Keywords
Magic fruit; Honey bee; Chronic HCV infection; HCV-RNA PCR (quantitative); FoxP3; CD4+CD25+expression
Introduction
Hepatitis C virus (HCV) attacks the liver and leads an inflammation [1]. 3% of the world’s population liver injure is infected with HCV as mentioned in recent WHO report; which was estimated that more than 170 million chronic carriers are developing liver cirrhosis and/or liver cancer [2].
HCV symptoms rarely occur in infected population; as dark urine, yellow tinged skin, fever and abdominal pain are occurring. 75% to 85% of chronic infection patients has no symptoms, hence leads to liver disease and cirrhosis [3,4] while cirrhotic patients will be developing other complications as liver failure, liver cancer [5].
Magic fruit (Ziziphus jujube) strengthens the liver and is used by Chinese practitioners for hepatitis and cirrhosis. It is stimulating the immune system [6]. The magic fruit (Ziziphus jujube) or the Chinese date is a member of Rhamnaceae family. It helps in many of vital activities and functions such as weight gain, antiallergenic, improving stamina and strength, mildly sedating, improving of liver function, and helping to efficiently get the energy from food and giving energy [7]. Ziziphus jujube is one of such medicinal plants used for curing liver ailments, insomnia, anemia, diarrhea, diabetic complications and cancer [8]. The practice of application of medicinal plants for relief from many illness dates back to ancient times, Ziziphus Jujuba Mill. (Z. Jujuba ) or the jujube, a herbal plant used in traditional medicine.
Honey is a natural product that produced in honey bees. In a complex process, they collect the nectar from different flower mixing with their salivary and enzymatic secretion [9]. Honey is well known as an antifungal, antibacterial and antioxidant behavior because of phenolic compound [10]. The main constituents of honey are 50% fructose and 44% glucose [11] which gives the sweety flavor, while the remaining constituents of honey, chrysin, pinobanksin, catalase, pincocembrin and vitamin C which acting as an antioxidant [12].
Materials and Methods
Patients
The present study has been conducted on a total of 50 patients, all with chronic hepatitis C (The male number was 35 while the female was 15; The median age was 45 years) taken from the outpatient clinics of the NCI-Cairo University in Egypt. Blood samples were obtained during the time from July 2014 to January 2015. Treatment the patients who have (virus C) by taken them 4 g from Magic fruit and 1 g from Honey bee 3 times daily for three months. Blood samples were collected from patients before and after treatment. Serum samples were permitted to clot for 30 min at 37°C [13,14]. Then, they were centrifuged at 3000 RPM for 10 min. The sera were collected and divided to several aliquots and were stored at -20°C until starting our tests. According to Giardina's recommendation, the AFU activity levels were done within 30 days after collection [15].
HCV-test, HCV PCR analysis, FoxP3, and T-regulatory cell (CD4, CD25) in HCV patients have been performed before and after treatment.
HCV-PCR levels were quantitatively determined by instrument of Applied Biosystems (AB) step one real-time PCR system (Singapore), while CD4, CD25 were analyzed by instrument of Flow Cytometery NAVIOS Flow Cytometery. Beckman Coulter (USA). The biochemical parameters as CBC, Liver function tests, kidney function tests were done by integrated automation in clinical laboratories in NCI.
Statistical Analysis
Data were expressed as Mean ± SD. The SPSS program version 15 was used in analysis. One-way analysis of variance (ANOVA) followed by Duncan post hoc test and/or t-test were used in analysis. Chi-square test was used for categorical data. Pearson correlation coefficient was used to study correlations. The percent change was calculated as (values after treatment - values before treatment)/values before treatment × 100, and tabulated as absolute values. P-values less than 0.05 were significant.
Results
Study population
Our study was done on 50 HCV patients from the outpatient clinics at the NCI, Egypt in the period between 2014-2015. They included 35 males and 15 females (66% / 34%). The age range was between 23 and 65 years with a median of 45 years.
Effect of treatment on HCV RNA/PCR (quantitative)
The median PCR was 0.53 × 106 before treatment, a value decreased to 0.12 × 106 after treatment with a percent reduction of 63.94 ± 27.51% (Table 1).
| Parameters |
Before treatment |
After treatment |
p-value |
r (p) |
% Change |
| ALT |
56.27 ± 35.43 |
39.68 ± 24.00 |
0.001 |
0.82 (0.001) |
29.15 ± 15.81 |
| AST |
54.44 ± 29.85 |
38.42 ± 17.37 |
0.001 |
0.78 (0.001) |
32.88 ± 18.74 |
| ALP |
4.36 ± 0.37 |
3.85 ± 0.52 |
0.001 |
0.48 (0.001) |
12.48 ± 9.46 |
| Bilirubin |
1.38 ± 0.57 |
1.04 ± 0.37 |
0.001 |
0.56 (0.001) |
29.26 ± 16.48 |
| AFU (μg/ml |
132.46 ± 27.19 |
125.08 ± 24.31 |
0.001 |
0.83 (0.001) |
7.77 ± 7.07 |
| AFP (ng/ml |
9.35 (2.1 - 51.0) |
5.15 (0.70 - 32.0) |
0.001 |
0.886 (0.001) |
45.89 ± 19.06 |
| Urea |
36.48 ± 7.38 |
27.97 ± 5.71 |
0.001 |
0.57 (0.001) |
22.48 ± 12.3 |
| Creatinine |
1.19 ± 0.32 |
0.94 ± 0.25 |
0.001 |
0.64 (0.001) |
25.19 ± 13.8 |
| Uric acid |
4.71 ± 1.05 |
4.55 ± 0.79 |
0.308 (NS) |
0.43 (0.002) |
17.67 ± 16.54 |
| Hb |
11.97 ± 1.53 |
13.35 ± 1.38 |
0.001 |
0.696 (0.001) |
12.77 ± 10.70 |
| RBCs |
4.23 ± 0.63 |
4.74 ± 0.54 |
0.001 |
0.749 (0.001) |
13.32 ± 10.35 |
| WBCs |
6.31 ± 2.33 |
6.33 ± 1.61 |
0.888 |
0.801 (0.001) |
20.48 ± 19.10 |
| Platelets |
154.78 ± 60.63 |
182.36 ± 71.40 |
0.001 |
0.927 (0.001) |
14.43 ± 8.33 |
| Significant: P<0.05 , Non-Significant:P> 0.05 |
Table 1: Routine tests: Liver function, Kidney function and Complete blood count for patients before and after treatment with magic fruit and honey bee.
Effect of treatment on human Fox 3p expression
The mean human Fox 3p after treatment, was 244.43 ± 107.6, that highly significantly decreased after treatment, 33.80 ± 31.14 (p<0.001) with a percent decrease of 85.67 ± 13.08% (Table 2).
| Molecular Parameters |
Before treatment |
After treatment |
p-value |
r (p) |
% change |
| PCR |
0.53× 106
(0.02-16.21) |
0.12 × 106
(0.0-14.25) |
0.001 |
0.449 (0.001) |
63.94 ± 27.51 |
| Fox P3 (ng/ml) |
244.43 ± 107.6 |
33.80 ± 31.14 |
0.001 |
0.548 (0.001) |
85.67 ± 13.08 |
| CD4 CD25 |
21.43 ± 8.63 |
9.13 ± 3.84 |
0.001 |
0.509 (0.001) |
54.55 ± 18.57 |
| Significant: P<0.05, Non-Significant: P = 0.05 |
Table 2: Molecular parameters; PCR values, Fox P3 and CD4 CD25 for HCV patients before and after treatment with magic fruit and honey bee.
Effect of treatment on human CD4CD25 expression
The same was observed for CD4CD25 where the mean expression was 21.43 ± 8.63 before treatment, a value significantly decreased to 9.13 ± 3.84 (P<0.001) after treatment with a percent reduction of 54.55 ± 18.57% (Table 3).
| Variables |
PCR |
Fox p3 |
CD4CD25 |
| Creatinine |
0.085 |
-0.196 |
-0.274 |
| Urea |
0.193 |
0.034 |
-0.119 |
| Uric acid |
-0.037 |
0.087 |
0.104 |
| Bilirubin |
-0.203 |
-0.116 |
0.107 |
| ALP |
-0.019 |
-0.039 |
-0.147 |
| ALT |
0.036 |
0.209 |
0.234 |
| AST |
-0.063 |
0.163 |
0.237 |
| AFU |
-0.116 |
0.019 |
0.218 |
| RBCs |
0.238 |
0.014 |
-0.147 |
| WBCs |
-0.108 |
-0.132 |
0.014 |
| Platelets |
0.054 |
-0.062 |
-0.084 |
| Hb |
0.251 |
0.017 |
-0.050 |
Table 3: Correlation between PCR, FoxP3 and CD4 CD25 and the traditional biochemical parameters before treatment.
Effect of treatment on liver function tests
After treatment with the magic fruit and honey bee, highly significant decreases were detected for bilirubin, ALP, ALT, AST and AFU (p<0.001). The percent of reductions were 29.26 ± 16.48, 12.48 ± 9.46, 29.15 ± 15.81, 32.88 ± 18.74 and 7.77 ± 7.07, respectively (Table 4).
| Variables |
PCR |
Fox p3 |
CD4 CD25 |
| Creatinine |
0.086 |
-0.332* |
-0.182 |
| Urea |
-0.114 |
-0.251 |
-0.210 |
| Uric acid |
0.299* |
0.175 |
-0.159 |
| Bilirubin |
-0.328* |
-0.125 |
0.162 |
| ALP |
-0.029 |
0.089 |
-0.330* |
| ALT |
-0.055 |
0.289* |
0.069 |
| AST |
-0.118 |
0.235 |
0.057 |
| AFU |
-0.054 |
-0.116 |
0.274 |
| RBCs |
0.155 |
0.025 |
-0.249 |
| WBCs |
-0.122 |
-0.199 |
0.192 |
| Platelets |
-0.086 |
0.001 |
0.210 |
| Hb |
0232 |
0.021 |
-0.331* |
Table 4: Correlation between PCR, FoxP3 and CD4 CD25 and the traditional biochemical parameters after treatment.
Effect of treatment on kidney function tests
After treatment with magic fruit and honey bee, highly significant decreases were detected for creatinine and urea (p<0.001 for each) whereas no significant effect was detected for uric acid level (p=0.308). The percent of reduction of creatinine and urea were 25.19 ± 13.8 and 22.48 ± 12.3, respectively (Table 5).
| Variables |
|
PCR after |
FoxP3 before |
FoxP3 after |
CD4CD25 before |
CD4CD25 after |
| PCR before |
R |
0.628 (**) 0 |
.105 0 |
-0.018 |
-0.183 |
-0.262 |
| P |
0.000 |
.467 0 |
.903 0 |
0.204 |
.066 0 |
| PCR after |
R |
1 |
-0.096 |
-0.096 |
-0.248 |
-0.027 |
| P |
|
.506 0 |
.508 0 |
.082 0 |
.850 0 |
| FoxP3 before |
R |
|
1 |
0.504 (**) |
.148 0 |
-0.177 |
| P |
|
|
.000 0 |
.305 0 |
0.218 |
| FoxP3 after |
R |
|
|
1 |
.204 0 |
-0.035 |
| P |
|
|
|
.156 0 |
0.810 |
| CD4CD25 before |
R |
|
|
|
1 |
.523 (**) 0 |
| P |
|
|
|
|
.000 0 |
| Treatment: **Correlation is significant at the 0.01 level (2-tailed). |
Table 5: Correlation between PCR, FoxP3 and CD4CD25 in patients before and after.
Effect of treatment on blood picture
After treatment with magic fruit and honey bee, highly significant increasing was detected for RBCs and platelets count as well as hemoglobin concentration (p<0.001). But for WBCs, the increase was non-significant. The increment percentages of RBCs, platelets and haemoglobin were 13.32 ± 10.35, 14.43 ± 8.33 and 12.77 ± 10.70, respectively (Table 6).
| Variables |
|
PCR after |
FoxP3 before |
FoxP3 after |
CD4CD25 before |
CD4CD25 after |
| PCR before |
R |
.644 (**) 0 |
0.377 (**) |
0.207 |
-0.215 |
-.359 (*) |
| P |
.0000 0 |
.007 0 |
.149 0 |
0.133 |
.011 0 |
| PCR after |
R |
1.000 |
.202 0 |
.161 0 |
-0.054 |
-0.081 |
| P |
. |
0.160 |
.263 0 |
0.710 |
.577 0 |
| FoxP3 before |
R |
-- |
1.000 |
0.469 (**) |
.195 0 |
-0.108 |
| P |
-- |
. |
.001 0 |
0.175 |
.454 0 |
| FoxP3 after |
R |
-- |
-- |
1.000 |
0.167 |
.112 0 |
| P |
-- |
-- |
. |
0.247 |
.439 0 |
| CD4 CD25 before |
R |
-- |
-- |
-- |
1.000 |
0.472 (**) |
| P |
-- |
-- |
-- |
. |
.001 0 |
**Correlation is significant at the 0.01 level (2-tailed).
*Correlation is significant at the 0.05 level (2-tailed). |
Table 6: Non-parametric correlations between PCR, FoxP3 and CD4CD25 in patient before and after treatment.
Correlation between PCR, human Fox P3 and CD4CD25 versus the traditional biochemical parameters before and after treatment
Figures 1A, 1B and 1C clarify the correlation between the traditional different biochemical parameters versus PCR, Fox P3 and CD4 CD25 values. Non-significant correlation was detected. While, Figures 2A, 2B and 2C clarify the correlation between the traditional different biochemical parameters after treatment. For PCR, positive significant correlation with uric acid (r=0.299, p<0.05) and negative significant correlation with bilirubin (r=- 0.328, p<0.05) were detected. For human Fox p3 expression, negative significant correlation with creatinine (r=-0.332, p<0.05) and positive significant correlation with ALT (r=0.289, p<0.05) were detected. For CD4 CD25 expression, two negative significant correlations could be detected; with ALP (r=- 0.330, p<0.05) and Hb (r=-0.331, p<0.05), respectively.
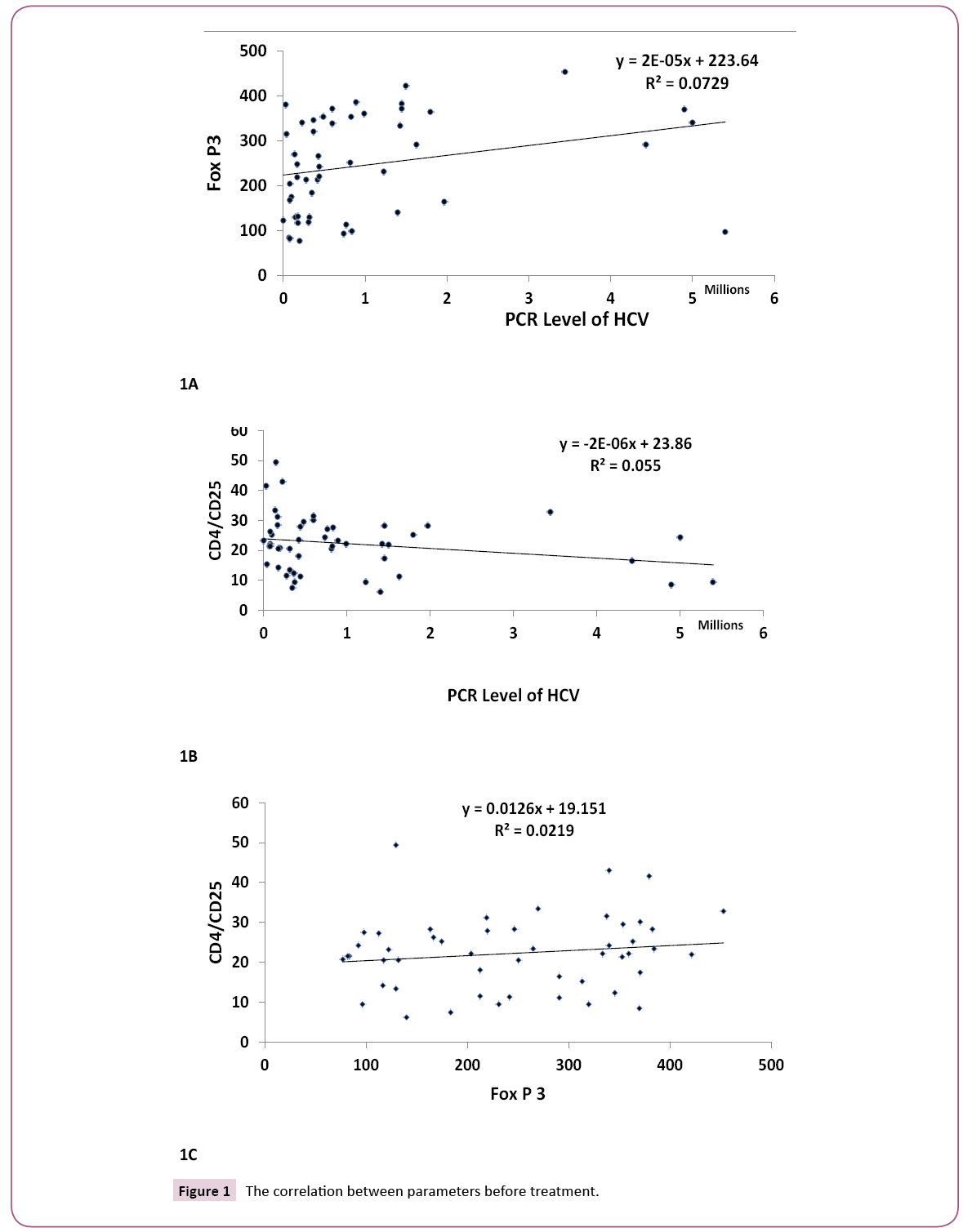
Figure 1: The correlation between parameters before treatment.
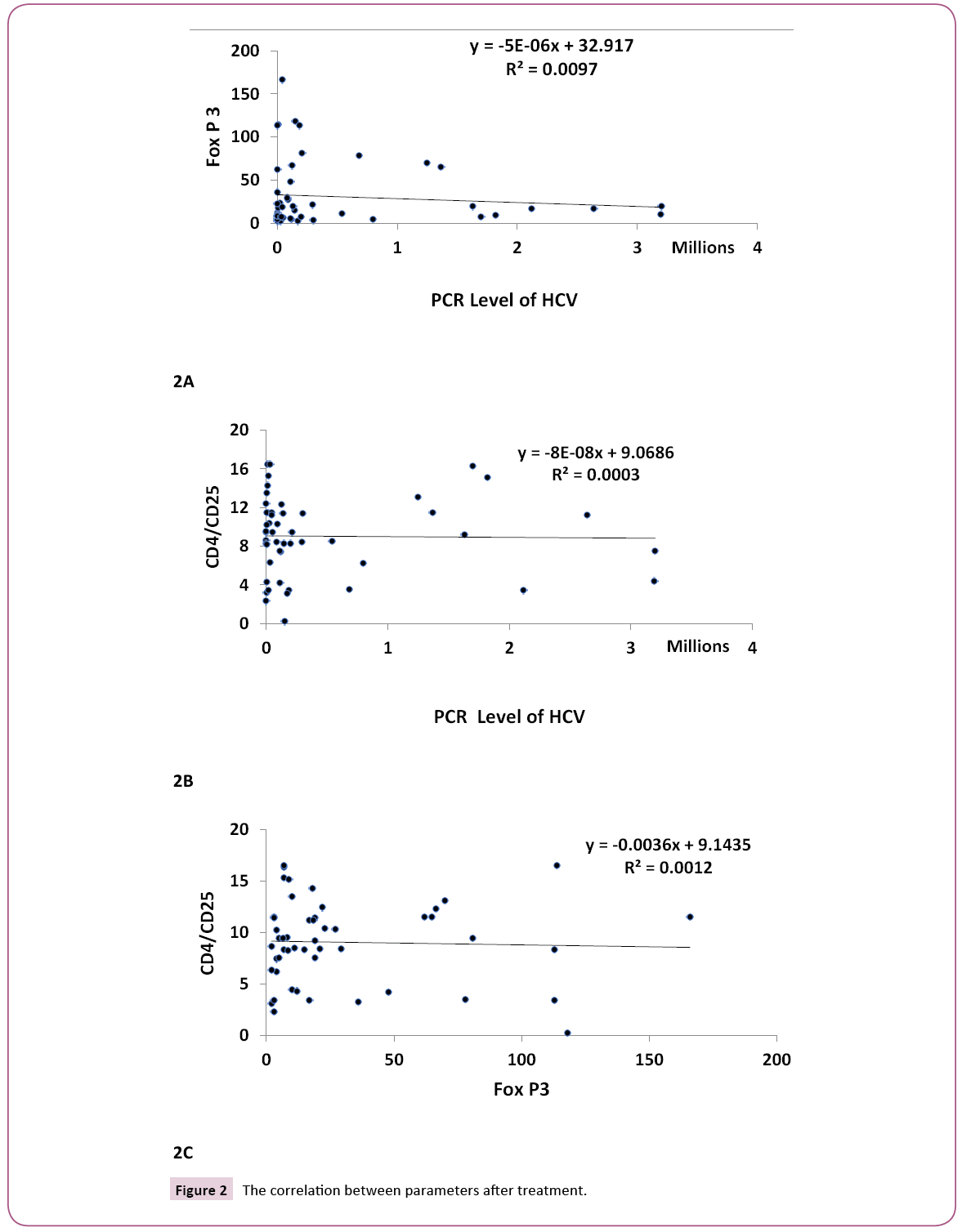
Figure 2: The correlation between parameters after treatment.
We observed that; there are correlations between PCR, Fox P3 and CD4 CD25 before and after treatment. There is positive correlation for PCR and Fox P3 before treatment (R=0.377, P<0.01), while a negative correlation was observed between PCR before treatment and CD4 CD25 after treatment (R=-0.359, P, 0.01).
Discussion
The researchers were emphasized a strong association of cirrhosis and HCC due to chronic viral hepatitis. Although the use of surgery, chemotherapy and radiotherapy have led to increasing the survival rates for patients with hepatocellular carcinoma, there are still problems of the marked side effects of the previously mentioned treatments in addition to cancer metastasis that sometimes occurs among those patients. Most cases of HCV infection are asymptomatic and clinical presentation is mild. Resolution of the infection occurs 3-4 months after, 70% of cases do not resolve but progress to chronic hepatitis [16].
Saeed in 1991 was the first to report in Saudi Arabia the very high incidence of HCV-Ab among Egyptian blood donors, which was 19.5%. Darwish in 18 reported that the incidence was 15% among healthy blood donors. Yet, Kamel in 1992 reported that the incidence is 6% among health care workers. The prevalence of HCV in Egyptian blood donors was reported to be the highest among the world [17-20].
Magic Fruit (Ziziphus jujube): In the last few decades there has been an exponential growth in the field of Herbal medicine [21]. Our result elucidates the protective ability of Z. jujube against hepatic injury and chronic inflammation that was confirmed in previous study [22].
Z. jujube showed significant protection against CCl4 intoxicated HepG2 cell lines by means of increased cell viability and decreased LDH levels compared to control group. It was reported for anticancer, anxiolytic, anti-complementary and hypoglycemic activities [23-26]. Jujuba methanol extraction with different concentrations inhibited the lipid peroxidation and significantly restored the liver function markers (Ast, Alt, Alp, LDH) IN Acute liver damaged) [27].
Previous study demonstrated the role of honey supplementation that is improving the immunological behavior which gives a useful result that prevents hepatic damage which caused by obstruction of the common bile duct.
The present work represents the first trial carried out in Egypt to show the effects of the mixture of magic fruit and honey bee on the hepatocarcinogenesis as well as the level of PCR, T-regulatory cell (CD4, CD25), and Fox P3 gene expression. The results showed the highly significant decrease in (PCR, T-regulatory cell (CD4, CD25), and Fox P3 gene expression) level after treatment by mixture of magic fruit and honey bee. The results also showed that the activation of virus-C has been significantly decreased after treatment by the mixture than before treatment indicating the possibility of using the mixture as an inhibitor the activation of virus-C in patients to be active.
Conclusion
In conclusion, the present work casted some light on the probability of using the mixture of magic fruit and honey bee in the natural therapy of virus C through the activation of immune system and decreasing the level of (PCR, T-regulatory cell (CD4, CD25), and Fox P3 gene expression). Future studies are recommended to examine the possibility of using the mixture in the treatment of other types of cancer.
Conflict of Interest
The authors declare that they have no conflict of interest that competes with any of the contents of the manuscript.
21342
References
- Ryan KJ, Ray CG (2004) Sherris Medical Microbiology.(4th edn) McGraw Hill, USA. 4:370.
- Maheshwari A, Thuluvath PJ (2010) Management of acute hepatitis C.Clin Liver Dis 14: 169-176.
- Poynard T, Yuen MF, Ratzin V, Lai CL (2003)Viral hepatitis C. Lancet 362: 2095-2100.
- Rusyn I, Lemon SM (2014) Mechanisms of HCV-induced liver cancer: What did we learn from in vitro and animal studies?. Cancer Lett 345:210-215.
- Ferri C, Sebastiani M, Giuggioli D, Colaci M, Fallah P (2015) Hepatitis C virus syndrome: A constellation of organ- and non-organ specific autoimmune disorders, B-cell non-Hodgkin’s lymphoma, and cancer. World J Hepatol 7: 327-343.
- Pawlowska AM, Camangi F, Bader A,Braca A (2009) Flavonoids of Zizyphus jujube L. and Zizyphusspina-christi (L) Wild (Rhamnaceae) fruits. Food Chem112:858-862.
- Ghaly IS, Said A, Abdel-Wahhab MA (2008) Zizyphus jujube and Origanummajorana extracts protect against hydroquinone-induced clastogenicity.EvironToxicolPharmacol25:10-19.
- ErguderBl, Kilicoglo SS, Namuslu M, Kilicoglu B, Devrim E, et al. (2005) Honey prevents hepatic damage Induced by obstruction of the common bile duct. World J Gastroenterol 14: 3729-3732.
- Martos I,ferreres F, Tomas-Barberian F (2000) Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J Agric Food Chem48: 1498-1502.
- Fiorani M, Accorsi A, Blassa M, Diamantini G, Piatti E (2006) Flavonoids from Italian multifloral honey reduce the extracellular ferricyanide inhuman red blood cell. J Agric Food Chem54: 8328-8334.
- Giardina MG, Matarazzo M, Napoli A, Martino R (1992) Serum alpha-L- fucosidase. A useful marker in the diagnosis of hepatocellular carcinoma. Cancer 70:1044-1048.
- Camp RL, Dolled- Filhart M, Rimm DL (2004) A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. ClinCancer Res 10: 7252-7259.
- El-Zayadi AR, Badran HM, Barakat EM (2005) Hepatocellular carcinoma in Egypt a single center study over a decade. World J Gastroenterol 11: 5193-5198.
- Habib M, Mohamed MK, Abdel-Aziz F (2001) Hepatitis C virus infection in a community in the Nile Delta: Risk factors for seropositivity. Hepatology 33: 248-253.
- Saeed AA, Al-Admawi AM, Al-Rasheed A, Faiicough B, Bacchus R (1991) Hepatitis C virus infection in Egyption voluntary blood donors in Riyadh. Lancet 338:459-460.
- Darwish NM, Abbas MO, Abdelfattah FM (1992) Hepatitis C virus Infection in blood donors in Egypt.Journal Egypt Health Assoc67:223-236.
- Kamel MA, Ghaffar YA, Wasef MA, Wright M, Clark LC, et al. (1992) High HCV prevalence in Egyptian blood donors. Lancet340: 347.
- Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, et al. (2000)Hepatitis C virus infection in a community in Nile Delta: Risk factors for seropositivity.Hepatology 33: 248-253.
- Plastina P,Bonofiglio D, Vizza D, Fazio A, Rovito D, et al. (2012) Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J Ethnopharmacol140:325-332.
- Shen X, Tang Y, Yang R, Yu L, Fang T, et al. (2009) The protective effect of Zizphus jujube fruit on carbon tetrachloride-induced hepatic injury in mice by anti-oxidative activities. J Ethnopharmacol122:555-560.
- Huang X, Kojima-Yuasa A, Norikura T, Kennedy DO, HasumaT et al. (2007) Mechanism of the anti-cancer activity of Zizphus jujube in HepG2 cells. Am jChin Med 35: 517-523.
- Peng WH, HsiehMT, Lee YS, Lin YC, LiaoJ (2000) Anxiolytic effect of seed of Ziziphus jujube in mouse models of anxiety. J. Ethnopharmacol 72: 435-439.
- Lee SM, Park JG, Lee CG, Min BS, Kim JH (2004) Anti-complementary activity of triterpenoides from fruits of Zizyphus jujube. Biol Pharm Bull 27: 1883-1892.
- Iganacimuthu S, Amalraj T (1998) Effect of leaf extract of Zizyphusjujube on diabetic rats.Indian JPharmacol 30: 107-112.
- Kandimalla R, Dash S, KalitaS,Choudhury B,Malampati S, et al. (2016) Protective effect of bioactivity guided fractions of Ziziphus jujube Mill: Root Bark against Hepatic injury and chronic inflammation via inhibiting inflammatory markers and oxidative stress. FrontPharmacol 7: 1-13.
- ErguderBl, Kilicoglu SS, Namuslu M, Kilicoglu B, Devrim E, et al. (2008) Honey prevents hepatic damage induced by obstruction of the common bile duct. J Agric Food Chem54: 8328-8334.







