Key words
|
| |
| S. torvum, marker enzymes, antioxidant defence enzyme, doxorubicin |
| |
Introduction
|
| |
| Doxorubicin is an anthracycline glycoside antibiotic that possesses a potent and broad spectrum antitumour activity against a variety of human solid tumours and haematological malignancies [1,2].The DOX antitumour effects include mechanisms related to alterations of DNA and the production of free radicals [3]. However its use in chemotherapy has been limited largely due to its diverse toxicities, including cardiac, hepatic, hematological and testicular toxicity [4, 5]. Several studies have shown that the combination of the inflammatory process, free radical oxidative stress, and lipid peroxidation is frequently associated with liver damage, induced by toxic agents such as DOX [6]. Persistent and irreversible liver damage has been as a side effect of DOX therapy. It has been observed that there is an increase in the apoptotic processes in liver tissue after a single dose of DOX [7,8]. It was confirmed that the therapeutic dose of DOX enhanced lipid peroxidation in microsomes and mitochondria in the liver, especially in the presence of Fe3+ ions [9]. DOX mediated hepatotoxicity includes focal damage in hepatocytes, vascular damage and steatosis [10]. |
| |
| Solanum torvum Sw. (Solanaceae), commomly known as Turkey berry is an erect spiny shrub of about 4 m tall, evergreen and widely branched. It is native and found cultivated in Africa and West Indies [11]. The fruits and leaves are widely used in Camerooninan folk medicine. The plant is cultivated in the tropics for its sharp tasting immature fruits. It is used in the treatment of stomach pain and skin infections [12]. It possesses antimicrobial [13, 14] antiviral [15], imuno-secretory [16], antiulcer [17], antioxidant [18], analgesic and anti-inflammatory [19], cardiovascular and anti-platelet aggregation activities [20]. Solanum torvum contains a number of potentially pharmacologically active chemicals like isoflavonoid sulfate and steroidal glycosides [21], chlorogenone and neochlorogenone [22] triacontane derivatives [23,24], 22-β-O-spirostanol oligoglycosides [25], 26-O- β -glucosidase [26]. The free radical scavenging effects of the Solanum torvum on DPPH (2, 2-diphenyl-1-picrylhydrazyl) was measured in-vitro. The antioxidant properties of flavonoids and their ability to chelate free iron could be effective in reducing hepatotoxicity of DOX [27]. In view of this, since DOX induced hepatotoxicity is linked to oxidative stress, we have investigated the possible hepatoprotective effect of Solanum torvum, against DOX in Wistar rats. |
| |
Materials and methods
|
| |
|
Extract preparation
|
| |
| Dried fruits of Solanum torvum Sw. (Solanaceae) were purchased locally and authenticated by Dr. S. C. Pal, NDMVP Samaj’s College of Pharmacy, Nashik, India. The voucher specimen (1731) has been deposited at Agharkar Research Institute, Pune, India. Mature fruits were collected, sundried and grounded. The powder obtained (1kg) was defatted using pet ether (60-800C). The marc was macerated in ethanol for 3-4 days at room temperature. The filterate was air dried and concentrated under reduced pressure to obtain 120 g, corresponding to a yield of 12.0 % w/w. The total flavanoid content of ethanolic extract of S.torvum was found to be 85.26±0.02 μg rutin equivalent/ mg of extract [28]. The total phenolic content of ethanolic extract of S.torvum was found to be 99.52± 0.42 μg gallic acid equivalent/ mg of extract [29].Appropriate concentrations of the extracts was made in distilled water. The phytoconstituents present in the crude extract are flavonoids, alkaloids, tannins and saponins [30]. |
| |
|
Animals
|
| |
| Laboratory breed Wistar albino rats of either sex weighing between 150-200 g, maintained under standard laboratory conditions of 25 ± 1°C, and photo period (12 hr dark/12 hr light) were used for the experiment. Commercial pellet diet (Amrut laboratory rat and mice feed, Sangli, India.) and water were provided ad libitum. The experiments were carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India, and approved by the Institutional Animal Ethical Committee. |
| |
|
Chemicals
|
| |
| Doxorubicin (Oncodria, Sun Pharmaceutical Ind. Ltd. Gujarat, India) was obtained from the local market. 1, 1-diphenyl, 2- picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich, Mumbai. All chemicals for sensitive biochemical assays were obtained from Sigma Chemicals Co. India and Hi media Chemicals, Mumbai, India. Distilled water was used for biochemical assays. ALT and AST kits were obtained from Span Diagnostics Ltd., Surat, India. |
| |
|
Experimental protocol
|
| |
| The animals were randomly divided into the following experimental groups with 5 animals in each group. |
| |
| Group 1: Vehicle treated group. |
| |
| Group 2: DOX (67.75 mg/kg, i.v) 2 days before the sacrifice of the animal. |
| |
| Group 3: Solanum torvum (100 mg/kg, p.o.) daily for four weeks. |
| |
| Group 4: Solanum torvum (300 mg/kg, p.o.) daily for four weeks. |
| |
| Group 5: Solanum torvum (100 mg/kg, p.o.) daily for four weeks+ DOX (67.75 mg/kg, i.v, 2 days before sacrifice). |
| |
| Group 6: Solanum torvum (300 mg/kg, p.o.) daily for four weeks + DOX (67.75 mg/kg, i.v, 2 days before sacrifice). |
| |
| At the end of the treatment schedule, the animals were sacrificed by a high dose of diethyl ether. Blood was withdrawn immediately for enzyme assays and the upper liver lobe was rapidly excised for microscopic examination and biochemical analysis. The hepatic tissue and blood was stored at -20°C until biochemical analysis [31]. |
| |
|
Preparation of Serum and tissue homogenate
|
| |
| Blood was collected and allowed to clot. Serum was separated using R-24 research centrifuge (Remi instruments Ltd., Mumbai) at 5000 rpm for 4 min and used for estimation of ALT and AST. The liver tissue was washed with ice-cold 0.9% saline and homogenized quickly with ice cold 0.1 M Tris HCl buffer (pH 7.5) using Remi homogenizer to give a 10% homogenate. The homogenate was centrifuged at 10,000 rpm for 20 min and the supernatants were used for estimation of SOD and CAT. |
| |
|
Antioxidant Parameters
|
| |
| Superoxide dismutase activity (SOD) |
| |
| The assay of SOD was based on the ability of SOD to inhibit spontaneous oxidation of adrenaline to adrenochrome [32]. 0.05 ml supernatant was added to 2.0 ml of carbonate buffer and 0.5 ml of 0.01mM EDTA solution. The reaction was initiated by addition of 0.5ml of epinephrine and the autooxidation of adrenaline (3x10-4 M) to adrenochrome at pH 10.2 was measured by following change in OD at 480 nm. The change in optical density every minute was measured at 480 nm against reagent blank. The results are expressed as units of SOD activity (mg/wet tissue). One unit of SOD activity induced approximately 50% inhibition of adrenaline. |
| |
| Catalase activity (CAT) |
| |
| Catalase(CAT): The reaction mixture consisted of 2 ml phosphate buffer (pH 7.0), 0.95 ml of hydrogen peroxide (0.019M) and 0.05ml of supernatant in a final volume of 3 ml. Absorbance were recorded at 240 nm every 10 sec for 1 min. One unit of CAT was defined as the amount of enzyme required to decompose 1 μmol of peroxide per min, at 25°C at pH 7.0. The results were expressed as units of CAT U/g of wet tissue [33]. |
| |
|
Liver marker enzymes
|
| |
| Blood samples were collected from each animal and the serum obtained by centrifugation was used for determination of ALT and AST (Span Diagnostics., Surat, India.). |
| |
|
Histopathological examination
|
| |
| The Liver tissue was excised and immediately fixed in 10% buffered formalin at the end of the experiment. The tissue specimen was embedded in paraffin after being dehydrated in alcohol and subsequently cleared with xylene. Five-micrometer thick serial histological sections were obtained from the paraffin blocks and stained with hematoxylin and eosin. The sections were examined under light microscope to evaluate pathological changes and photomicrographs were taken. |
| |
|
Statistical analysis
|
| |
| All data were expressed as the mean ± SEM. For statistical analysis of the data, group means were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s test, P<0.05 was considered significant. |
| |
Results
|
| |
|
Liver marker enzymes
|
| |
| Animals treated with DOX (67.75 mg/kg, i.v, 2 days before sacrifice) significantly increased the levels of liver enzymes i.e. ALT and AST levels. Animals treated with S. torvum extract (100 mg/kg and 300 mg/kg) alone have not shown any significant change as compared to control group. Pretreatment with S. torvum extract (100 mg/kg and 300 mg/kg) significantly (p<0.05) decreased the levels of ALT and AST in DOX treated animals as compared to DOX group (Table 1). |
| |
|
Antioxidant enzymes
|
| |
| Animals treated with DOX (67.75 mg/kg, i.v, 2 days before sacrifice) significantly (p<0.05) decreased the levels of SOD and CAT. Animals treated with S. torvum extract (100 mg/kg and 300 mg/kg) alone have not shown any significant change as compared to control group. Pretreatment with S. torvum extract (100 mg/kg and 300 mg/kg) significantly (p<0.05) increased the levels of SOD and CAT in DOX treated animals as compared to DOX group (Table 2). |
| |
|
Histopathology
|
| |
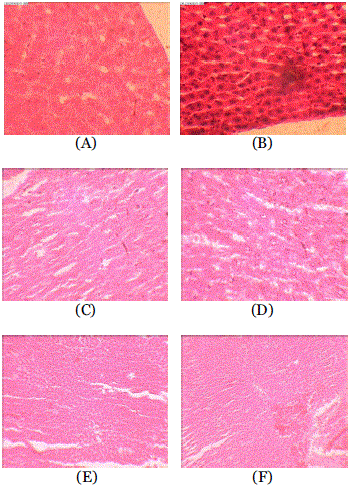
| In histopathological examination, normal architecture was observed in control animals whereas leukocytic inflammation, centrilobular necrosis, apoptosis, congestion and fatty changes were observed in the liver of DOX treated rats (Fig 1). The lesions were reduced significantly in animals which received Solanum torvum (100 mg/kg and 300 mg/kg) prior to DOX. |
| |
Discussion
|
| |
| Results of the present study indicate that the ethanolic extract of S.torvum significantly protected DOX –induced hepatotoxicity. The dose of DOX used in this study corresponds to the dose that is currently being used in the clinical practice [34]. The overall incidence of cardiotoxicity is about 3% at a total dose of 400 mg/m2 in human subjects [35]. In rats the corresponding dose was found to be 67.75 mg/kg using the dose calculator results. Our pilot study data indicated that the dose of DOX used in this study was effective to induce hepatotoxicity in rats. |
| |
| DOX in the form of DOX semiquinone has been suggested to play a major role in its hepatotoxic action [36]. Semiquinones are unstable under aerobic conditions thereby generating superoxide anion radicals by reacting with molecular oxygen [37]. Hepatocytes are the likely targets of reactive oxygen species attack in the failing liver. It is conceivable that free radicals cause damage at their formation. Consequently, a major source of ROS production, mitochondria could also be major target susceptible to ROS attack [38]. |
| |
| Serum transaminases have long been considered as sensitive indicator of hepatic injury [39]. Injury to the hepatocytes alters their transport function and membrane permeability, leading to leakage of enzymes from the cells [40], this leakage causes a decrease in the levels of ALT and AST in hepatic cells but increase in levels of ALT and AST in serum [41]. Administration of DOX to rats significantly increased serum ALT and AST levels. ALT is a more liver specific enzyme. Increase in AST activity is also roughly proportional to the extent of cardiac damage. Our results are in good agreement with those previously reported [42, 43, 44, 45]. However treatment with S. torvum extracts (100 and 300 mg/kg) has resulted in significant (p<0.05) decrease of enzyme activities in DOX treated animals thus offering considerable protection against hepatotoxicity (Table 2). |
| |
| The nephroprotective mechanism also appears to be through modulation of various anti-oxidant parameters thereby improving the overall antioxidant defence of the renal tissue. Free radical scavenging enzymes such as catalase, superoxide dismustase are the first line cellular defense enzymes against oxidative injury, decomposing O2 and H2O2 before their interaction to form the more reactive hydroxyl radical (OH•). The equilibrium between these enzymes is an important process for the effective removal of oxygen stress in intracellular organelles [46]. In our study, a decrease in concentration of SOD and CAT levels in DOX treated group was observed. S. torvum extract (100 mg/kg and 300 mg/kg) treatment significantly reversed the changes in antioxidant levels induced by DOX treatment. A decrease in the activity of SOD can result in the decreased removal of superoxide ion, which can be harmful to the hepatic cells. Moreover the enhanced SOD activity in the S. torvum treated group might be involved in the scavenging of O-2 generated from DOX. There is a general agreement that flavonoids act as scavengers of reactive oxygen species [47]. The antioxidant properties of Solanum torvum could be attributed to the presence of flavonoid phytoconstituent in it. Moreover, the histopathology data has revealed that treatment with S.torvum has protected the renal tissues from necrosis induced by DOX. |
| |
| Thus, this study revealed that pretreatment with Solanum torvum protected hepatic tissues against DOX induced hepatotoxicity. |
| |
Conflict of interest
|
| |
| The authors express no potential conflict of interest. |
| |
Acknowledgements
|
| |
| The authors acknowledge the technical assistance provided by Mr. Pradeep for histopath study and Dr. Meena Kulkarni, HOD, Dept of histopathology, KBH Dental College, Nashik. Section of liver from Group A (Control) showing normal architecture. Section of the liver from Group B (DOX (67.75 mg/kg) rats reveals leukocytic inflammation, centrilobular necrosis, apoptosis, congestion and fatty changes Section of liver from Group C (ST extract-100 mg/kg) and Group D (ST extract-300 mg/kg, p.o.) rats showing normal architecture. Section of the liver from Group E (ST extract -100 mg/kg + DOX-67.75 mg/kg) and Group F(ST extract -300 mg/kg + DOX-67.75 mg/kg ) rats shows few inflammatory cells and mild congestion. |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
| Figure 1 |
|
| |







