marrow cells treated with DOX. These findings are in conformation with earlier studies where DOX treatment has been found to increase lipid peroxidation [35,43-45,68-70,83]. Naringin treatment reduced the DOX-induced lipid peroxidation in rat bone marrow is in agreement with the earlier studies, where a similar effect has been reported [35,43-45,69,70]. Naringin has been reported to protect against the bleomycin-induced lipid peroxidation in rat lung [52]. Likewise, L-glutamine has been also reduced the DOXinduced lipid peroxidation in rat heart in an earlier study [71].
The exact mechanism of protection of DOX-induced biochemical lesions by naringin in rat bone marrow is not known. It is speculated that naringin may have used different mechanisms to reduce DOX-induced toxicity. The scavenging/ inhibition of DOX-induced free radicals by naringin may be one of the important mechanisms of action, which would have reduced the burden on GSH and other enzymes resulting in their increase in the bone marrow when compared to DOX alone treatment. DOX-utilizes NADPH oxidase system to trigger free radical formation and the presence of naringin has been reported to inhibit the activation of NADPH oxidase, which may have arrested the free radical formation [84,85]. The presence of iron increases the DOX-induced free radical formation and iron chelating action of naringin would have blocked this action, which would have led to an increase in the antioxidants in the rat bone marrow in the present study [35]. Naringin has been reported upregulate the gene expression of catalase, and SOD that may have resulted in the elevated antioxidant status in the present study [40]. The nuclear factor erythroid 2-related factor 2 (Nrf2) is constitutively expressed by all tissues and DOX has been reported to inhibit its expression causing depletion in the endogenous antioxidants. The presence of naringin may have reduced this ability of DOX leading to a rise in the antioxidant status when compared to DOX alone. Naringin has been reported to upregulate Nrf2 [86].
Conclusion
DOX treatment caused an attrition in the endogenous antioxidants in the rat bone marrow which may due to its ability to produce free radical-induced oxidative stress. The naringin pretreatment may have inhibited the DOX-induced free radical formation leading to elevated antioxidant status in the rat bone marrow. This increase in endogenous antioxidants by naringin may be due to the inhibition of NADH oxidase and upregulation of Nfr2 elements in the rat bone marrow.
Acknowledgements
This work was supported by a grant no. F4-10/2010(BSR) UGC from the University Grants Commission, New Delhi, India.
Conflict of Interest Statement
Authors do not have any conflict of interest statement to declare.
22275
References
- Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, et al. (1969) Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng. 9: 1101–1110.
- Weiss RB (1992) The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 19: 670-686.
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56: 185-229.
- Quiles JL, Ochoa JJ, Huertas JR, Lopes-Frias M, Mataix J (2006) Olive oil and mitochondrial oxidative stress: studies on adriamycin toxicity, physical exercise and ageing. In Quiles JL, CABI Publishing, Oxford, UK. pp. 119-151.
- Otterson GA, Villalona-Calero MA, Hicks W, Pan X, Ellerton JA, et al. (2010) Phase I/II study of inhaled doxorubicin combined with platinum-based therapy for advanced non–small cell lung cancer. Clin Cancer Res 16: 2466-2473.
- Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, et al. (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16: 3267-3285.
- Tam K (2013) The roles of doxorubicin in hepatocellular carcinoma. ADMET & DMPK 1: 29-44.
- Calvo E, Moreno V, Flynn M, Holgado E, Olmedo ME, et al. (2017) Antitumor activity of lurbinectedin (PM01183) and doxorubicin in relapsed small-cell lung cancer: results from a phase I study. Ann Oncol 28: 2559-2566.
- Kusyk CJ, Hsu TC (1976) Adriamycin‚ÂÃÂinduced chromosome damage: elevated frequencies of isochromatid aberrations in G2 and S phases. Experientia 32: 1513–1514.
- Kizek R, Adam V, Hrabeta J, Eckschlager T, Smutny S, et al. (2012) Anthracyclines and ellipticines as DNA-damaging anticancer drugs: recent advances. Pharmacol Ther 133: 26-39.
- Dolin TD, Himmelfarb J (2008) “Drug-induced kidney disease,” in Pharmacotherapy: A Pathophysiologic Approach, JT Dipiro (ed) (7th edn) pp: 795–810.
- Lipshultz SE, Lipsitz SR, Kutok JL, Miller TL, Colan SD, et al. (2013) Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer 119: 3555-3562.
- Mancuso A, Migliorino M, De Santis S, Saponiero A, De Marinis F (2006) Correlation between anemia and functional/cognitive capacity in elderly lung cancer patients treated with chemotherapy. Ann Oncol. 17: 146-150.
- Chatterjee K, Zhang J, Honbo N, Karliner JS (2010) Doxorubicin cardiomyopathy. Cardiol 115: 155-162.
- Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 65: 157-170.
- Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, et al. (2014) Doxorubicin-induced cardiotoxicity: From bioenergetic failure and cell death to cardiomyopathy. Med Res Rev 34: 106–135.
- Myers C (1998) The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol 25: 10-14.
- Minotti G, Cairo G, Monti E (1999) Role of iron in anthracycline cardiotoxicity: New tunes for an old song? FASEB J. 13: 199-212.
- Ichikawa Y, Ghanefar M, Wu R, Khechaduri A, Prasad SVNga, et al. (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124: 617–630.
- Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U (2006) New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol. 41: 389-405.
- Olson RD, Mushlin PS (1990) Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J 4: 3076-3086.
- Šim‚¯nek T, Štºrba M, Popelová O, Adamcová M, Hrdina R, et al. (2009) Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 61: 154-171.
- Gutteridge JMC, Halliwell B (1989) 1 Iron toxicity and oxygen radicals. Bailliere's Clin Haematol 2: 195-256.
- Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF (1984) Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 226: 466–468.
- Yang ES, Huh YJ, Park JW (2013) RNA interference targeting sensitive-to-apoptosis gene potentiates doxorubicin- and staurosporine-induced apoptosis of PC3 cells. Anticancer Res 33: 847–855.
- Sinha BK, Mimnaugh EG, Rajagopalan S, Myers CE (1989) Adriamycin activation and oxygen free radical formation in human breast tumor cells: protective role of glutathione peroxidase in adriamycin resistance. Cancer Res 49: 3844–3848.
- Kostrzewa-Nowak D, Paine MJ, Wolf CR, Tarasiuk J (2005) The role of bioreductive activation of doxorubicin in cytotoxic activity against leukaemia HL60-sensitive cell line and its multidrug-resistant sublines. Br J Cancer 93: 89-97.
- Deavall DG, Martin EA, Horner JM, Roberts R (2012) Drug-induced oxidative stress and toxicity. J Toxicol 2012.
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52: 213–1225.
- Swiader K, Lamer-Zarawska E (1996) Flavonoids of rare artemisia species and their antifungal properties. Fitoterapia 67: 77-78.
- Zhang J (2007) Flavonoid in grape fruit and commercially grape fruit juices: concentration, distribution, and potential health benefits. Proc Fla State Hort Soc 120: 288-294.
- Gammal AA, Mansour RM (1986) Antimicrobial activities of some flavonoid compounds. Zentrabl Mikrobiol 141: 561–565.
- Aboobaker VS, Balgi AD, Bhattacharya RK (1994) In vivo effect of dietary factors on the molecular action of aflatoxin B1: Role of non-nutrient phenolic compounds on the catalytic activity of liver fraction. In Vivo 8: 1095–1098
- Gordon PB, Holen I, Seglen PO (1995) Protection by naringin and some other flavonoids of hepatocytic autophagy and endocytosis against inhibition by okadaic acid. J Biol Chem 270: 5830–5838.
- Jagetia GC, Reddy TK (2014) The grape fruit flavonone naringin protects mice against doxorubicin-induced cardiotoxicity. J Mol Biochem 3.
- Kim HD, Jeong KH, Jung UJ, Kim SR (2016) Naringin treatment induces neuroprotective effects in a mouse model of Parkinson's disease in vivo, but not enough to restore the lesioned dopaminergic system. J Nutr Biochem 28: 140-146.
- Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacoma C, et al. (2000) Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol Toxicol 16: 91–98.
- Gorinstein S, Leontowicz H, Leontowicz M, Krzeminski R, Gralak M, et al. (2007) of hesperidin and naringin on the plasma lipid profile and plasma antioxidant activity in rats fed a cholesterol-containing diet. J Sci Food Agric 87: 1257–1262.
- Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, et al. (2001) Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 69: 2855-2866.
- Jeon SM, Bok SH, Jang MK, Kim YH, Nam KT, et al. (2002) Comparison of antioxidant effects of naringin and probucol in cholesterol-fed rabbits. Clin Chim Acta 317: 181-190.
- Jagetia GC, Venkatesha VA, Reddy TK (2003) Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow. Mutagenesis 18: 337-343.
- Jagetia GC, Reddy TK (2011) Alleviation of iron induced oxidative stress by the grape fruit flavanone naringin in vitro. Chem Biol Interactions. 190: 121-128
- Jagetia GC, Lalnuntluangi V (2016) The citrus flavanone naringin enhances antioxidant status in the albino rat liver treated with doxorubicin. Biochem Mol Biol J. 2016 2: 2.
- Jagetia GC, Lalrinengi C (2017) Naringin, a grape fruit bioflavonoid protects mice bone marrow cells against the doxorubicin-induced oxidative stress. SOJ Biochem 3: 1-9.
- Jagetia GC, Lalrinengi C (2017) Treatment of mice with naringin alleviates the doxorubicin –induced oxidative stress in the liver of Swiss albino mice. MOJ Anat Physiol 4: 00130.
- Jagetia GC, Reddy TK (2002) The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: a micronucleus study. Mutat Res 519: 37-48.
- Jagetia GC, Reddy TK (2016) The grape fruit bioflavonoid naringin protects against the doxorubicin-induced micronuclei formation in mouse bone marrow. Int J Mol Biol. 1: 6.
- Jagetia GC, Reddy TK (2004) Chemopreventive effect of naringin on the benzo(a)pyrene-induced forestomach carcinoma in mice. International J Cancer Prev 1: 429-444.
- You Q, Wu Z, Wu B, Liu C, Huang R, et al. (2016) Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J Endocrinol. 230: 197-214.
- Kumar A, Dogra S, Prakash A (2010) Protective effect of naringin, a citrus flavonoid, against colchicine-induced cognitive dysfunction and oxidative damage in rats. J Med Food 13: 976-984.
- Adebiyi OO, Adebiyi OA, Owira PM (2015) Naringin reverses hepatocyte apoptosis and oxidative stress associated with hiv-1 nucleotide reverse transcriptase inhibitors-induced metabolic complications. Nutrients 7: 12
- Turgut NH, Kara H, Elagoz S, Deveci K, Gungor H, et al. (2016) The protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med 2016: 7601393.
- Ma X, Lv J, Sun S, Ma J, Xing G, et al. (2016) Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Sci Rep 6: 24562
- Jagetia GC, Reddy TK, Venkatesha VA, Kedlaya R (2004) Influence of naringin on ferric iron induced oxidative damage in vitro. Clinica Chimica Acta. 347: 189-197.
- Camargo CA, Gomes-Marcondes MC, Wutzki NC, Aoyama H (2012) Naringin inhibits tumor growth and reduces interleukin-6 and tumor necrosis factor α levels in rats with Walker 256 carcinosarcoma. Anticancer Res 32: 129-133.
- Li H, Yang B, Huang J, Xiang T, Yin X, et al. (2013) Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol Lett 18: 219-228.
- Zeng L, Zhen Y, Chen Y, Zou L, Zhang Y, et al. (2014) Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NFcaspase-1 pathway in HeLa cervical cancer cells. Int J Oncol 45: 1929-1936.
- Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophy Acta 582: 67-78.
- Habig WH, Pabst MJ, JakobyWB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation, J Biol Chem 249: 7130–7139.
- Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469-474.
- McCord J M, Fridovich I (2000) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049-6055.
- Gelvan D, Saltman P (1990) Different cellular targets of Cu- and Fe-catalyzed oxidation observed using a Cu-compatible thiobarbiturate acid assay. Biochimica Biophysica Acta 1035: 353-360.
- Bhinge KN, Gupta V, Hosain SB, Satyanarayanajois SD, Meyer SA, et al. (2012) The opposite effects of Doxorubicin on bone marrow stem cells versus breast cancer stem cells depend on glucosylceramide synthase. Int J Biochem Cell Biol 44: 1770–1778.
- Meister A, Anderson ME (1983) Glutathione. Ann Rev Biochem. 52: 711-760.
- Lushchak VI (2012) Glutathione homeostasis and functions: Potential targets for medical interventions. J Amino Acids Article 26: 1
- Schumacker PT (2015) Reactive oxygen species in cancer: A dance with the devil. Cancer Cell 27: 156-157.
- Jagetia GC, Venkatesh P (2015) An Indigenous plant Bael (Aegle marmelos (L.) Correa) extract protects against the doxorubicin-induced cardiotoxicity in mice. Biochem Physiol 4: 3.
- Papasani VMR, Hanumantharayappa B, Annapurna A (2014) Cardioprotective effect of naringin against doxorubicin induced cardiomyopathy in rats. Indo American J Pharm Res 4: 2593-2598.
- Kwatra M, Kumar V, Jangra A, Mishra M, Ahmed S, et al. (2016) Ameliorative effect of naringin against doxorubicin-induced acute cardiac toxicity in rats. Pharm Biol 54: 637-647.
- Todorova VK, Kaufmann Y, Hennings L, Klimberg VS (2010) Oral glutamine protects against acute doxorubicin-induced cardiotoxicity of tumor-bearing rats. J Nutr 140: 44–48.
- Laborde E (2010) Glutathione transferases as mediators of signalling pathways involved in cell proliferation and cell death. Cell Death Different 17: 1373-1380.
- Schnekenburger M, Karius T, Diederich M (2014) Regulation of epigenetic traits of the glutathione -S-transferase P1 gene: from detoxification toward cancer prevention and diagnosis. Front Pharmacol. 5: 170.
- McIlwain CC Townsend, DM, Tew KD (2006) Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene 25: 1639–1648.
- Sies H (2014) “Role of metabolic H2O2 generation: redox signalling and oxidative stress,” J Biol Chem 289: 8735-8741.
- Alfonso-Prieto M, Biarnés X, Vidossich P, Rovira C (2009) The molecular mechanism of the catalase reaction. J Am Chem Society 131: 11751-11761.
- Kodydková J, Vávrová L, Kocík M, ák A (2014) Human catalase, its polymorphisms, regulation and changes. of its activity in different diseases. Folia Biologica 60: 153-167.
- Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, et al. (2014) Superoxide dismutases and superoxide reductases. Chem Reviews 114: 3854-918.
- Miller AF (2012) Superoxide dismutases: Ancient enzymes and new insights. FEBS Letters 586: 585–595.
- Vasquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard Jr KA, et al. (1997) Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 36: 11293–11297.
- Yin H, Xu L, Porter NA (2011) Free radical lipid peroxidation: Mechanisms and analysis. Chem Rev 111: 5944–5972.
- Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidat Med Cell Long.
- Pieniek A, Czepas J, Piasecka-Zelga J, Gwoski K, Koceva-Chya A (2014) Oxidative stress induced in rat liver by anticancer drugs doxorubicin, paclitaxel and docetaxel. Adv. Medi Sci 58: 104-111.
- Gilleron M, Marechal X, Montaigne D, Franczak J, Neviere R, et al. (2009) NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis. Biochem Biophys Res Commun 388: 727-731.
- Li W, Wang C, Peng J, Liang J, Jin Y, et al. (2014) Naringin inhibits TNF-α induced oxidative stress and inflammatory response in HUVECs via Nox4/NF-κ B and PI3K/Akt pathways. Curr Pharm Biotechnol 15: 1173-1182.
- Kulasekaran G, Ganapasam S (2015) Neuroprotective efficacy of naringin on 3-nitropropionic acid-induced mitochondrial dysfunction through the modulation of Nrf2 signaling pathway in PC12 cells. Mol Cell Biochem 409: 199-211.











