Keywords
Virgin coconut oil; Atrazine; Glucose; Oestrogen; Diabetes; Environmental pollutants
Introduction
Our health can be greatly affected by environmental stressors such as pollutants and chemicals that we are daily exposed to, and these have detrimental effects on the human body, which substantially contribute to major public health diseases [1]. Pollution is one of the most common risk factor for the increased rate of human diseases and has a link to several chronic ailments such as respiratory disease, cancers, cardiovascular diseases diabetes mellitus. In 2015, pollution killed over 9 million people in the world [2-7].
Diabetes mellitus is referred to a syndrome affecting the body’s metabolism whose hallmark is excessive blood glucose or hyperglycemia which is due to a total reduction of insulin secretion or reduced insulin sensitivity in the tissues [8]. There is a high epidemiological and mortality rate of diabetes mellitus which has greatly affected our health, economic and social development [9]. By 2015, close to four hundred and fifiteeen million people have been reported to have diabetes worldwide and the rate in Nigeria is about 4 million. Diabetes mellitus was reported to cause about 105,091 deaths in Nigeria [9].
The most common risk factors for diabetes mellitus are genetics, diet, overweight or obesity and physical inactivity but it has been shown that these factors cannot completely account for the sudden increase in the prevalence of this disease; environmental pollutants are believed to be a major risk factor [10]. These pollutants can have a deleterious effect on a variety of biochemical and physiological processes and on structural organization within the cells [11]. Long-term exposure to environmental pollution is discovered to be linked to a greater risk of death in diabetic patient in comparison to patients without diabetes [12]. Additionally, people that have diabetes are more prone to dying or have an increased risk of being hospitalized due to heart or cardiovascular when they are highly exposed to environmental pollution [13,14]. The assumption that environmental exposures are linked to metabolic disease has been shown by persistent organic pollutants and toxins that have consistently shown to be associated with Insulin Resistance (IR) and type II DM [15]. One of such endocrine disruptor of interest is Atrazine (ATZ) and the focus of the present study. It is one of the most commonly used herbicides worldwide and its use is on the increase in Nigeria [16]. Humans are exposed to ATZ in the air, water and food [17,18].
The use of Atrazine (ATZ) has been banned in Europe since 2005 because of some suspected detrimental effects on health and the environment [19].
It has been reported that substantial utilization of Atrazine (ATZ) might be related with the danger of obesity which is a pre- disposing risk factor for type II DM. Atrazine (ATZ) has been found to prompt overweight and insulin resistance in experimental rats by causing an impairment of the mitochondrial activity [20]. Atrazine (ATZ) has also been reported to cause inflammation by increasing inflammatory markers and chemicals which can incite diabetes mellitus [21]. Another report from showed a significant increase in fasting blood glucose levels in diabetic untreated and diabetic atrazine administered rats in comparison with normal rats [22]. In any case, no noteworthy difference in levels of plasma glucose was seen amid the diabetic rat administered Atrazine (ATZ) and diabetic untreatedrats demonstrating a conceivable connection among atrazine and diabetes mellitus [22].
It was initially accepted that oestrogens deleteriously affect metabolism of glucose, because elevated oral contraceptives dosages have incited intolerance of blood sugar in some females [23]. However, researches carried out on healthy and postmenopausal women affected by type II DM have demonstrated that utilization of oestrogen only, can decrease levels of blood sugar and boost sensitivity of insulin [24,25]. Additionally, report has shown the insulinotropic activity of oestrogen, as research in dogs and primates has demonstrated that oestrogens have the ability to ameliorate hyperglycemia after incomplete pancreatectomy [26]. Estrogens are main controllers of blood glucose homeostasis via metabolic homeostasis advancement, improvement of resistance to insulin and maintaining the function and survival of β-cell. Loss of the primary circulating estrogen, 17-Esftroge n (E2) either through natural or surgically-induced menopause possesses impacts which surpass reproductive health. Lack of estrogen and the cellular activity disruption gives rise to a sudden decrease in metabolism, an increase in central obesity, lipid abnormalities, and worsening of the metabolic disorder. Collectively, all these progressions cause an increment in the danger of type II diabetes, nonalcoholic steatohepatitis, heart diseases and the factors aggravating it [27].
Virgin Coconut Oil (VCO) is dietary and medical nourishment of the customary regions growing coconut. Virgin Coconut Oil (VCO) refers to unprocessed oil extracted out of dried, matured, and fresh white part, i.e., the kernel of a coconut fruit (cocos nucifera ) either via natural or mechanized method, without or with the aid of low heat [28]. It is dominatingly made up of saturated fats (about 94%), with a good content (above 62%) of medium chain fatty acids having highest fatty acid content of 45%-52% lauric acids. The lauric acid changes into monolaurin acid that is discovered to battle viral pathogens and shield the body from parasites [29]. Other than lauric acid, short-chain fatty acids, like caprylic and capric caproic, acids are present in Virgin Coconut Oil (VCO), which have been reported to be anti-viral and anti-microbial [29]. Virgin Coconut Oil (VCO) has been found to have hypoglycaemic actions, enhance secretion of insulin and ameliorate oxidative strain in rats induced with non-insulin dependent diabetes [30].
Therefore, this research was designed to observe the effect of Atrazine on body weight, fasting blood glucose and oestrogen levels in experimental rats and any probable ameliorative effect of Virgin Coconut Oil (VCO).
Materials and Methods
Experimental animals
Matured albino male rats (180-200 g) Body Weight (BW) were purchased and maintained at the animal house Unit of the Department of Physiology, Faculty of Basic Medical Sciences, and University of Calabar. The animals were kept in a well-ventilated space and acclimatized for 2 weeks. The rats ate rat chow and drinking water freely. After acclimatization duration, they were weighed on an electronic weighing balance; their Fasting Blood Glucose (FBG) levels were measured and reassigned before commencing the experimental treatment. The cages were cleared and kept clean throughout the period of the experiment.
Experimental design and treatment of animals
The rats were randomly separated into two major experimental groups (the test and recovery groups) of 35 rats in each major group. Experiment for the test group lasted for two weeks while experiment for the recovery group lasted for four weeks.
Thirty-five (35) rats in the test group were randomly divided into five sub-groups of 7 rats per sub-group (n=7) and were oral gavaged and treated thus: Sub-Group (SG)1 served as normal control and received 10 ml/kg body weight of distilled water, Sub- Group (SG) 2 received 10 ml/kg of Virgin Coconut Oil (VCO), Sub- Group (SG) 3 received 123 mg/kg (20% of lethal dose) of Atrazine (ATZ), Sub-Group (SG) 4 was the diabetic control that were left untreated and Sub-Group (SG) 5 was the diabetic group that were treated with 10 ml/kg of Virgin Coconut Oil (VCO). Treatment in the test group lasted for 2 weeks, after which the animals were sacrificed and blood collected for analysis.
During these 2 weeks’ period, thirty-five rats for the recovery group were also divided into 5 sub-groups of 7 rats per sub- group (n=7) and were treated as follows: (SG) 1 served as normal control and received 10 ml/kg body weight of distilled water, SG 2 received 10 ml/kg of Virgin Coconut Oil (VCO), SG 3, 4 and 5 received 123 mg/kg of ATZ. After 2 weeks, the animals were re-treated for recovery and were treated thus: (SG) 1 served as normal control and received 10 ml/kg body weight of distilled water, SG 2 received 10 ml/kg of Virgin Coconut Oil (VCO), SG 3 received 123 mg/kg of ATZ, SG 4 was treated with 10 ml/kg of Virgin Coconut Oil (VCO) and SG 5 was given 10 ml/kg of distilled water. Treatment for recovery also lasted for 2 weeks, after which the animals were sacrificed and blood collected for analysis.
Induction of Diabetes Mellitus (DM)
150 mg/kg body weight of alloxan monohydrate was injected intraperitoneally to induce Diabetes Mellitus (DM) [31,32]. The diabetic state was observed from about 48 hours by the symptoms of polyuria, polydypsia and glucosuria. After 72 hours, DM was confirmed with Fasting Blood Glucose (FBG) concentration above 180 to 200 mg/dL using a glucometer (ACCU-CHECK Active) and ACCU-CHECK compatible glucose test strips [30].
Preparation of Virgin Coconut Oil (VCO)
Matured dried coconut fruit (cocos nucifera) were harvested and identified by a botanist at the Department of Botany, University of Calabar. The method employed in extracting Virgin Coconut Oil (VCO) was the modified wet extraction method of [33]. The hard white endosperm of the matured coconut was grated; 500 ml H2O was poured into the grated coconut and pressed via musflfin cloth to get coconut milk. The obtained milk was allowed to stay for around 18 hrs to encourage gravitational segregation into different segments. The demulsification process created three segments; water or aqueous segment at the lowest part, the coconut oil segment in the middle layer and cream or emulsion segment on top. The cream on the top segment was removed; the coconut oil was scooped and placed on low heat for about 5 mins for evaporation of moisture. The coconut oil gotten was then filtered with the aid of cotton wool, after which it was stored for further use at room temperature.
Determination of body weight
The body weight of the rats was determined before the commencement and at the end of the experimental treatment. This was done with the aid of an electronic weighing balance.
Determination of fasting blood glucose
Fiber Bragg Grating (FBG) was evaluated with the aid of ACCU- Check blood glucose meter with recommended blood glucose test strips. The tail tip was pricked and blood dropped gently on the test strip, after which the readings were noted. Fiber Bragg Grating (FBG) readings were taken before diabetes induction and 72 hours after induction; after which it was done weekly.
Estimation of serum oestrogen concentration
The Calbiotech, Inc (CBI) Oestrogen (E2) ELISA Kit (USA) for the quantitative evaluation of E2 concentration in rat serum and plasma was used as applied by [34].
Assay procedure
25 μl of the standard solution, control solution and test sample were separately pipetted to recommended wells and then 100 μl of oestrogen enzyme conjugate which is the working solution was added into each of the wells. A shaker was used to mix the combined solution very well for ten to twenty seconds, after which they were allowed to incubate at 18-25°C, room temperature for sixty minutes. At the incubation period, a specific quantity of Horseradish Peroxidase-labeled Estrogen competes with oestrogen endogenously available in standard solution, control solution or test sample for specific binding sites number of oestrogen antibody. As oestrogen concentration in each specimen increases, the concentrations of oestrogen peroxidase conjugate that is bound immunologically to the well gradually declined. The resultant solution of E2 peroxidase conjugate that which remain unbound was poured out from the wells and washed with 300 μL of wash buffer 3 times; the plate was then turned upside down and blotted on clean paper towels. Then, 100 microlitre of 3,3,5,5'-tetramethylbenzidine reagent was then poured into all the wells and mixed lightly for about ten seconds and incubated for 30 minutes at 18-25°C, resulting in development of blue color. The reactivity was halted by addition of 50 μl stop solution to all the wells and mixed lightly for 30 seconds for complete change of colour from blue to yellow. Finally, the absorbance was calculated at 450nm with the aid of a microplate reader within 15 minutes.
Statistical analysis
Windows SPSS package (SPSS 20.0) was used for the statistical analysis. One-way ANOVA were used to analyse the obtained data, after which it was subjected to Tukey’s post hoc test. The obtained values were expressed as Mean+Standard Error of Mean (Mean ± SEM). Results with values of P<0.05 were accepted as significant.
Results
Body weight changes in normal control, ATZ and diabetic groups
The initial body weight of all experimental groups were not significantly (p<0.05) different as shown in Table 1; but the final body weight of the Atrazine (ATZ) significantly (p<0.05) decreased when compared with the NC+H2O and NC+VCO. There was a significant (p<0.05) decrease in the final body weight of the diabetic untreated group when compared to the NC+H2O, NC+VCO and Atrazine (ATZ) groups. In the diabetic group treated with VCO, the final body weight significantly (p<0.05) increased when compared to the diabetic untreated group.
| Groups |
Treatments |
| Normal control+H20 |
10 ml/kg of distilled water (H2O) |
| Normal control+VCO |
10 ml/kg of Virgin Coconut Oil (VCO) |
| Atrazine treated |
123 mg/kg (20% of lethal dose) of Atrazine |
| Diabetic control |
10 ml/kg of distilled water (H2O) |
| Diabetic+VCO |
10 ml/kg of Virgin Coconut Oil (VCO) |
Table 1: Experimental grouping and treatment test group (2 weeks).
Body weight changes in normal control and ATZ recovery groups
Table 2 showed a significant (p<0.05)decrease in body weight with continuous use of ATZ in the ATZ continued group while the body weight of both the Virgin Coconut Oil (VCO) recovered and untreated groups were significantly (p<0.05) higher than the ATZ group.
| Groups |
Treatment (1st 2 weeks) |
Treatment (2nd 2 weeks) |
| Normal control+H20 |
10 ml/kg of distilled water (H2O) |
10 ml/kg of distilled water (H2O) |
| Normal control+VCO |
10 ml/kg of Virgin Coconut Oil (VCO) |
10 ml/kg of Virgin Coconut Oil (VCO) |
| Atrazine treated |
123 mg/kg (20% of lethal dose) of Atrazine |
123 mg/kg (20% of lethal dose) of Atrazine |
| VCO after ATZ |
123 mg/kg (20% of lethal dose) of Atrazine |
10 ml/kg of Virgin Coconut Oil (VCO) |
| Untreated after ATZ |
123 mg/kg (20% of lethal dose) of Atrazine |
10 ml/kg of distilled water (H2O) |
Table 2: Recovery group (4 weeks).
Fasting blood glucose level in normal control, ATZ and Diabetic groups
Initially, there was no significant difference in the fasting blood glucose level of all the experimental rats. After diabetes induction with alloxan, the fasting blood glucose level of the diabetic untreated and diabetic treated with VCO increased significantly (p<0.05) when compared with the other groups. After one week of atrazine administration, there was no significant difference in the Atrazine (ATZ) group when compared with the normal control (NC+H2O) and normal control+VCO groups (NC+VCO). But at the end of week 2, there was a significant (p<0.05) increase in the Fasting Blood Glucose (FBG) of the ATZ group when compared with the NC+VCO. Also, by the end of week 2, the fasting blood glucose level of the diabetic+VCO group was significantly (p<0.05) lower than the diabetic untreated group, though still significantly (p<0.05) higher than NC+H2O, NC+VCO and ATZ treated groups (Figure 1).
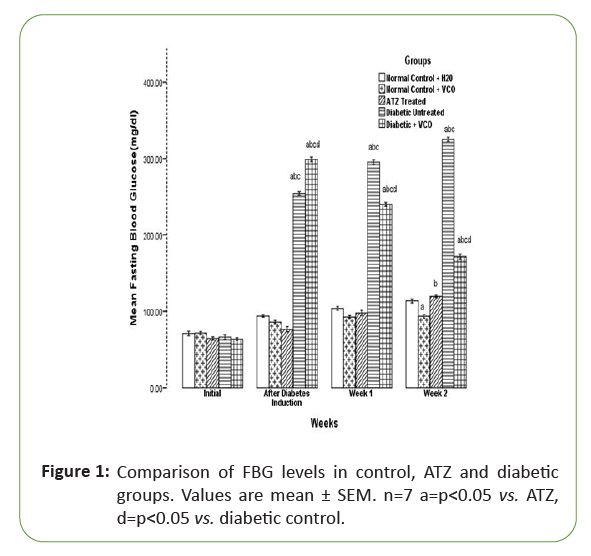
Figure 1: Comparison of FBG levels in control, ATZ and diabetic groups. Values are mean ± SEM. n=7 a=p<0.05 vs. ATZ, d=p<0.05 vs. diabetic control.
Fasting blood glucose level in normal control and ATZ recovery groups
In the recovery groups, there was no significant (p<0.05) difference in the Fasting Blood Glucose (FBG) of all the experimental groups in the first two weeks of experimentation but with continuous use of Atrazine (ATZ) till the end of week 4, in the Atrazine (ATZ) continued group, there was a significant (p<0.05) increase in the Fasting Blood Glucose (FBG) level when compared with the NC+H2O and NC+VCO. By week 4, the Fasting Blood Glucose (FBG) level in the Virgin Coconut Oil (VCO) recovered group became significantly (p<0.05) lower than the Atrazine (ATZ) continued group (Figure 2).
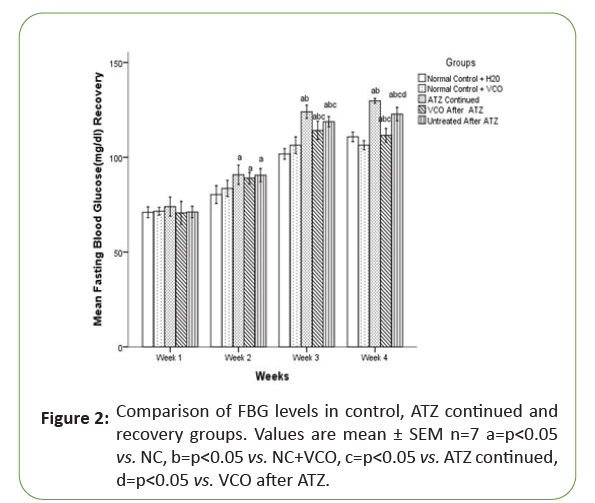
Figure 2: Comparison of FBG levels in control, ATZ continued and recovery groups. Values are mean ± SEM n=7 a=p<0.05vs. NC, b=p<0.05 vs. NC+VCO, c=p<0.05 vs. ATZ continued, d=p<0.05 vs. VCO after ATZ.
Oestrogen levels (pg/ml) in normal control, ATZ and Diabetic groups
The mean values for oestrogen in the NC+H2O, NC+VCO, Atrazine (ATZ) treated, Diabetic control and Diabetic+VCO groups are 12.90 ± 0.13, 13.63 ± 0.19, 7.92 ± 0.06, 9.31 ± 0.18 and 11.21 ± 0.19 respectively. The oestrogen levels in the Atrazine (ATZ) treated group significantly (p<0.05) decreased when compared with the NC+H2O and NC+VCO. There was a significant (p<0.05) decrease in oestrogen levels in the diabetic untreated group when compared with the NC+H2O and NC+VCO but a significant (p<0.05) increase when compared with the Atrazine (ATZ) treated group. There was no significant (p<0.05) difference in the oestrogen levels between the diabetic untreated and diabetic treated with Virgin Coconut Oil (VCO) (Figure 3).
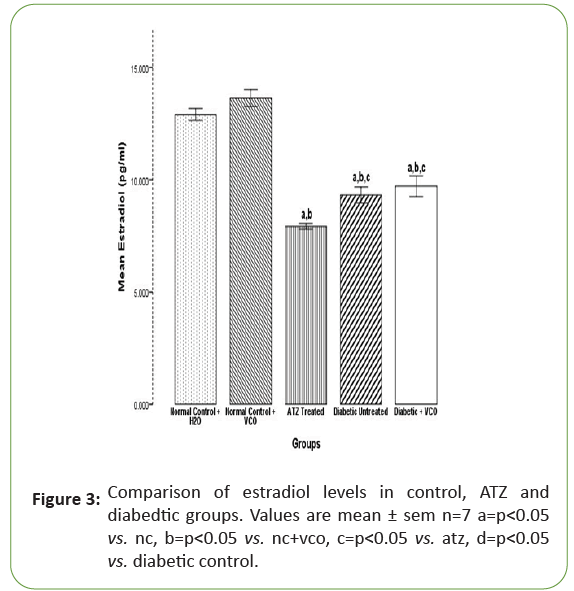
Figure 3: Comparison of estradiol levels in control, ATZ and diabedtic groups. Values are mean ± sem n=7 a=p<0.05vs. nc, b=p<0.05 vs. nc+vco, c=p<0.05 vs. atz, d=p<0.05 vs. diabetic control.
Oestrogen levels in normal control and ATZ recovery groups
Figure 4 showed a significant (p<0.05) decrease in the oestrogen levels in the Atrazine (ATZ) continued group (3.82 ± 0.29) when compared with the NC+H2O (12.89 ± 0.17) and NC+VCO (13.54 ± 0.21). Virgin Coconut Oil (VCO) treatment (6.47 ± 0.17) significantly (p<0.05) increased oestrogen when compared with the Atrazine (ATZ) continued group but significantly (p<0.05) lower than the NC+H2O and NC+VCO. Oestrogen levels also increased significantly (p<0.05) in the Atrazine (ATZ) untreated group (7.22 ± 0.09) when compared with the Atrazine (ATZ) continued group in Tables 3 and 4.
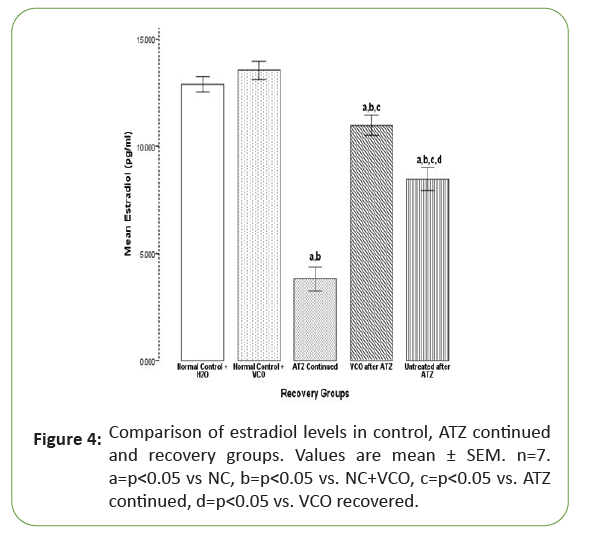
Figure 4: Comparison of estradiol levels in control, ATZ continued and recovery groups. Values are mean ± SEM. n=7. a=p<0.05 vs NC, b=p<0.05 vs. NC+VCO, c=p<0.05 vs. ATZ continued, d=p<0.05 vs. VCO recovered.
| Groups/Body weight |
Initial (g) |
Final (g) |
Percentage weight change (%) |
| Normal control |
179.83 ± 1.22 |
242.80 ± 9.60 |
25.93 (Increase) |
| Normal control+VCO |
185.20 ± 4.56 |
252.40 ± 6.94 |
26.62 (Increase) |
| ATZ treated |
183.75 ± 4.66 |
202. 76 ± 8.45a,b |
9.3 (Increase) |
| Diabetic control |
192.20 ± 2.45 |
163.80 ± 9.65 a,b,c |
17.33 (decrease) |
| |
183.75 ± 4.66 |
225.00 ± 7.78 a,b,c,d |
18.33 (increase) |
Values are mean ± SEM. n=7
a=p<0.05 vs. NC, b=p<0.05 vs. NC+VCO, c=p<0.05 vs. ATZ, d=p<0.05 vs. Diabetic control
Table 3: Body weight changes in normal, ATZ and diabetic groups.
| Groups/Body weight |
Initial (g) |
Final (g) |
Percentage weight change (%) |
| Normal control |
249. 64 ± 6.22 |
280.30 ± 2.67 |
10.93 (Increase) |
| Normal control+VCO |
242. 55 ± 5.82 |
268.17 ± 6.73 |
9.55 (Increase) |
| ATZ continued |
192. 76 ± 8.45 |
180.33 ± 3.58 a,b |
6.89 (Decrease) |
| VCO after ATZ |
190. 58 ± 3.42 |
225.03 ± 5.44 a,b,c |
15.31 (Increase) |
| Untreated after ATZ |
195. 76 ± 8.45 |
216.00 ± 2.78 c,d |
9.37 (Increase) |
Values are mean ± SEM. n=7
a=p<0.05 vs. NC, b=p<0.05 vs. NC+VCO, c=p<0.05 vs. ATZ continued, d=p<0.05 vs. VCO recovered
Table 4: Body weight changes in normal and ATZ recovery groups.
Environmental pollutant is inevitably taken into the body through inhaling, swallowing or from skin contact [35].These have been found to cause devastating effect on human health causing a whole lot of diseases one of which is diabetes mellitus [36]. Persistent hyperglycaemia in diabetes mellitus is associated with derangement in metabolic signal transduction pathway which is responsible for most of the pathological symptoms associated with this syndrome. Persistent hyperglycaemia in diabetes mellitus is associated with derangement in metabolic signal transduction pathway which is responsible for most of the pathological symptoms associated with this syndrome. Atrazine toxicity has been reported by many researchers this toxicity is probably responsible for the metabolic derangements observed in the present study [22, 37-40].
There was a significant increase in Body Weight (BW) in both the Co+H2O and Co+VCO groups. The increased body weights of the Co+H2O and Co+VCO groups probably suggest an increased synthesis of tissue proteins in these groups of rats. Also observed an increase in body weight gain in normal rats and normal rats treated with VCO [41,42]. Body weights in the Co+H2O and Co+VCO groups were not significantly different. Similarly, rodents administered with VCO recorded no significant body weight change in relation to the control rats [43,44]. Normally, exposure to pesticide can cause body and organ weight reduction in animals [45,46]. In the present study, we observed an increased weight of the body in Atrazine (ATZ) treated rats though this weight gain was significantly lower than the Co+H2O and Co+VCO groups in the first phase of the experiment. Observed that ATZ treatment caused an increment in body weight in the entire tested rats after 15 days [22]. They also observed that the weight of the kidney and liver significantly increased in atrazine treated rats in relation to untreated nor-diabetic rats, while in atrazine treated diabetic rats the weight of the liver increased but that of kidney decreased in relation to the diabetic untreated rats. They attributed this increased weight gain to decreased tissue sensitivity to insulin which might lead to excessive weight increase Increased body weight was also observed in a research by chronic atrazine concentration (0.03 or 0.3 mg kg−1) for 6 months led to an increase in body weights in rodents [47]. The principal manifestations of Diabetes Mellitus (DM) in both humans and in experimental conditions are glucosuria, hyperglycaemia and weight loss these principal symptoms also occured in this research [48]. In alloxan- induced diabetic rats, a significant drop in BW was recorded in comparison with Co+H2O and Co+VCO groups which might be a consequence of breakdown of tissue proteins in an attempt to arrest the alloxan assault. The DT+VCO group, an increase in body weight gain was observed probably due to reduced loss of tissue proteins due to treatment of hyperglycemia. Reported reduced BW in diabetic rodents and an increased BW gain when treated with VCO [41]. Udia and colleagues (2016) also observed that treatment of alloxan-initiated diabetic rats with leaf extracts of Rothmannia Hispida (RHE) effectively reversed alloxan-induced hyperglycaemia and body weight loss consequent upon diabetic induction [49].
Discussion
In the recovery group, it was observed that with continuous use of ATZ in the ATZ continued group, a significant reduction in body weight was observed. Besides, unchanged or decreased body weights with the administration of atrazine were also reported [50,51]. Son and colleague (2003) documented that the treatment with high dosage of atrazine significantly diminished BW but increased relative weight of the liver [52]. The diminished body/ organs weight observed in atrazine treated rats might be because of decrease in food intake or because of necrotic alterations in various body tissues [53]. Laws and colleagues also noted a fall in BW in atrazine treated rat model mainly due to a decrease in food intake demonstrated that exposure to 100 μg of ATZ can cause osmoregulatory disturbance, physiological stress and reduction in food intake and growth of Atlantic salmon smolts [54,55]. But also, with Virgin Coconut Oil (VCO) administration and withdrawal of ATZ, an increase in body weight was observed.
The fasting blood glucose levels in the normal control group and normal+VCO group were not significantly different at the end of the first phase of the experiment. This supports the report by which showed that there were no significant differences in the blood glucose concentration of VCO group compared with the normal control [41]. The non-significant effect Virgin Coconut Oil (VCO) administration had on the blood glucose concentrations of the normal rats as compared with the NC, suggests that Virgin Coconut Oil (VCO) did not negatively impact on their blood glucose concentrations [41]. Triggering the production of Reactive Oxygen Species (ROS), which in turn cause the destruction of the beta cells and an eventually result in a condition of insulin- dependent diabetes is been documented as the main mechanism of action of alloxan-induced diabetes [56]. Thus, alloxan- diabetic model is used to investigate diabetes arising from beta cell destruction caused by ROS [30]. The basic marker of DM is hyperglycemia owing to decrease in pancreatic insulin secretion or defective insulin action and insulin resistance by target cells [57]. Type 1 DM results from idiopathic or cellular-mediated autoimmune destruction of pancreatic β- cells, patients thus depend totally on exogenous insulin. Following alloxan injections, significant increase in FBG of alloxan induced diabetic animals in comparison to Co+H2O, Co+VCO and ATZ rats were noted. Similarly, several studies have documented a marked increase in blood glucose levels following alloxan induction [30,41,56-59]. It was observed that treatment of the diabetic group with 10 ml/kg of VCO for two weeks brought about a significant drop in fasting blood glucose levels. This further supports the hypoglycemic ability of Virgin Coconut Oil (VCO) [60,61]. Several other studies have also reported on the hypoglycemic effect of VCO on alloxan induced diabetic rats [30,41,59]. This beneficial potential of Virgin Coconut Oil (VCO) on blood glucose level was attributed to its antioxidant ability and observed that the administration of coconut oil diet to diabetic mice significantly reduced blood glucose, which could be mediated through its antioxidant effect [30,62]. A study demonstrated that the administering coconut oil prevented diabetes (Houssay and, Martínez, 1947 According to our report, significant elevation of fasting blood glucose occured in Atrazine (ATZ) treated group comparable to Co+H2O and Co+VCO groups but the level diminished in relation to the Diabetic Untreated (DUT) group. This probably showed that Atrazine (ATZ) does not have a hyperglycemic effect on the fasting blood glucose for the 14 days, i.e. the fasting blood glucose level was still below 120 mg/dl. This supports the report by where FBG levels of rodents treated with Atrazine (ATZ) and placed on a normal diet increased in comparison with the controls [20]. Recorded a significant increment in FBG levels in diabetic untreated and diabetic rats administered Atrazine (ATZ) comparable to control rats; nevertheless, no significant differnce in Fasting Blood Glucose (FBG) concentration occurred comparing diabetic untreated and diabetic rats administered Atrazine (ATZ), showing that Atrazine (ATZ) did not further aggravate the diabetic blood glucose during the two weeks’ experimentation period. In the recovery group, consistent elevation of the Fasting Blood Glucose (FBG) concentration in the Atrazine (ATZ) continued group was recorded; the fasting blood glucose level actually increased to a hyperglycemic level (125 mg/dl-140 mg/dl) but not up to the diabetic level (>140 mg/dl) relative to this research [22]. Significant reduction in Fasting Blood Glucose (FBG) levels in the atrazine sub-group that were administered Virgin Coconut Oil (VCO) when compared with the other groups was observed. Our report hereby suggests that Virgin Coconut Oil (VCO) has a mitigating effect on atrazine induced hyperglycemia.
Oestrogen activities in fat tissue, immune cells, skeletal muscle and liver are engaged in insulin tissue sensitivity, inflammation and accumulation of lipid. Oestrogen activities in pancreatic islet beta cells control insulin discharge, cellular nutrient homeostasis and survival. Oestrogen inadequacy elevates metabolic breakdown, which prompts metabolic disorder type II diabetes and obesity [63]. The result of this study showed no significant effect of Virgin Coconut Oil (VCO) on oestrogen level in normal rats showing that Virgin Coconut Oil (VCO) administration did not affect oestrogen levels in normal rats. There are conflicting reports about the oestrogenic potential of atrazine as an endocrine disruptor. Some reports suggest that atrazine is an environmental oestrogen while others have shown that atrazine could actually be an oestrogen antagonist [37-40,64]. Oestrogen level in the atrazine treated group decreased significantly comparable to the oestrogen levels in Co+H2O and Co+VCO groups. Several clinical investigations have documented the powerful defensive potential of estrogen against metabolic disorder and diabetes showing that the risk of diabetes increase as oestrogen level decreases [65,66]. This observation supports the regenerative toxicology research in rats sustained with atrazine diet at 500 ppm concentration; they observed a diminished oestrogenic response on vaginal cytology and oestrous cycling patterns following atrazine administration [67,68]. Documented that atrazine exposure to carp showed no impact on oestrogen-actuated generation of vitellogeni [69]. Found no evidence that atrazine effect had any naturally occurring oestrogen-mediated responses [70]. Hypothesized that triazines (atrazine, simazine and diaminochlorotriazine) might be able to express or modulate oestrogen activity using a rat uterine model system; they concluded that these triazines possess no oestrogen-like activity but possess a weak oestrogenic antagonist property [39]. From above reports, it seems that Atrazine (ATZ did not bind directly to oestrogen receptor and have no in vivo oestrogenic action, but was demonstrated to hinder estrogen- activated incorporation of 3H-thymidine and development of uterine invivo [39].Revealed that atrazine simazine, and cyanazine blocked estrogen-stimulated reaction in yeast cells, that is they have oestrogenic abilities [37]. However, did not uphold these findings; they reported a nor-inhibitory effect of atrazines on 17β-oestrogen-induced trans-activation in yeast [71]. Also evaluated the potential of atrazine to trigger G Protein Oestrogen Receptor (GPER)-mediated signaling in cancer cells and cancer-associated fibroblasts and concluded that atrazine should be included among the environmental contaminants that may elicit oestrogenic activity through G Protein Oestrogen Receptor (GPER) mediated signalling [38]. This study therefore support that Atrazine (ATZ) might not possess an oestrogenic activity but might be an oestrogen antagonist. Various investigations have recommended that oestrogens have a significant influence on glucose homeostasis. They have shown that treatment with oestrogen reduces diabetic complications, and normalizes the endothelium role in diabetic condition [72,73]. Oestrogen receptors exist in islets of Langerhans (57 et al.) and the oestrogen effects in some functional part of this islet are recorded [74,75]. In the alloxan induced diabetic untreated group, there was a significant increase in the oestrogen levels when compared with the oestrogen levels of the atrazine treated group, but significant decrease in the level of oestrogen was observed when compared with the normal control+VCO and normal control+H2O groups. This showed that oestrogen levels are reduced in diabetes mellitus. Hypogonadotropic hypogonadism, hypooestrogenism, menstrual irregularities, polycystic ovaries and early menopause have been described in T1DM women, which may be due to the relationship between decreased oestrogen levels and insulin action [76]. Clinical and experimental investigations have demonstrated a solid connection between estrogen inadequacy and metabolic disorder [77,78]. Women at their premenopausal age show more sensitivity to INL and lower occurrence of diabetes type II in relation to men at the same age. However, this beneficial effect vanishes in postmenopausal age with upset glucose homeostasis, which might be to an extent due to a decrease in plasma oestrogen [79]. In affirmation to this claim, clinical investigation on estrogen replacement treatment in women at their postmenopausal age showed a fall in INL resistance and a decrease in FBG concentration [80,81]. These reports further support the reduced level of oestrogen in the alloxan diabetic condition in this study. There was a significant increase in serum oestrogen level in diabetic group treated with Virgin Coconut Oil (VCO) when compared to the diabetic untreated group, though the level was significantly lower than the normal control groups. Osteoporosis one of the postmenopausal effects in elderly women is typically connected with estrogen inadequacy [82]. Worked on the impact of Virgin Coconut Oil (VCO)supplementation on bone loss in osteoporotic rat model; they reported that Virgin Coconut Oil (VCO) maintained structure of the bone, also prevented loss of the bone in oestrogen-deficient rodents [83]. They attributed these positive abilities of Virgin Coconut Oil (VCO) on bone micro-structure to its antioxidant ability. They deduced that the antioxidative properties of Atrazine, Virgin Coconut Oil (VCO) counteracted ROS-stimulated bone loss related to oestrogen inadequacy [83-85].
Conclusion
Therefore, in our oestrogen deficient diabetic rat model, Virgin Coconut Oil (VCO) was also effective in improving oestrogen level. It is suggested that if used for a longer period of time, the oestrogen level could possibly be restored to the normal level. These observations suggest that Virgin Coconut Oil (VCO) probably possess an oestrogen stimulating effect or uses another pathway for stimulating oestrogen synthesis and release which could be attributed to its polyphenolic constituent or lauric acid content.
39004
References
- Franco SS, Nardocci AC, Gunther WM (2008) PAH biomarkers for human health risk assessment: A review of the state-of-the-art. Cad Saude Publica 24:S569-S580.
- Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S (2013) Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol 41:361-373.
- Kelly FJ, Fussell JC (2011) Air pollution and airway disease. Clin Exp Allergy 41:1059-1071.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, et al. (2012) Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380:219-229.
- Michael B, Hadley R, Valentin F (2018) Air pollution and cardiovascular disease: a window of opportunity. Nature Reviews Cardiology 15:193-194.
- Bowe B, Xie Y, Li T, Yan Y, Xian H, et al. (2018) The 2016 global and national burden of diabetes mellitus attributable to PM2·5 air pollution: a longitudinal cohort study using the Global Burden of Disease 2016 data and methodologies. Lancet Planet Health 7:301-312.
- Carrington D (2017) Global pollution kills over 9 million a year and threatened survival of human societies.
- Guyton A C, Hall JE (2011) Textbook of Medical physiology, (12th Edn).
- International Diabetes Federation IDF (2015) Nigeria vs World Prevalence of Diabetes.
- Hectors TL, Vanparys C, Van der VK, Martens GA, Jorens PG, et al. (2011) Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia 54:1273-1290.
- Malhotra SS, Khan AA (1984) Biochemical and PhysiologicalImpact of Major Pollutants.
- Jerrett M, Burnett R, Ma R, Pope C, Krewski D, et al.(2005) Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology 16:727-736.
- Goldberg MS, Burnett RT, Bailar JC, Brook J, Bonvalot Y, et al. (2001) The association between daily mortality andambient air particle pollution in Montreal, Quebec. 2. Causes pecific mortality. Environ Res 86:26-36.
- Zanobetti A, Schwartz J (2002) Cardiovascular damage by airborneparticles: Are diabetics more susceptible?. Epidemiology 13:588-592.
- Rajagopalan S, Brook RD (2012) Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 61:3037-3045.
- Chikoye D, Udensi EU, Fontem AL (2006) Performance of a new formulation of ATZ for weed control in maize in Nigeria. Food Agriculture Environment.
- Mair DC, Yoo JY, Baker BE (1978) Residues of ATZ and N-dealkylated ATZ in water from five agricultural watersheds in Quebec. Arch Environ Contam Toxicol 7:221-235.
- Funari E (1989) Preliminary report on the ATZ and molinate water supply contamination in Italy. Chemosphere 18:2339-2343.
- Mercola A (2016) Environmental Signs Suggest ATZ Is Becoming a Serious Health Threat.
- Lim S, Ahn SY, Song IC, Chung MH, Jang HC, et al. (2009) Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. Plos One 4:e5186.
- Stanko JP, Enoch RR, Rayner JL, Davis CC, Wolf DC, et al. (2010) Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate inflammation of male Long-Evans rats. Reprod Toxicol 10:115-178.
- Jestadi D, Phaniendra A, Babji U, (2014) Effect of short term exposure of atrazine on the liver and kidney of normal and diabetic rats.
- Wynn V, Doar JW (1966) Some effects of oral contraceptives on carbohydrate metabolism. Lancet 2:715-719.
- Brussaard HE, Gevers JA, Frolich M, Kluft C, Krans HM (1997) Short-term oestrogen replacement therapy improves insulin resistance, lipidsand fibrinolysis in postmenopausal women with NIDDM. Diabetologia 40:843-849.
- Crespo CJ, Smit E, Snelling A, Sempos CT, Andersen RE (2002) Hormone replacement therapy and its relationship to lipid and glu-cose metabolism in diabetic and nondiabetic postmenopausal women: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 25:1675-1680.
- Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, et al. (2011) Estrogen receptor activation reduces lipid synthesis in pancreaticislets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest 121:3331-3342.
- Carr MC (2003) The emergence of the metabolic syndrome with menopause. J Clini Endocrinol Metabol 88:2404-2411.
- Villarino B, Dy L, Lizada C (2007) Descriptive sensory evaluation of virgin coconut oil and refined, bleached and deodorized coconut oil. LWT-Food Science and Technology 40:193-199.
- Bergsson G, Arnfinnsson J, Karlsson S, Steingrimsson O, Chormar H (1998) In vitro inactivation of Chlamydia tracomatis by fatty acids and monoglycerides. Antimicrorb Agents Chemothe 42:2290-2294.
- Iranloye B, Oludare G, Olubiyi M (2013) Anti-diabetic and antioxidant effects of virgin coconut oil in alloxan induced diabetic male Sprague Dawley rats. J Diab Mellitus 3:221-226.
- Katsumata K, Katsumata Y, Ozawa T (1993) Potentiating effects of combined usage of three sulfonylurea drugs on the occurrence of alloxan diabetes in rats. Hormone Metab Res 25:125-126
- Etuk EU (2010) Animals models for studying diabetes mellitus. Agric Biol J N Am 1:130-134.
- Nevin KG, Rajamohan T (2006) Virgin coconut oil supplemented diet increases the antioxidant status in rats. Food Chemistry 99:260-266.
- Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC (2011) Estimation of oestrogen in mouse serum samples: evaluation of commercial oestrogen immunoassays. Endocrinology 152: 4443-4447.
- Agency for Toxic Substances and Disease Registry (2003) Public Health Statement for Atrazine Buford Hwy NE, Atlanta.
- Rylander L, Rignell-Hydbom A, Hagmar L (2005) A cross-sectional study of the association between persistent organochlorine pollutants and diabetes.
- Tran DQ, Kow KY, McLachlan JA, Arnold SF (1996) The inhibition of estrogen receptor-mediated responses by chloro-s-triazine-derived compounds is dependent on oestrogen concentration in yeast. Biochem Biophys Res Commun 227:140-146.
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, et al. (2007) G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-oestrogen and selec-tive GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67: 1859-1866.
- Tennant MK, Hill DS, Eldridge JC, Wetzel LT, Breckenridge CB, et al. (1994) Anti-estrogenic properties of chloro-s-triazines in rat uterus. J Toxicol Environ Health 43:183-196.
- Tanaka T, Hiroyuki K, Rikako S, Shigeyuki S (2004) Lack of modifying effects of an estrogenic compound atrazine on 7,12-dimethylbenz(a)anthracene-induced ovarian carcinogenesis in rats. Cancer Letters 210:129-137.
- Eleazu C, Egedigwe-Ekeleme C, Famurewa A, Mohamed M, Akunna G, et al. (2019) Modulation of the lipid profile, hepatic and renal antioxidant activities, and markers of hepatic and renal dysfunctions in alloxan-induced diabetic rats by virgin coconut oil. Endocr Metab Immune Disord-Drug Targets 19:1-8.
- Eleazu CO, Ezekwibe I, Egbe M, Saidu S, Eleazu K, et al. (2017) Dietary intake of boiled breadfruit (Treculiaafricana) seeds did not improve hyperglycemia in streptozocin induced diabetic rats: Effect on the oral glucose tolerance of normoglycemicrats. Acta Scientiarum Polonorum Technologia Alimentaria 16:93-99.
- Nevin KG, Rajamohan T (2004) Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clini Biochem 37:830-835
- Nevin KG, Rajamohan T (2008) Influence of virgin coconut oil on blood coagulation factors, lipid levels and LDL oxidation in cholesterol fed Sprague-Dawley rats. Clini Nutr Metaboli 3:1-8.
- Sharma Y, Bashir S, Irshad M, Nag TC, Dogra TD (2005) Dimethoate-induced effects on antioxidant status of liver and brain of rats following subchronic exposure. Toxicology 215:173-181.
- Dutta AL, Sahu CR (2013) Emblica officinalis garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. Springer Plus 2:541-554.
- Gojmerac T, Kartal B, Zuric M, Curic S, Mitak M (1995) Serum biochemical and histopathological changes related to the hepatic function in pigs following atrazine treatment. J Appl Toxicol 15:233-236.
- WHO/UNEP, Åke Bergman, Jerrold J Heindel, Susan Jobling, Karen A Kidd, R. Thomas Zoeller (2012) State of the science of endocrine disrupting chemicals (Edn).
- Udia PM, Takem LP, Ufot UF, Antai AB, Owu DU (2016) Insulin and alpha amylase levels in alloxan-induced diabetic rats and the effect of Rothmannia hispida(K. Schum) Fagerl leaf extract. J Phytopharmacol 5:1-5.
- Roloff BD, Belluck DA, Meisner LF (1992) Cytogenetic studies of herbicide interactions in vitro and in vivo using atrazine and linuron. Arch Environ Contam Toxicol 22:267-271.
- Cantemir C, Cozmei C, Scutaru B, Nicoara S, Carasevici E (1997) p53 Protein expression in peripheral lymphocytes from atrazine chronically intoxicated rats. Toxicology Letters 93:87-94.
- Son HY, Nishikawa A, Okazaki K, Lee KY, Imazawa T, et al. (2003) Lack of modifying effects of atrazine and/or tamoxifen on thyroid carcinogenesis in rats pretreated with Nbis(2-hydroxypropyl) nitrosamine (DHPN). Food Chem Toxicol 41:1811-1816.
- Fukamachi K, Seok Han B, Kyu Kim C (2004) Possible enhancing effects of atrazine and nonylphenol on 7,12- dimethylbenz[a]anthracene-induced mammary tumor development in human c-Ha-ras proto-oncogene transgenic rats. Cancer Science 95:404-410.
- Laws SC, Hotchkiss M, Ferrell J, Jayaraman S, Mills L, et al. (2009) Chlorotriazine herbicides and metabolites activate an ACTH-dependent release of corticosterone in male Wistar rats. Toxicol Sci 112:78-87.
- Nieves-Puigdoller K, Bjorn T, Stephen D (2007) Effects of hexazinone and atrazine on the physiology and endocrinology of smolt development in Atlantic salmon. Aquatic Toxicol 84:27-37.
- Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced Diabetes. Diabetologia 51:216-226.
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, et al. (2000) Nongenomic actions of estro-gens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci 97:11603-11608.
- Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiology Res 50:537-546.
- Maidin NH, Norhayati Ahmad (2015) Protective and Antidiabetic Effects of Virgin Coconut Oil (VCO) On Blood Glucose Concentrations in Alloxan Induced Diabetic Rats. Int J Pharm Pharm Sci 7:57-60.
- Garfinkel M, Lee S, Opara E, Akwari OC (1992) Insulunotropic potency of lauric acid. A menabolic rationale for medium chain fatty acids (MCF) in TPN –formulation. J sur res 52:328-333.
- Ngala R, Isaac A, Samuel A, Enoch O (2016) Effect of dietary vegetable oil consumption on blood glucose levels, lipid profile and weight in diabetic mice: an experimental case-control study. BMC Nutrition 2:28-36
- Mauvais-Jarvis F, Clegg DJ, Hevener AL (2013) The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34:309-338.
- Bisson M, Hontela A (2002) Cytotoxic and endocrine-disrupting potential of atrazine, diazinon, endosulfan, and mancozeb in adrenocortical steroidogenic cells of rainbow trout exposed in vitro. Toxicol Appl Pharmacol 180:110-117.
- Margolis KL, Bonds DE, Rodabough RJ (2004) Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the Women’s Health Initiative Hormone Trial. Diabetologia 47:1175-1187.
- Pentti K, Tuppurainen MT, Honkanen R (2009) Hormone therapy protects from diabetes: The Kuopio osteoporosis risk factor and prevention study. Europe J Endocrinol 160:979-983.
- Hauswirth JW, Wetzel LT (1998) Toxicity characteristics of the 2-chlorotriazines atrazine and simazine: In Ballantine LG, McFarland JE, Hackett DS (edns).
- Eldridge JC, Wetzel LT, Stevens JT, Simpkins JW (1999) The mammary tumor response in triazine-treated female rats: A threshold-mediated interaction with strain and species-specific reproductive senescence. Steroids 64:672-678.
- Sanderson JT, Letcher RJ, Henewer M, Giesy JP, van den Berg M (2001) Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect 109:1027-1031.
- LeGoff G, Hilliou F, Siegfried BD, Boundy S, Wajnberg E, et al. (2006) Xenobiotic response in Drosophilia melanogaster: sex dependence of P450 and GST gene induction. Insect Biochem Mol Biol 36:674-682.
- Graumann K, Breithofer A,Jungbauer A (1999) Monitoring of estrogen mimics by a recombinant yeast assay: Synergy between natural and synthetic compounds?. Sci Total Environ 225:69-79.
- Ohmichi M, Tasaka K, Kurachi H, Murata Y (2005) Molecular mechanism of action of selective estrogens receptor modulator in target tissues. Endocr J 52:151-162.
- Vina J, Borras C, Gomez-Cabrera MC, Orr W (2006) Role of reactive oxygen species and (phyto) oestrogens in the modulation of adaptive response to stress. Free Radic Biol Med 40:111-119.
- Sutter-Dub MT (2002) Rapid non - genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic β-cells, the adipocyte and other cell types. Steroids 67:77-93.
- Stubbins RE, Holcomb VB, Hong J, Nunez NP (2012) Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 51:861-870.
- Codner E (2008) Estrogen and type 1 diabetes mellitus. Pediatr Endocrinol Rev 6:228-234.
- Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, et al. (2003) Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology 144:1474-1480.
- Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, et al. (2006) Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 8:538-554.
- Yan H, Yang W, Zhou F, Li X, Pan Q, et al. (2019) Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 68:291-304.
- Kim CH (2013) Expression of extracellular superoxide dismutase protein in diabetes. Arch Plast Surg 40:517-521.
- Manson JE, Chlebowski RT, Stefanick ML (2013) The Women’s Health Initiative Hormone Therapy Trials: Update and Overview of Health Outcomes during the Intervention and Post-Stopping Phases. JAMA 310:1353-1368.
- Riggs BL, Khosla S,Melton LJ (2002) Sex steroids andthe construction and conservation of the adult skeleton. Endocrine Revi 23:279-302.
- Hayatullina Z, Muhammad N, Mohamed N, Soelaiman I (2012) Virgin coconut oil supplementation prevents bone loss in osteoporosis rat model.
- Connor K, Howell J, Chen I, Liu H, Berhane K, Sciarretta C, et al. (1996) Failure of chloro-S-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam Appl Toxicol 30:93-101.
- Houssay B, Martinez C (1947) Experimental diabetes and diet. Sci 105:548-549.









