Keywords
Neurobehavioral development; Physical development; Postnatal; Protein malnutrition; Lead; Zinc; Rats
Introduction
Lead (Pb) is ubiquitous and one of the earliest metals discovered by the human race [1]. Unfortunately, exposure to Pb is unavoidable because of many applications of this metal in the current life of human and its accumulation in environment [2]. Lead may enter the body by ingestion through the intestines, through the lungs by inhalation or through the skin [3]. Lead cannot be considered safe or acceptable even at low blood levels as it causes alterations in the developing brain neurotransmitters [4].
Throughout pregnancy, lead readily crosses from the maternal to the infant circulation. Infant blood lead concentration becomes virtually identical to that of the mother [3]. Studies in human or in experimental animals have shown that Pb accumulates in the fetus from the second trimester onwards [5] and also during lactation since it is excreted into the milk leading to continues the risk to nursing offspring [6]. It was found that level of lead in milk and plasma during lactation are similar [7] and the lactation transfer of lead was considerably higher than the placental transfer [8]. Lead can also penetrate the blood brain barrier (BBB) of the infant and enter the developing brain [9]. The developing human brain is highly susceptible to lead even at very low concentrations [10]. Chronic lead exposure during perinatal or early postnatal development causes CNS impairments indicated by different behavioral, physiological and biochemical measures [11,12]. In children, exposure to low levels of Pb can lead to disorders of brain functions as impaired cognition, hearing and sight as well as different neurobehavioral functioning disorders including aggression. It was also found that Pb may be an important factor in inducing lower IQ in schoolchildren [13,14].
Lead poisoning exerts its most severe consequences during development, thus its developmental toxicity has become a vital area of research [15]. This can be attributed to the intense cellular proliferation, differentiation, and synaptogenesis in the developing brain [16]. Moreover, developing organism lacks of functional BBB in addition of being fivefold greater absorption of pb [17,18]. It is worthy to mention that, Pb exposure during gestation and early childhood has been reported to cause a variety of toxic effects in both human and animals [15]. Regarding this point, studies of animals and human have reached to similar results, since behavioral deficits induced by lead are seen at approximately the same blood levels in both [15,19]. Lead may produces its deleterious effects by causing oxidative stress which ultimately alters the cellular processes. Such effects might be affecting the morphological developments as well as the sensory motor reflexes of the pups and the behavior of the young adult offspring [15].
Nutritional inadequacy remains one of the most important non-genetic factors affecting development of brain. It continues to be of interest because of the widespread incidence of fetal and infantile nutritional deficiencies and the growing body of evidence that its effects on the developing brain are long-lasting and lead to permanent deficits in learning and behavior [20,21]. It has been shown that status of mother nutrition during gestation and lactation is essential to normal growth and development in both human and experimental animals [22]. Unfortunately, there are limited published data regarding human fetal physical and neurodevelopment delays under nutritional deficiency, but appropriate animal studies help to understand the effect of maternal malnutrition on fetal development [23].
As dietary protein is an important source of essential amino acids that can serve as intracellular antioxidant, its restriction may lead to an increase in oxidative damage by diminishing antioxidant defenses of tissue [24]. Protein malnutrition can occur throughout the lifespan from fetal life through adulthood. The most significant neurodevelopment effects appear to occur when severe PM is imposed on a rapidly growing brain during fetal and early postnatal life [25]. In rats, there are two principal types of PM experimental models: prenatal and early postnatal malnutrition [26]. During prenatal period, the fetus can take the essential elements from the dam thus may moderate the effect of malnutrition on the developing brain, whereas postnatal malnutrition can induce marked physiological, behavioral and cognitive deficits [20]. Protein malnutrition in early life was demonstrated to produce memory impairments in different experimental conditions [27].
Zinc (Zn) is a known fundamental component of the endogenous enzymatic antioxidant system. It plays an essential role in cell membrane integrity and functions in many aspects of cellular metabolism [28]. It acts as antioxidant [29] or a cofactor for the antioxidant enzyme [2]. Zn is notable among individual nutrients that have been designated as problem nutrients, adequate intake of which is difficult from complementary foods without fortification [30]. Zn can easily penetrate the placenta, the blood-brain barriers and also transferred through milk during nursing [31]. Importantly, maternal zinc status can influence the pregnancy and lactation outcomes. Many features of fetus and offspring such as growth, birth weight and morbidity, mental development or behavior depend on neonatal and early postnatal zinc availability [32,33]. Zinc deprivation during fetal life, lactation and early postnatal period induces several neurobehavioral abnormalities as anxiety, memory deficits and learning impairments [34,35]. Dose-dependent improvement of spatial learning and memory in rat pups has been evidenced after pre- and postnatal zinc administration. The influence of Zn on cognitive function is attributed to its anti-oxidative activity [35].
Thus, the aim of the current study was to investigate the influence of postnatal Pb exposure on physical development, neurobehavioral maturation and behavioral functions in the neonates of both NF and PM dams. Additionally, we aimed also to roll out and compare the possible protective effect of Zn against postnatal Pb-induced developmental and neurobehavioral alterations in both conditions of normal feeding and under concomitant protein malnutrition.
Materials and Methods
Animals: The study was performed in accordance with the ethical guidelines of Faculty of Pharmacy, Al-Azhar University, Egypt. Adult male Sprague-Dawley rats weighing 200-220 g and female Sprague-Dawley rats weighing 170-200 g were utilized. Rats were obtained from The NILE Co. for Pharmaceuticals and Chemical Industries, Cairo, Egypt. They were kept under the same adequate environmental conditions and provided with their daily dietary requirements consisting of standard diet pellets (El-Nasr Chemical Co., Abu Zaabal, Cairo, Egypt) and water was given ad-libitum. Either males or females rats were housed in stainless-steel cages (three to four per cage) and kept at the animal house for an acclimation period of one week prior to mating. Each male rat was introduced into a cage together with two to three pro-estrous females overnight and vaginal smears from females were examined microscopically in the following morning. The presence of sperms in the vaginal smears marked the occurrence of mating and was considered as day 1 of gestation. One week prior to the projected delivery date the females were caged individually.
Nourishment: From parturition which considered postnatal day 0, experimental lactating female (dams) were maintained on special diet containing standard quantities of all the required dietary components [36]. They were allowed food and water ad-libitum. Two preparations of diet were utilized in this study, a protein sufficient diet (20% casein) for NF groups; each 100 g diet contain (20 g) casein, (70 g) sucrose-starch mixture 1:1, (4 g) salt mixture, (5 g) oil and oil-soluble vitamins and (0.6 g) vitamin mixture in starch [37] and protein deficient one (8% casein) for protein PM groups [38]. It has the same composition as protein sufficient diet except that casein was reduced to 8 g instead of 20 g per 100 g of the diet. The remained 12 g was replaced by sucrose-starch mixture.
Drugs and chemicals: Lead acetate and Zinc sulfate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Both were freshly dissolved in distilled water.
Experimental design: After parturition dams were divided randomly into two major groups. Group A (NF groups): Dams were maintained on a protein sufficient diet (20% casein) from parturition (PND 0) until PND 25. Group B (PM groups): Dams were maintained on a protein deficient diet (8% casein) from parturition (PND 0) until PND 25. Both PM and PM groups were further subdivided into three subgroups (6 dams/each). Group 1: Dams received saline daily from parturition (PND 0) until weaning (PND 21) and served as control group. Group 2: Dams were gavaged daily with 1 ml solution of lead acetate containing 12 mg/ml pb [39] from parturition until weaning (PND 21) and served as a Pb groups Group 3: Dams were gavaged daily with 1 ml solution of lead acetate containing 12 mg/ml pb, they were also administered Zinc sulphate orally 32 mg/kg [35] from parturition until weaning (PND 21) and served as zinc treated groups. All treatments were at a dose volume not exceeding 0.5 ml/100 g body weight. The litter size was holding constant between 6-8 offspring per dam which represents the optimal size [40]. Tests were conducted on 3-4 offspring per each litter which were selected at random and marked for testing on postnatal day 1.
All dams and their offspring were taken to the test situation for examination of the testing parameters after removing food and water from the home cage. Physical and neurobehavioral testing began on postnatal day 1 and extended until the end of all examinations at PND 25. Rat pups were evaluated for postnatal physical and neurobehavioral development as well as for functional behavioral deficits using the following developmental test battery which used as indicator for postnatal adversities: Change in body weight, Development of physical signs including Pinna detachment, Downy hair, Incisor eruption, Fur development, Ear opening and Eye opening [40,41]. Development of reflexes and sensory functions (neurobehavioral development) including Righting reflex, Cliff avoidance, Negative geotaxis 25°, Palmar grasp, Negative geotaxis 45° and Auditory startle [41]. In addition rat pups were evaluated in two types of behavioral experiments: performance in the Neonatal T-maze: in which number of correct choices /10 trials was measured and performance in the Open-field test: in which latency time, ambulation frequency, rearing frequency and grooming time were measured.
1. Physical development:
• Body weight changes: Rat pups were weighed at different ages: PND 0, 5, 10, 15, 20 [15,42].
• Development of physical signs: The test infant rats (3-4/ litter) in all groups were daily examined carefully according to a suitable time schedule where the time at which the following physical signs had been evident was recorded for each rat pup in all groups [41,43].
• Pinna detachment: Observed until unfolding of the external ear was evident for each infant rat.
• Downy hair: Observed until the appearance of primary coat of downy hair in all infants.
• Incisor eruption: Upper and lower incisors were observed daily until they had erupted through the gum-line for each test pup.
• Fur development: Observed daily until complete development of fur for each infant rat.
• Ear opening: Observed until complete opening of both ears for each neonate.
• Eye opening: It was observed daily for each pup until all test pups exhibited complete opening of both eyes.
2. Neurobehavioral development: Time of appearance of various reflexes and sensory functions were daily recorded for all examined rat pups (3-4 pups/litter) in all groups according to a time schedule which determine the approximate time for development of these reflexes and sensory functions [41,43].
• Righting reflex: Rat pup turns over to rest in normal position when placed on its back (from a supine to prone position), two trials per day with a maximum time allowance of 30 sec. per trial.
• Cliff avoidance: Infant rats crawl away from the edge of a cliff or a table top with the forepaws and face over the edge to avoid drop, they show retraction and backward or sideward movement within 60 sec.
• Negative geotaxis 25°: Test pups rotate up by 180° on 25° inclined plywood plane where placed in a head down position, pups were given one trial per day and allowed a maximum time of 60 sec.
• Palmar grasp: Infant rats try to grasp a paper clip with forepaws if stroked.
• Negative geotaxis 45°: Infant rat rotates 180° on 45° inclined plywood plane from a head-down to head-up orientation; each infant was given one trial per day up to a limit of 60 sec. It must be noticed that the approximate time for development of negative geotaxis behavior is directly proportional with the angle of the slope.
• Auditory startle: Observed until a positive startle response (jerk of the head and extension of the hind limbs) occurred to an auditory stimulus 15 cm over the head of the infant rat, infants were given one trial per day and the presence or absence of startle response can be readily recognized.
3. Behavioral experiments: They include evaluation of the behavioral development in an unfamiliar environment by using learning and memory tests for infant rats [40,41,43].
• Performance in the neonatal T-maze: Infant rats were alternatively tested in the neonatal T-maze which was used as index of learning and memory. The neonatal T-maze is made of black acrylic and measured 5.1 × 30.5 × 5.1 cm in the stem and 5.1 × 25.4 × 5.1 cm in the cross arm [41]. A correct choice resulted in a door being opened to a connected chamber containing the pup's home bedding and its littermates while an incorrect choice led to a dead end, followed by being picked up and placed in an empty cage for 1 min of isolation. Gates at the entrance of each choice arm prevented retracing once a decision was made. Number of correct choices out of ten trials was recorded for each neonate.
• Open-Field Test (OFT): It is a general test for motor activity, excitability, emotionality and exploratory behavior in rodents. OFT is considered as one of the most important procedures in the majority of behavioral studies. It consists of a square wooden box 40 cm × 40 cm × 25 cm height [44], with red sides and white floor. The floor of the field was divided by black lines into 16 equal squares 4 × 4 [45,46]. The test was performed under white light in a quiet room. The experiment performed between 10:00 A.M. - 4:00 P.M. One hour before the experiment, all animals were taken to test situation after removing food and water from the home cage. Experimental animal were taken from their cages alternately; placed into the central squares of the open-field and videotaped for 3 min [47]. The open-field was thoroughly wiped using 10% isopropyl alcohol and dried before application of a new subject in order to obviate possible effect on its behavior due to odor remained from previous rats [48].
The behavior of the experimental rat in the open-field was continuously recorded during the 3 min. observation period [46] using coded symbols for the following parameters: Latency, time from putting the animal at the middle of the arena until decided to move [43]. It was measured in seconds using a stopwatch. Ambulation frequency; number of squares crossed by the animal [45,49]. It was scored as a total count during a 3 min. period. Rearing frequency, number of time the animal stood stretched on its hind limbs with and without forelimbs support [49,50]. It was scored during a 3 min. observation period. Grooming time, time elapsed while the animal was scratching face, licking paws, fur or genitals [50].
Statistical analysis: Data was expressed as the mean + SEM. and statistical analysis was carried out by one way ANOVA followed by Tukey-Kramer multiple comparisons test to calculate significance of the difference between treatments. Values of p<0.05 were considered significant. All statistical analyses were performed using Instat (version 3) software package. Graphs were sketched using GraphPad Prism (ISI®, USA) software (version 5).
Results
1. Change in body weight: As shown in Figure 1 (i and ii), postnatal Pb administration induced a significant reduction in the body weight of only NF rat pups at PND 5, 10, 15, 20 to (55.38%, 50.93%, 59.19%, 49.39% of normally fed control value) respectively as compared to their corresponding control group.
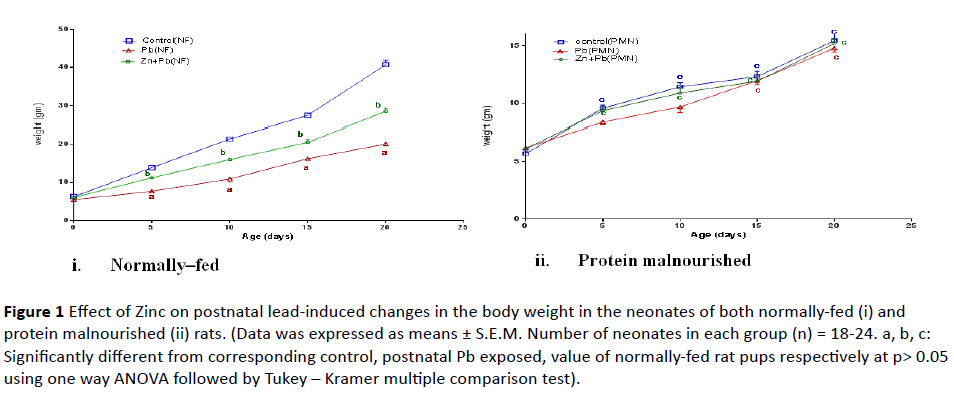
Figure 1: Effect of Zinc on postnatal lead-induced changes in the body weight in the neonates of both normally-fed (i) and protein malnourished (ii) rats. (Data was expressed as means ± S.E.M. Number of neonates in each group (n) = 18-24. a, b, c: Significantly different from corresponding control, postnatal Pb exposed, value of normally-fed rat pups respectively at p> 0.05 using one way ANOVA followed by Tukey – Kramer multiple comparison test).
Induction of protein malnutrition in lactating dams significantly decreased the body weight of their pups at PND 5, 10, 15 and 20 to (68.8%, 53.32%, 44.88% and 37.93% of normally fed control value) respectively as compared to normally-fed control rat pups. Zinc co-administered postnatal with lead caused a significant increase in the body weight of only NF pups at PND 5, 10, 15, 20 to (80.74%, 74.79%, 74.81% and 69.95% of normal control value) respectively but it didn’t significantly alter the body weight of PM pups as compared to corresponding postnatal lead alone exposed.
2. Development of physical signs: As shown in Figure 2 (ivi), postnatal Pb administration induced a significant prolongation in the time of appearance of downy hair, fur development, ear opening and eye opening in NF rat pups to (138.64%, 124.19%, 122.21% and 111.61% of normal control value) respectively as compared to their corresponding control. Zinc co-administered postnatal with lead induced a significant decrease in the time of appearance of downy hair, fur development, ear opening and eye opening in NF pups to (108.07%, 99.2%, 104.41% and 101.23% of normal control value) respectively as compared to corresponding postnatal lead alone exposed pup.
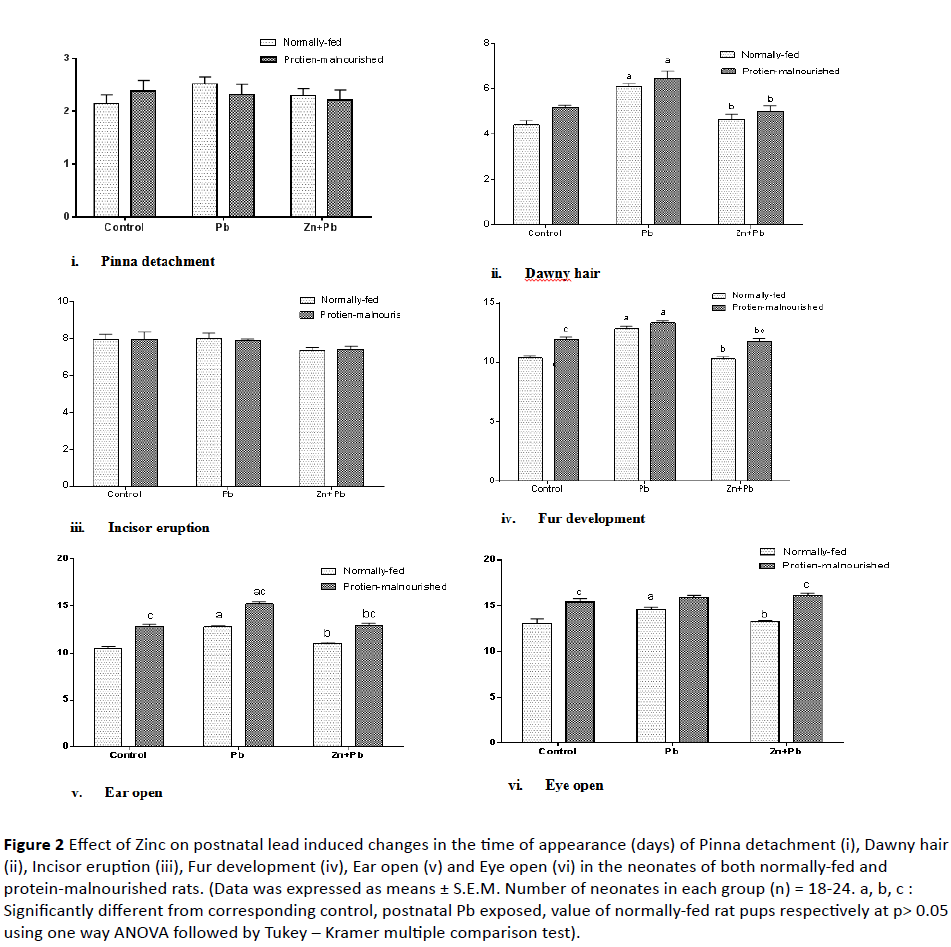
Figure 2: Effect of Zinc on postnatal lead induced changes in the time of appearance (days) of Pinna detachment (i), Dawny hair (ii), Incisor eruption (iii), Fur development (iv), Ear open (v) and Eye open (vi) in the neonates of both normally-fed and protein-malnourished rats. (Data was expressed as means ± S.E.M. Number of neonates in each group (n) = 18-24. a, b, c : Significantly different from corresponding control, postnatal Pb exposed, value of normally-fed rat pups respectively at p> 0.05 using one way ANOVA followed by Tukey – Kramer multiple comparison test).
Induction of protein malnutrition in lactating dams significantly prolonged the time of appearance of fur development, ear opening and eye opening in their pups to (115.21%, 122.65% and 117.95% of normal control value ) respectively as compared to normal control group. In PM pups postnatal Pb exposure significantly increased the time of appearance of downy hair, fur development and ear opening to (147.09%, 128.78% and 145.44% of normally fed control value) respectively as compared with their corresponding control. While with respect to corresponding value of NF rat pups there was a significant prolongation in the time of appearance of ear opening only. Zinc significantly reduced the time of appearance of downy hair, fur development and ear open to (113.64%, 114.15% and 123.48% of normal control value) respectively in PM pups as compared to corresponding postnatal lead alone exposed pups. But with respect to corresponding value of NF rat pups; there was a significant prolongation in the time of appearance of fur development, ear opening and eye open.
3. Development of reflexes and sensory functions (neurobehavioral development): As illustrated in Figure 3 (i-vi) postnatal lead administration induced a significant prolongation in the time of appearance of Negative geotaxis 25°, Negative geotaxis 45° and auditory startle in NF rat pups to (127.99%, 118.1% and 120.89% of normally-fed control value) respectively as compared to their corresponding control. Zinc co-administered postnatal with lead induced a significant decrease in the time of appearance of Negative geotaxis 25°, Negative geotaxis 45° and auditory startle in NF pups to (98.14%, 100.2% and 103.54% of normal control value) respectively as compared to corresponding postnatal lead alone exposed pups.
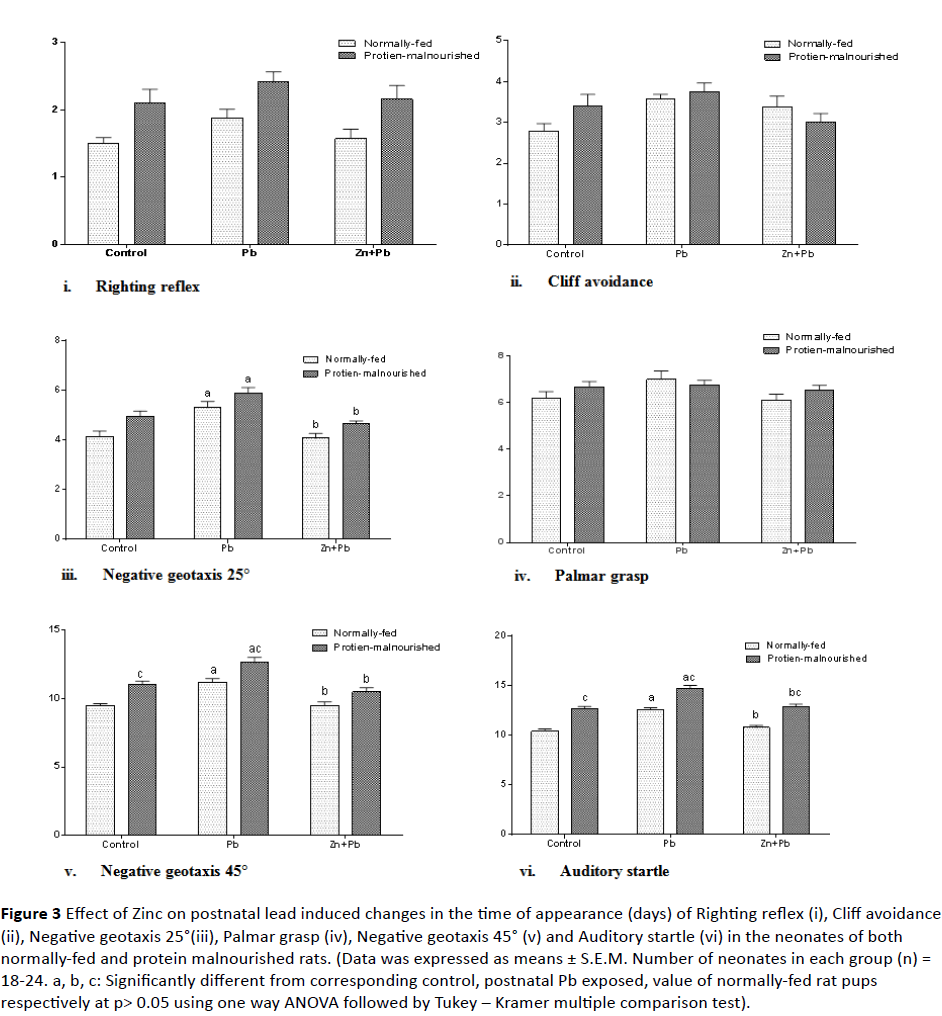
Figure 3: Effect of Zinc on postnatal lead induced changes in the time of appearance (days) of Righting reflex (i), Cliff avoidance (ii), Negative geotaxis 25°(iii), Palmar grasp (iv), Negative geotaxis 45° (v) and Auditory startle (vi) in the neonates of both normally-fed and protein malnourished rats. (Data was expressed as means ± S.E.M. Number of neonates in each group (n) = 18-24. a, b, c: Significantly different from corresponding control, postnatal Pb exposed, value of normally-fed rat pups respectively at p> 0.05 using one way ANOVA followed by Tukey – Kramer multiple comparison test).
Induction of protein malnutrition in lactating dams significantly prolonged the time of appearance of Negative geotaxis 45° and auditory startle in their pups to (116.34% and 121.74% of normally-fed control value) respectively. In PM pups postnatal lead exposure significantly increased the time of appearance of Negative geotaxis 25°, Negative geotaxis 45° and auditory startle to (142.27%, 133.06% and 141.16% of normal control value) respectively as compared to their corresponding control. With respect to corresponding value of NF rat pups there was a significant prolongation in the time of appearance of Negative geotaxis 45° and auditory startle only. In PM pups zinc significantly reduced the time of appearance of Negative geotaxis 25°, Negative geotaxis 45° and auditory startle to (112.18%, 110.47% and 110.47% of normal control value) respectively as compared to corresponding postnatal lead alone exposed pups. But With respect to corresponding value of NF rat pups; there was a significant prolongation in the time of appearance of auditory startle.
4. Performance in the neonatal T-maze: As shown in Figure 4 postnatal Pb administration induced a significant reduction in the number of correct choice in NF pups to 77.6% of normally fed control value as compared to their corresponding control. Induction of postnatal protein malnutrition in lactating dams significantly reduced the number of correct choice in their pups to 80.54% of normally fed control value. In PM pups postnatal Pb exposure showed an additional significant reduction in the number of correct choice to (55.42% of normal control value) as compared to their corresponding control. Also, with respect to corresponding value of NF rat pups there was a significant decrease in the number of correct choice. Zinc co-administered postnatal with lead induced a significant elevation in the number of correct choice in both NF and PM pups to (96.51% and 77.81% of normally fed control value) respectively as compared to the corresponding postnatal lead alone exposed pups. However, with respect to corresponding value of NF rat pups; there was a significant decrease in the number of correct choice in PM pups.
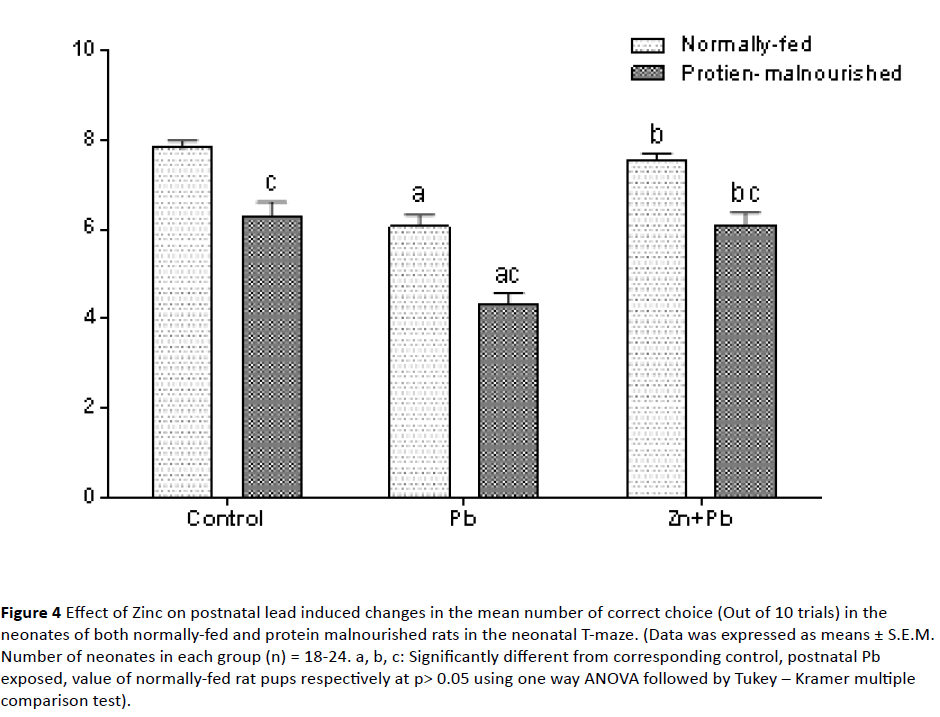
Figure 4: Effect of Zinc on postnatal lead induced changes in the mean number of correct choice (Out of 10 trials) in the neonates of both normally-fed and protein malnourished rats in the neonatal T-maze. (Data was expressed as means ± S.E.M. Number of neonates in each group (n) = 18-24. a, b, c: Significantly different from corresponding control, postnatal Pb exposed, value of normally-fed rat pups respectively at p> 0.05 using one way ANOVA followed by Tukey – Kramer multiple comparison test).
5. Performance in the open-field test: As illustrated in Figure 5 (i-iv) postnatal Pb administration induced a significant reduction in the ambulation and rearing frequencies to (66.41% and 63.38% of normal control value) respectively while it showed a significant elevation in the grooming time to 143.32% of normal control value) in NF rat pups as compared to their corresponding control.
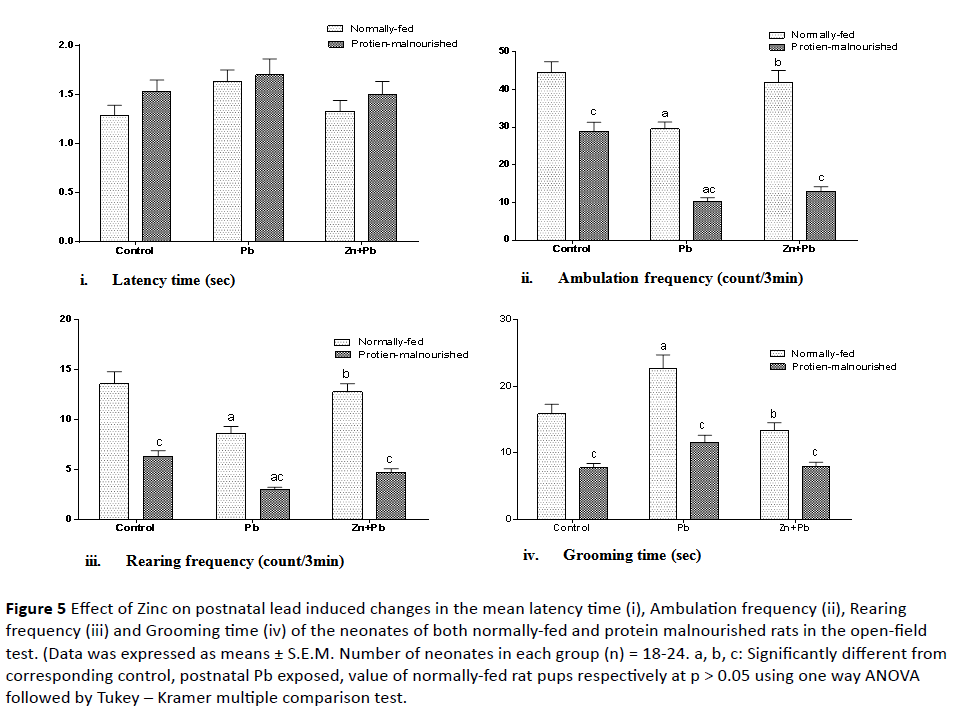
Figure 5: Effect of Zinc on postnatal lead induced changes in the mean latency time (i), Ambulation frequency (ii), Rearing frequency (iii) and Grooming time (iv) of the neonates of both normally-fed and protein malnourished rats in the open-field test. (Data was expressed as means ± S.E.M. Number of neonates in each group (n) = 18-24. a, b, c: Significantly different from corresponding control, postnatal Pb exposed, value of normally-fed rat pups respectively at p > 0.05 using one way ANOVA followed by Tukey – Kramer multiple comparison test.
Induction of protein malnutrition in lactating dams significantly decreased the ambulation frequency, rearing frequency and grooming time to (64.75%, 46.54% and 48.74% of normal control value) respectively as compared to normal control group. In PM pups postnatal lead exposure caused an additional significant reduction in the ambulation and rearing frequencies to (23.22% and 22.06% of normal control value) as compared to their corresponding control. With respect to corresponding value of NF rat pups there was a significant decrease in the ambulation frequency, rearing frequency and grooming time.
Zinc co-administered postnatal with lead induced a significant increase in the ambulation and rearing frequencies to (94.04% and 93.97% of normally fed control value) respectively and a significant decrease in the grooming time to (84.55% of normally fed control value) only in NF pups as compared to corresponding postnatal lead alone exposed pups. But with respect to corresponding value of NF rat pups,
Discussion
Lead is an environmental and industrial pollutant that has been detected in almost all biological system. Developing brain has been shown to be more susceptible to the neurotoxic effect of Pb [51]. Thus, Pb exposure/toxicity continues to be a leading environmental health issue for children and women of childbearing age [15]. In the present study, developmental lead exposure was carried out postnatally during lactation period. Lactational transfer of Pb from mother to fetus has been demonstrated in several studies [15]. A Pervious study has shown that the level of Pb in milk are thus similar to those in plasma [7] and that lactational transfer after current or recent exposure to Pb in dams was considerably higher than placental transfer [8].
Results of the current study showed that, postnatal administration of Pb to lactating dams induced suppression in the rate of somatic growth of their pups as reflected by significant decrease in the body weight which was evident very early at PND 5. Body weight reductions in dams and offspring are a frequent observation in standardized testing protocols designed to assess the developmental toxicity of environmental contaminants [52,53]. These results are in agreement with the previous studies of Kahloula et al. and Luthman et al. which showed that Pb induce reduction of food intake. The Pb induced reduction of food intake, may be due of Pb action on certain centers responsible to the control of the hunger in the brain [54,55]. In addition, the endocrine and biochemical mechanisms underlying the growth suppression produced mainly by gestational and lactational lead exposure are related with decreases in growth hormones and associated factors [56].
Current investigation showed that induction of protein malnutrition in lactating dams significantly decreased the body weight of their pups which reflected as decline in the rate of somatic growth. The protein malnutrition induced weight reduction was also demonstrated in the studies of Fukuda et al., da Silva Hernandes et al. and Falcao-Tebas et al. [57-59]. PM induced weight reduction may be explained by the fact that, maternal protein restriction is associated with lower stores of maternal nutrients and, subsequently, lesser transfer of nutrients to the offspring, which is related to reduced postnatal growth [60].
In the present study lead didn’t show any significant alteration in the body weight of PM pups as compared to PM control pups which revealed that lead didn’t cause additional decline in the body weight over that induced by protein malnutrition. The severity of malnutrition-induced body weight reduction is a function of the type (malnutrition or under nutrition) and the age at onset of diet restriction. Malnutrition-induced decrease in body weight was higher when it occurred at birth, during lactation and through the month following weaning [1]. Thus, in the present study induction of protein malnutrition during the lactation period has large inhibitory effect on the body weight which may explain the lack of Pb-induced reduction in the body weight of PM pups. On the other hand, current results showed that lead induced a significant reduction in the body weight of PM pups with respect to corresponding value of normally fed rat pups. However this significance was achieved just at PND 15. These data indicated that the deleterious effect of postnatal lead exposure on the rate of somatic growth of rat pups was markedly greater under condition of concomitant protein malnutrition however, this difference was clear only after longer period of Pb exposure.
There are increasing considerations have been given to the interactions occurring in the organism between toxic and essential metals. So, one can use a beneficial or less harmful element to combated damages induced by toxic element. In this regard Zn has been shown to reverse some toxicity of Pb in experimental animals [51,61]. It was demonstrated that, Zn easily penetrates the placenta and blood-brain barriers, and is transferred to a suckled child via mother’s milk [62]. In the current work Zn, administered concomitantly with Pb induced a significant increase in the body weight of NF pups without any significant alteration in the body weight of PM pups. These data reflect the protective effect of zinc against lead induced deficit in the rate of somatic growth of NF rat pups. This protective role of zinc was abolished under a condition of protein malnutrition which may be due to the additional effect of protein malnutrition itself on the body weight. The findings of the current work are in harmony with several previous studies which demonstrated the protective role of zinc against lead toxicity in different body system. A decrease in blood Pb level of rats during Zn co-administration was reported [63]. Additionally, the maternal zinc status influences the pregnancy and lactation outcomes [35]. Many features of fetus and offsprings such as growth, birth weight and morbidity, mental development or behavior depend on neonatal and early postnatal zinc availability [32,33].
Results of the current work revealed that developmental Pb exposure induced a decline in the postnatal physical and neurobehavioral development in both NF and PM rat pups as indicated by the delay in the time of appearance of some landmarks of physical growth (including downy hair, fur development, ear opening, eye opening) and by prolongation in the time of appearance of some reflexes and sensory function (including Negative geotaxis 25°, Negative geotaxis 45° and auditory startle). In addition, there was a significant prolongation in the time of appearance of ear open, Negative geotaxis 45° and auditory startle in PM pups with respect to corresponding value of NF pups which revealed that some of the postnatal lead induced deteriorations in the physical and neurobehavioral maturation was markedly greater when a condition of concomitant protein malnutrition is coexist. The most of the physical sign and neurobehavioral reflexes that didn’t altered by Pb exposure are those which are evident early in the postnatal life as pinna detachment, righting reflex and cliff avoidance. These finding are parallel to the findings which showed delayed eye opening and prolongation in the time of Negative geotaxis [55]. In agreement with our study the delay in the appearance of ear unfolding and Negative geotaxis was also demonstrated by Shallie et al. [15]. However the Shallie et al. study pointed to an advance in the day of appearance in some physical maturation as accelerated eye open and incisor eruption [15]. It was reported that Pb treatment (in contrast to our result) hastened the day of appearance of eye opening, startle reflex and negative geotaxis while it (in agreement with our result) caused no effect on the date of ear unfolding or incisor eruption in the pups [42]. This disagreement among results may be attributed to different onset, duration, dosage and method of imposing heavy metal intoxication, and alterations in maternal behavior.
The present study showed that protein malnutrition during the critical period of development resulted in a deterioration in the postnatal physical and neurobehavioral development in rat pups as reflected by the delay in the time of appearance in some landmarks of physical growth (fur development, ear opening, eye opening) and some reflexes and sensory functions as Negative geotaxis 45° and auditory startle. The physical signs and neurobehavioral reflexes that were significantly delayed by postnatal protein malnutrition are those which are evident lastly in the postnatal life (when the protein malnutrition was well established). These results are in accordance with the results of the study of Falcao-Tebas et al. which demonstrated that pups born to mothers provided with the low-protein diet during gestation and lactation showed a delay in the appearance of physical feature and most reflexes and a deficit in somatic growth [59]. Additional evidence was also provided by numerous studies [23,64].
There are some factors which can contribute to the delay in the physical growth and neurobehavioral development of pups induced by maternal under nutrition, such as the nonavoidable stress and neurotransmitter systems [23]. Many investigators found biochemical alterations in the nervous system in experimental malnutrition models [9], especially those related to neurotransmitter systems [65]. Also it was reported that rats submitted early in life to low-protein diets revealed altered brain levels of noradrenalin, dopamine, and serotonin [66]. Moreover, this deterioration in brain development may be due to increased oxidative stress since dietary protein is an important source of essential amino acids that can be used as intracellular antioxidants. Therefore, its restriction may lead to an increase in oxidative damage [9,65].
Results of the current work suggested the protective role of postnatal zinc against developmental lead induced deterioration in the physical and neurobehavioral development in both NF and PM rats as indicated by acceleration in the time of appearance of some sign of physical and neurobehavioral maturation which were delayed by lead (as downy hair, fur development, ear opening, eye opening, Negative geotaxis 25°, Negative geotaxis 45° and auditory startle) when Zn was co-administered along with lead to NF, PM dams. However, there was a significant prolongation in the time of appearance of fur development, ear opening, eye opening and auditory startle in PM pups with respect to corresponding value of normally fed rat pups. These data suggested that the protective role of Zn against some aspect of lead induced deficit in the physical and neurobehavioral development may be attenuated under a condition of protein malnutrition. This decline may be attributed to the additional deteriorating effect of protein malnutrition itself on these parameters.
There are several mechanisms were suspected to be involved in the lead induced defect in the physical and neurobehavioral development and zinc has been demonstrated in several studies to inhibit most of these mechanisms. Reduction in the level of Ach may explain why the motor reflexes of lead exposed pups were compromised [15]. Pb-exposure mainly affects cholinergic system by reducing Ach release [66,67], uptake [68] and turnover rates [69]. Zinc supplementation was demonstrated to protect the brain from the Pb-induced alterations in cholinergic system [70]. Additionally, lead produces its deleterious effects probably by causing oxidative stress which ultimately alters the overall morphological developments and sensory motor reflexes of the developing pups and behavior of the young adult rat [15]. Zinc supplementation significantly protects against lead-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain, a major contributing factor to neurotoxicity [71]. The neonatal reflexes may be considered as an index of brain maturation [23]. Thus, the observed actions of lead may be related to impaired maturation of sensitive brain regions which develop postnatal [15]. Zinc was shown to possess a critical role for correct brain development and functioning of the CNS in many experimental studies in animals [35].
The current results showed that Pb significantly decreased the number of correct choice in the neonatal T-maze in both NF and PM pups. Additionally there was a significant reduction in the number of correct choice in PM pups with respect to corresponding value of NF pups. These data revealed that postnatal lead exposure induced a significant impairment in the learning and memory in both NF and PM pups. The impairment in learning and memory was significantly higher in the PM pups than in NF pups. The present findings are in harmony with numerous studies which demonstrated a significant impairment of learning and memory after developmental exposure to lead either postnatal during lactation [72-74] or from beginning of gestation (prenatal) until weaning [75,76]. Abundant evidence suggests that many of the neurochemical, neuroanatomical, and neurophysiologic sites sensitive to Pb may play crucial roles in the regulation of cognitive processes [77]. It has been shown that hippocampus is particularly sensitive to Pb in animal models [78] and in humans [79]. Spatial memory is a hippocampus-dependent process and it is well established that Pb impairs this arm of cognition [73]. Morphologically, Pb exposure affects neurogenesis in hippocampus [80]. Neurochemically, pb replaces calcium resulting in altered calcium channel; and metabolically, it causes oxidative stress and damages [81]. All these effects can lead to learning and memory deficits [73].
The data of the present work revealed that early postnatal malnutrition induced an impairment of learning and memory as indicated by a significant reduction in the number of correct choice in the neonatal T-maze in PM pups. The malnutrition induced learning and memory impairment was supported with several studies which showed learning and memory deficit after malnourishment imposed postnatal only as revealed by Valadares et al. and Reyes-Castro et al. [43,82] or prenatal and/or postnatal as demonstrated by Reyes-Castro et al. [82]. There are several mechanisms suggested for malnutrition induced impairment in learning and memory. It has been shown that early protein deprivation affects the glutamatergic, cathecolaminergic, cholinergic, serotoninergic, GABAergic and opioids neurotransmitter systems [83]. Additionally, it was known that the hippocampus has been associated with spatial and non-spatial learning and memory [84]. It is very sensitive to different stressful challenges such as malnutrition [85]. Long periods of malnutrition decrease hippocampus volume and number of neurons [86] which may partly explain the emotional, motivational, and memory disturbances commonly observed in malnourished rats [87].
Results of the present study revealed that Zn effectively reverses the Pb induced deficit in learning and memory in both NF and PM pups as reflected by increased the number of correct choice in neonatal T-maze. The protective role of Zn against Pb induced impairment in learning and memory decline to some extent when PM is coexist. This may be attributed to the additional deleterious effect of protein malnutrition itself on learning and memory. The findings of the current work are in harmony with other findings [88]. A direct evidence of the dose-dependent improvement of spatial learning and memory in rat pups after pre- and postnatal zinc administration was provided by another study [35]. Our results are in consistency with several studies which suggested that zinc supplementation may be an effective treatment option for improving behavioral deficits such as cognitive impairment induced by various insults such as maternal zinc deficiency [89] and following traumatic brain injury (TBI) [90].
Zinc is believed to play an important role in cognition and memory via its function as a neuronal messenger, modulator of synaptic transmission and cortical plasticity [91]. It is important for myelination and for the release of the neurotransmitters GABA and glutamate, which are key modulators of neuronal excitability [92]. The neuromodulatory effect of zinc may be connected with its antioxidant properties. Thus the influence of zinc supplementation on cognitive function may depend also on its anti-oxidative action [35]. Additionally, Jaako-Movits et al. demonstrated that developmental lead exposure induces persistent inhibition of neurogenesis in the rat hippocampus which could, at least partly, contribute to behavioral and cognitive impairments [93]. The key roles for the essential trace element zinc in the control of both developmental and adult neurogenesis has been revealed [94].
Data obtained from the current work suggested that postnatal Pb exposure induced a significant reduction in locomotor and exploratory activities in both NF and PM rat pups as reflected by a significant reduction in the ambulation and rearing frequencies. The reduction in locomotor and exploratory activities was markedly greater in case of protein malnutrition which may be attributed to the additional effect of protein malnutrition itself. Regarding the emotional behaviour, lead resulted in an increase in the emotional behavior as indicated by a significant increase in grooming time in the NF rat pups only while lead induced alteration in the emotionality was not detected in PM rat pups. The current results are supported by the results of previous studies which showed that Pb poisoning caused a reduction of locomotor activity and decrease of environment exploration in offspring born from lead exposed dams either during lactation only as previously demonstrated [55] or starting from gestations untill weaning [95]. While in contrast to the present study a significant increase in spontaneous locomotor activity as revealed by Moreira et al. [96] as well as rearing which was reported by Hassan and Jassim [97]. Regarding the emotionality the current findings are in conformity with the findings of Lisboa et al. [98]. However, in contrary to present findings, Basha et al. [95] reported a decrease in grooming activity. Generally studies assessing the behavioral effects of Pb exposure have found conflicting results. The exposure regimen and behavioral protocols are very variable and have led to different results in the literature [96]. Based on this consideration, the discrepancy between our findings and results of some study could be ascribed to differences in Pb dose, route of administration, developmental stage (through gestation, lactation or post weaning), duration of exposure and the PND at which the test is conducted.
It was reported that the open-field behavioral alterations induced by lead can be attributed to the perturbations in the cholinergic and aminergic systems in the Pb-exposed hippocampus [95]. There are increasing numbers of reports regarding Pb related changes in dopaminergic system [99] which play critical roles in the regulation of cognitive processes and locomotor activity [54]. The current results showed that induction of postnatal protein malnutrition resulted in a significant reduction in locomotor and exploratory activity as well as a reduction in the emotional behavior as indicated by OFT. The reported hypoactivity in OFT is supported by other findings [82]. The present study is not in harmony with that of Ohishi et al. [100] in which there no statistically significant difference between the normal was and low protein diet group in male offspring while in female offspring, statistically significant high values were recorded in the motor activity in the low protein diet group. This contradiction may be due to the difference in the malnutrition regimen that has been applied in different stages of development, the period of nutritional rehabilitation and to the age of animals at which locomotor activity were evaluated. Regarding emotionality the findings of the present study agreed with that obtained by Vicente et al. [101]. However, it was found that severe protein malnutrition resulted in hyperactivity in the OFT [101]. It has been shown that early protein deprivation affects the glutamatergic, cathecolaminergic, cholinergic, serotoninergic, GABAergic and opioids neurotransmitter systems [83]. Most of these neurotransmitter systems play critical roles in the regulation of locomotor activity [54] the observed hypoactivity in the OFT may be due to the alteration in the neurotransmitter systems induced by protein malnutrition.
These data revealed that postnatal zinc administration inhibited lead induced alterations in the locomotor, exploratory activities as well as in the emotional behavior (as indicated by a significant increase in the ambulation and rearing frequencies and a significant decrease in the grooming time) in the neonates of NF dams while it didn’t exert this action in the neonates of PM dams. These findings are in conformity with the findings of Piechal et al. and Prasanthi et al. studies [35,88]. Also it was demonstrated that zinc deficiency during the last trimester of pregnancy and during lactation impaired motor activity [102]. Zinc was suggested to alter the kinetics of Pb toxicity and reduce the Pb burden on the body; thereby confirming that intake of Zn nutrient may be beneficial in preventing the toxic effects of Pb [88]. The lead induced behavioral disorders seen in the open-field experiments could be due to alterations in any of the various neurotransmitter systems [88] such as the cholinergic [103], aminergic [95] glutaminergic, or dopaminergic systems [104,105]. Zinc was shown to protect against these lead induced alterations which account for the behavioral impairment. Zinc supplementation reversed the Pb-induced perturbations both in the levels of monoamines and in the activity of MAO [106]. Additionally, Zinc was reported to protect the brain from the Pb-induced alterations in cholinergic system [70]. Moreover, Zn supplementation reversed the Pb-induced effects on antioxidant enzymes, lipid peroxidation and free radical formation. These findings strongly support that zinc supplementation significantly protect the Pb-induced oxidative stress, a major contributing factor to neurotoxicity [71].
Conclusion
Postnatal lead exposure caused delay in some aspect of physical and neurobehaviol development as well as in some behavioral functions as learning, locomotor and exploratory activities in addition to increased emotionality. Most of the lead-induced alterations were much greater under condition of protein malnutrition. Zinc was shown to protect against these lead-induced alterations in both NF and PM rat pups. However, the protective effect of Zinc sometimes declined to some extent under concomitant protein malnutrition which can be attributed to and also explained by the additive deteriorating effect of protein malnutrition itself.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Financial support
This research has not received any specific grant from any funding agency in the public, commercial or not for profit sectors. The work has been done on the expense of the authors.
9744
References
- Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: A review with recent updates. InterdiscipToxicol 5: 47-58.
- Malekirad AA, Oryan S, Fani A, Babapor V, Hashemi M, et al. (2010) Study on clinical and biochemical toxicity biomarkers in a zinc-lead mine workers. ToxicolInd Health 26: 331-337.
- Bijoor AR, Sudha S, Venkatesh T (2012) Neurochemical and neurobehavioral effects of low lead exposure on the developing brain. Indian J ClinBiochem 27: 147-151.
- Bhattacharyya MH (1983) Bioavailability of orally administered cadmium and lead to the mother, fetus, and neonate during pregnancy and lactation: an overview. Sci Total Environ 28: 327-342.
- Hallen IP, Jonsson S, Karlsson MO, Oskarsson A (1996) Toxicokinetics of lead in lactating and nonlactating mice. ToxicolApplPharmacol 136: 342-347.
- Astrup-Jensen A, Slarach SA (1991) Chemical contamination in human breast milk. Boston Rouge: CRC Press: LA.
- Hallen IP, Jorhem L, Oskarsson A (1995) Placental and lactational transfer of lead in rats: a study on the lactational process and effects on offspring. Arch Toxicol 69: 596-602.
- Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126: 5-19.
- Antonio MT, Corredor L, Leret ML (2003) Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. ToxicolLett 143: 331-340.
- Lanphear BP, Hornung R, Ho M, Howard CR, Eberly S, et al. (2002) Environmental lead exposure during early childhood. J Pediatr 140: 40-47.
- Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348: 1517-1526.
- Jakubowski M (2011) Low-level environmental lead exposure and intellectual impairment in children--the current concepts of risk assessment. Int J Occup Med Environ Health 24: 1-7.
- Shallie PD, Adefule AK, Akpan HB, Olubiyi OO, Fakunle PB, et al. (2010) Lead toxicity and some subsets of motor skill: Comparative evaluation of adult and prenatally exposed rats. Journal of Neuroscience and Behavioural Health 2: 23-29.
- Burdette LJ, Goldstein R (1986) Long-term behavioral and electrophysiological changes associated with lead exposure at different stages of brain development in the rat. Brain Res 394: 101-110.
- Lockitch G (1993) Perspectives on lead toxicity. ClinBiochem 26: 371-381.
- Goyer RA (1990) Transplacental transport of lead. Environ Health Perspect 89: 101-105.
- Annau Z (1990) Behavioral toxicology and risk assessment. NeurotoxicolTeratol 12: 547-551.
- Alamy M,Bengelloun WA (2012) Malnutrition and brain development: an analysis of the effects of inadequate diet during different stages of life in rat. NeurosciBiobehav Rev 36: 1463-1480.
- Morgane PJ, Mokler DJ, Galler JR (2002) Effects of prenatal protein malnutrition on the hippocampal formation. NeurosciBiobehav Rev 26: 471-483.
- De Andrade FM, Judice LF, Cardoso GP, Cisne R, Ramos Cada F, et al. (2012) Maternal malnutrition during lactation in Wistar rats: effects on elastic fibers of the extracellular matrix in the trachea of offspring. J Bras Pneumol 38: 588-594.
- Zhang Y, Li N, Yang J, Zhang T, Yang Z (2010) Effects of maternal food restriction on physical growth and neurobehavior in newborn Wistar rats. Brain Res Bull 83: 1-8.
- Bonatto F, Polydoro M, Andrades ME, da FrotaJunior ML, Dal-Pizzol F, et al. (2005) Effect of protein malnutrition on redox state of the hippocampus of rat. Brain Res 1042: 17-22.
- Georgieff MK, Rao R (2001) The role of nutrition in cognitive development. In: Nelson CA, Luciana M,(eds). Hand book of developmental cognitive neuroscience: Massachusetts Institute of technology pp: 491-504.
- Sato S, Nakagawasai O, Tan-No K, Niijima F, Suzuki T, et al., (2011) Executive functions of post-weaning protein malnutrition in mice. Biol Pharm Bull 34: 1413-1417.
- Valadares CT, Fukuda MT, Francolin-Silva AL, Hernandes AS, Almeida SS (2010) Effects of postnatal protein malnutrition on learning and memory procedures. NutrNeurosci 13: 274-282.
- Formigari A, Irato P, Santon A (2007) Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp BiochemPhysiol C ToxicolPharmacol 146: 443-459.
- Catania AS, Barros CR, Ferreira SR (2009) Vitamins and minerals with antioxidant properties and cardiometabolic risk: controversies and perspectives. Arq Bras EndocrinolMetabol 53: 550-559.
- Hambidge KM, Krebs NF (2007) Zinc deficiency: a special challenge. J Nutr 137: 1101-1105.
- Nowak P, Bielaczyc G, Szkilnik R, Labus Ã…ÂÂÂÃÂ, Dąbrowska J, et al. (2006) Effects of pre- and postnatal zinc exposure on adult rat brain dopamine activity and behavior. Polish J of Environ Stud 15: 565-572.
- Railey AM, Micheli TL, Wanschura PB, Flinn JM (2010) Alterations in fear response and spatial memory in pre- and post-natal zinc supplemented rats: remediation by copper. PhysiolBehav 100: 95-100.
- Shahbazi M, Naghdi N, Tahmasebi S, Sheikh M, NamvarAsl N, et al. (2009) The effect of iron and zinc dietary restriction of pregnant rats on physical growth of litters. Biol Trace Elem Res 128: 232-238.
- Chowanadisai W, Kelleher SL, Lonnerdal B (2005) Maternal zinc deficiency reduces NMDA receptor expression in neonatal rat brain, which persists into early adulthood. J Neurochem 94: 510-519.
- Piechal A, Blecharz-Klin K, Pyrzanowska J, Widy-Tyszkiewicz E (2012) Maternal zinc supplementation improves spatial memory in rat pups. Biol Trace Elem Res 147: 299-308.
- Bamji MS, Sharada D (1972) Hepatic glutathione reductase and riboflavin concentrations in experimental deficiency of thiamin and riboflavin in rats. J Nutr 102: 443-447.
- Ali AA, Ezzeldin E (2004) Evaluation of the effect of caffeine and nicotine on postnatal neuorobehavioral and functional development in normally-fed and protein malnourished rats. J. Egypt. Soc. Toxicol 31: 77-89.
- Plagemann A, Harder T, Rake A, Melchior K, Rohde W (2000) Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. J Nutr 130: 2582-2589.
- Dearth RK, Hiney JK, Srivastava V, Burdick SB, Bratton GR (2002) Effects of lead (Pb) exposure during gestation and lactation on female pubertal development in the rat. ReprodToxicol 16: 343-352.
- Alder S, Zbinden G (1977) Methods for the evaluation of physical, neuromuscular and behavioral development of rats in early postnatal life. In: Neubert D, Merker HJ, Kwasigroch TE, (eds). Method in prenatal toxicology: Thieme, Stuttgart pp: 175-185.
- Vorhees CV (1983) Behavioral teratogenicity testing as a method of screening for hazards to human health: a methodological proposal. NeurobehavToxicolTeratol 5: 469-474.
- Mello CF, Kraemer CK, Filippin A, Morsch VM, Rodrigues AL, et al. (1998) Effect of lead acetate on neurobehavioral development of rats. Braz J Med Biol Res 31: 943-950.
- Zbinden G (1981) Experimental methods in behavioral teratology. Arch Toxicol 48: 69-88.
- Hamed MR, Kandil AK, shorbagy OE, Abass ZMA (2009) Influence of protein malnutrition on prenatal toxicity of fluoxetine. The Egyptian Journal of Hospital Medicine 35: 229-245.
- Volosin M, Cancela L, Molina V (1988) Influence of adrenocorticotrophic hormone on the behaviour in the swim test of rats treated chronically with desipramine. J Pharm Pharmacol 40: 74-76.
- Vorhees CV (1974) Some behavioral effects of maternal hypervitaminosis A in rats. Teratology 10: 269-273.
- Lo Pumo R, Bellia M, Nicosia A, Micale V, Drago F (2006) Long-lasting neurotoxicity of prenatal benzene acute exposure in rats. Toxicology 223: 227-234.
- Lazarini CA, Florio JC, Lemonica IP, Bernardi MM (2001) Effects of prenatal exposure to deltamethrin on forced swimming behavior, motor activity, and striatal dopamine levels in male and female rats. NeurotoxicolTeratol 23: 665-673.
- van den Buuse M, de Jong W (1989) Differential effects of dopaminergic drugs on open-field behavior of spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. J PharmacolExpTher 248: 1189-1196.
- Cunha JM, Masur J (1978) Evaluation of psychotropic drugs with a modified open field test. Pharmacology 16: 259-267.
- Ani M, Moshtaghie AA, Aghadavood M (2007) protective effects of selenium and zinc on the brain acetyl cholinestrase activity in lead intoxified rat Reasearch in Pharmaceutical Sciences 2: 80-84.
- Chernoff N, Rogers EH, Gage MI, Francis BM (2008) The relationship of maternal and fetal toxicity in developmental toxicology bioassays with notes on the biological significance of the ‘no observed adverse effect level’. ReprodToxicol 25: 192-202.
- Schwetz BA, Harris MW (1993) Developmental toxicology: status of the field and contribution of the National Toxicology Program. Environ Health Perspect 100: 269-282.
- Kahloula K, Slimani M, Aoues A (2009) Behavioural and neurochemical studies of perinatal lead exposed in rat Wistar. European Journal of Scientific Research 35: 603-614.
- Luthman J, Oskarsson A, Olson L, Hoffer B (1992) Postnatal lead exposure affects motor skills and exploratory behavior in rats. Environ Res 58: 236-252.
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Templer L, et al. (1998) Endocrine mechanisms underlying the growth effects of developmental lead exposure in the rat. J Toxicol Environ Health A 54: 101-120.
- Fukuda MT, Francolin-Silva AL, Almeida SS (2002) Early postnatal protein malnutrition affects learning and memory in the distal but not in the proximal cue version of the Morris water maze. Behav Brain Res 133: 271-277.
- Da Silva Hernandes A, Francolin-Silva AL, Valadares CT, Fukuda MT, Almeida SS (2005) Effects of different malnutrition techniques on the behavior of rats tested in the elevated T-maze. Behav Brain Res 162: 240-245.
- Falcao TF, Bento SA, Fidalgo MA, De Almeida MB, dos Santos JA, et al. (2012) Maternal low-protein diet-induced delayed reflex ontogeny is attenuated by moderate physical training during gestation in rats. Br J Nutr 107: 372-377.
- Passos MC, Toste FP, Dutra SC, Trotta PA, Lisboa PC, et al. (2009) Role of neonatal hyperleptinaemia on serum adiponectin and suppressor of cytokine signalling-3 expression in young rats. Br J Nutr 101: 250-256.
- Brocardo PS, Pandolfo P, Takahashi RN, Rodrigues AL, Dafre AL (2005) Antioxidant defenses and lipid peroxidation in the cerebral cortex and hippocampus following acute exposure to malathion and/or zinc chloride. Toxicology 207: 283-291.
- Romeo IA, Abbott NJ, Bradbury MWB (1996) The blood-brain barrier in normal CNS and in metal-induced neurotoxicity. In: Chang LW, editor. Toxicology of Metals. Boca Raton: Lewis pp: 561.
- Flora SJ, Jain VK, Behari JR, Tandon SK (1982) Protective role of trace metals in lead intoxication. ToxicolLett 13: 51-56.
- Barros KM, Manhaes-De-Castro R, Lopes-De-Souza S, Matos RJ, Deiro TC, et al. (2006) A regional model (Northeastern Brazil) of induced mal-nutrition delays ontogeny of reflexes and locomotor activity in rats. NutrNeurosci 9: 99-104.
- Feoli AM, Siqueira IR, Almeida L, Tramontina AC, Vanzella C, et al. (2006) Effects of protein malnutrition on oxidative status in rat brain. Nutrition 22: 160-165.
- Chen JC, Turiak G, Galler J, Volicer L (1995) Effect of prenatal malnutrition on release of monoamines from hippocampal slices. Life Sci 57: 1467-1475.
- Suszkiw J, Toth G, Murawsky M, Cooper GP (1984) Effects of Pb2+ and Cd2+ on acetylcholine release and Ca2+ movements in synaptosomes and subcellular fractions from rat brain and Torpedo electric organ. Brain Res 323: 31-46.
- Silbergeld EK (1977) Interactions of lead and calcium on the synaptosomal uptake of dopamine and choline. Life Sci 20: 309-318.
- Shih TM, Hanin I (1978) Effects of chronic lead exposure on levels of acetylcholine and choline and on acetylcholine turnover rate in rat brain areas in vivo. Psychopharmacology (Berl) 58: 263-269.
- Prasanthi RPJ, Basha DC, Reddy GR (2010) Protective effects of calcium and zinc on lead induced perturbations in brain acetylcholinesterase activity. Current trends in Biotechnology and Pharmacy 4.
- Prasanthi RP, Devi CB, Basha DC, Reddy NS, Reddy GR (2010) Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J DevNeurosci 28: 161-167.
- Niu R, Sun Z, Cheng Z, Li Z, Wang J (2009) Decreased learning ability and low hippocampus glutamate in offspring rats exposed to fluoride and lead. Environ ToxicolPharmacol 28: 254-258.
- Rahman A, Khan KM, Al-Khaledi G, Khan I, Al-Shemary T (2012) Over activation of hippocampal serine/threonine protein phosphatases PP1 and PP2A is involved in lead-induced deficits in learning and memory in young rats. Neurotoxicology 33: 370-383.
- Rahman A, Khan KM, Al-Khaledi G, Khan I, Attur S (2012) Early postnatal lead exposure induces tau phosphorylation in the brain of young rats. ActaBiol Hung 63: 411-425.
- Barkur RR, Rao MS, Bairy LK (2011) Low lead exposure during foetal and early postnatal life impairs passive avoidance learning in adulthood in rats. ArhHigRadaToksikol 62: 147-153.
- Betharia S, Maher TJ (2012) Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology 33: 1117-1127.
- Soodi M, Sharifzadeh M, Naghdi N, Ostad N, Abdollahi M, et al. (2007) Systemic and developmental exposure to lead causes spatial memory deficits and a reduction in COX-2 immunoreactivity in the hippocampus of male rats. J Neurosci Res 85: 3183-3192.
- Zhong Z, Zhang C, Rizak JD, Cui Y, Xu S, et al. (2010) Chronic prenatal lead exposure impairs long-term memory in day old chicks. NeurosciLett 476: 23-26.
- Jiang YM, Long LL, Zhu XY, Zheng H, Fu X, et al. (2008) Evidence for altered hippocampal volume and brain metabolites in workers occupationally exposed to lead: a study by magnetic resonance imaging and (1)H magnetic resonance spectroscopy. ToxicolLett 181: 118-125.
- Verina T, Rohde CA, Guilarte TR (2007) Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience 145: 1037-1047.
- Zhang R, Lu H, Tian S, Yin J, Chen Q, et al. (2010) Protective effects of pre-germinated brown rice diet on low levels of Pb-induced learning and memory deficits in developing rat. ChemBiol Interact 184: 484-491.
- Reyes-Castro LA, Rodriguez JS, Charco R, Bautista CJ, Larrea F, et al. (2012) Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. Int J DevNeurosci 30: 39-45.
- Rotta LN, Schmidt AP, Mello e Souza T, Nogueira CW, Souza KB, et al. (2003) Effects of undernutrition on glutamatergic parameters in rat brain. Neurochem Res 28: 1181-1186.
- Lipton PA, Eichenbaum H (2008) Complementary roles of hippocampus and medial entorhinal cortex in episodic memory. Neural Plastpp: 258467.
- Martinez Y, Diaz CS, Leon JU, Aguilar VA, Medina AC, et al. (2009) Effects of postnatal malnutrition and senescence on learning, long-term memory, and extinction in the rat. Behav Brain Res 203: 48-53.
- Andrade JP, Castanheira-Vale AJ, Paz-Dias PG, Madeira MD, Paula-Barbosa MM (1996) The dendritic trees of neurons from the hippocampal formation of protein-deprived adult rats. A quantitative Golgi study. Exp Brain Res 109: 419-433.
- Francolin-Silva AL, da Silva Hernandes A, Fukuda MT, Valadares CT, Almeida SS (2006) Anxiolytic-like effects of short-term postnatal protein malnutrition in the elevated plus-maze test. Behav Brain Res 173: 310-314.
- Prasanthi RPJ, Reddy GH, Reddy GR (2006) Calcium or zinc supplementation reduces lead toxicity: assessment of behavioral dysfunction in young and adult mice. Nutrition Research 26: 537 - 545.
- Yu X, Jin L, Zhang X (2013) Effects of maternal mild zinc deficiency and zinc supplementation in offspring on spatial memory and hippocampal neuronal ultra-structural changes. Nutrition 29: 457-461.
- Cope EC, Morris DR, Scrimgeour AG, Levenson CW (2012) Use of zinc as a treatment for traumatic brain injury in the rat: effects on cognitive and behavioral outcomes. Neurorehabil Neural Repair 26: 907-913.
- Nakashima AS, Dyck RH (2009) Zinc and cortical plasticity. Brain Res Rev 59: 347-373.
- Hardy AB, Serino AS, Wijesekara N, Chimienti F, Wheeler MB (2011) Regulation of glucagon secretion by zinc: lessons from the beta cell-specific Znt8 knockout mouse model. Diabetes ObesMetab 13 Suppl 1: 112-117.
- Jaako-Movits K, Zharkovsky T, Romantchik O, Jurgenson M, Merisalu E, et al. (2005) Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int J DevNeurosci 23: 627-635.
- Levenson CW, Morris D (2011) Zinc and neurogenesis: making new neurons from development to adulthood. AdvNutr 2: 96-100.
- Basha DC, Rani MU, Devi CB, Kumar MR, Reddy GR (2012) Perinatal lead exposure alters postnatal cholinergic and aminergic system in rat brain: reversal effect of calcium co-administration. Int J DevNeurosci 30: 343-350.
- Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. NeurotoxicolTeratol 23: 489-495.
- Hassan AA, Jassim HM (2010) Effect of treating lactating rats with lead acetate and its interaction with vitamin E or C on neurobehavior, development and some biochemical parameters in their pups. Iraqi Journal of Veterinary Sciences 24: 45-52.
- LisboaSFdS, Gonçalves G, Komatsu F, Queiroz CA, Almeida AA, et al. (2005) Developmental lead exposure induces depressive-like behavior in female rats. Drug ChemToxicol 28: 67-77.
- Cory-Slechta DA (1995) Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annu Rev PharmacolToxicol 35: 391-415.
- Ohishi T, Wang L, Akane H, Shiraki A, Sato A, et al. (2012) Adolescent hyperactivity of offspring after maternal protein restriction during the second half of gestation and lactation periods in rats. J ToxicolSci 37: 345-352.
- Vicente Fd, Rodriguez-Perez MC, Gomez-Jarabo G (1991) The effects of protein malnutrition and cortisol treatment on motor activity of rats Behavioural Processes 25: 1-14.
- TahmasebiBoroujeni S, Naghdi N, Shahbazi M, Farrokhi A, Bagherzadeh F, et al. (2009) The effect of severe zinc deficiency and zinc supplement on spatial learning and memory. Biol Trace Elem Res 130: 48-61.
- Ohno M, Yamamoto T, Watanabe S (1992) Effects of intra-hippocampal injections of N-methyl-D-aspartate receptor antagonists and scopolamine on working and reference memory assessed in rats by a three-panel runway task. J PharmacolExpTher 263: 943-950.
- Devi CB, Reddy GH, Prasanthi RP, Chetty CS, Reddy GR (2005) Developmental lead exposure alters mitochondrial monoamine oxidase and synaptosomal catecholamine levels in rat brain. Int J DevNeurosci 23: 375-381.
- Ma T, Chen HH, Ho IK (1999) Effects of chronic lead (Pb) exposure on neurobehavioral function and dopaminergic neurotransmitter receptors in rats. ToxicolLett 105: 111-121.
- Jaya Prasanthi RP, Hariprasad Reddy G, Bhuvaneswari Devi C, Rajarami Reddy G (2005) Zinc and calcium reduce lead induced perturbations in the aminergic system of developing brain. Biometals 18: 615-626.










